Iron Deficiency Is Associated with Elevated Parathormone Levels, Low Vitamin D Status, and Risk of Bone Loss in Omnivores and Plant-Based Diet Consumers
Abstract
:1. Introduction
2. Results
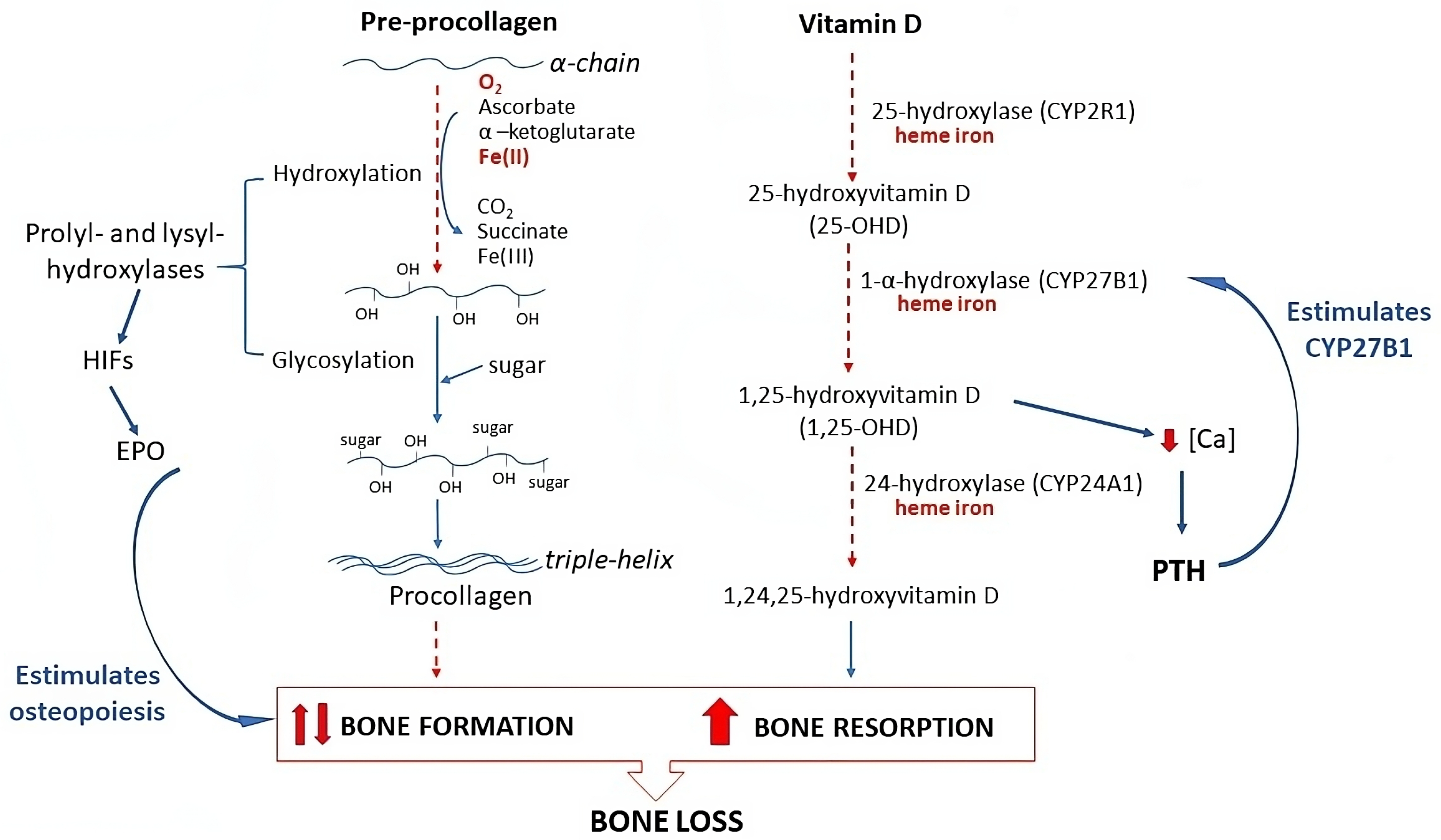
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Determinations
4.3. Cut-Off Values
- PTH status: PTH > 10 and ≤65 pg/mL, normal; PTH > 65 pg/mL, high.
- Vitamin D status: 25-OHD ≥ 75 nmol/L, sufficiency; 25-OHD < 75 nmol/L and ≥50 nmol/L, insufficiency; 25-OHD < 50 nmol/L, deficiency.
- Iron status: ferritin > 30 ng/mL, sufficiency; ferritin ≤ 30 and >15 ng/mL, deficiency; ferritin ≤ 15 ng/mL, depletion; hemoglobin < 12 g/dL for women and <13 g/dL for men, or use of iron supplements, iron deficiency anemia.
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTH | parathormone |
| BAP | bone alkaline phosphatase |
| BMD | bone mineral density |
| BMI | body mass index |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCV | mean corpuscular volume |
| MPV | mean platelet volume |
| NTx | cross-linked N-telopeptides of type I collagen |
| RDW | red cell distribution width |
| WHO | World Health Organization |
| 25-OHD | 25-hydroxycholecalciferol; 25-hydroxy vitamin D |
| 1,25-(OHD)2D | 1,25-hydroxycholecalciferol; 1,25-hydroxy vitamin D |
| 1,24,25-(OH)3D | 1,24,25-hydroxycholecalciferol; 1,24,25-hydroxy vitamin D |
References
- International Osteoporosis Foundation. International Osteoporosis Foundation. 2024. Available online: www.osteoporosis.foundation (accessed on 25 June 2024).
- Safiri, S.; Kolahi, A.A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Yang, J.; Li, Q.; Feng, Y.; Zeng, Y. Iron Deficiency and Iron Deficiency Anemia: Potential Risk Factors in Bone Loss. Int. J. Mol. Sci. 2023, 24, 6891. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Song, L. Chapter One—Calcium and Bone Metabolism Indices. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–46. Available online: https://www.sciencedirect.com/science/article/pii/S0065242317300471 (accessed on 25 June 2024).
- Bienaimé, F.; Prié, D.; Friedlander, G.; Souberbielle, J.C. Vitamin D metabolism and activity in the parathyroid gland. Mol. Cell. Endocrinol. 2011, 347, 30–41. [Google Scholar] [CrossRef]
- Proff, P.; Römer, P. The molecular mechanism behind bone remodelling: A review. Clin. Oral Investig. 2009, 13, 355–362. [Google Scholar] [CrossRef]
- Toxqui, L.; Vaquero, M.P. Chronic Iron Deficiency as an Emerging Risk Factor for Osteoporosis: A Hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef]
- Toxqui, L.; Perez-Granados, A.; Blanco-Rojo, R.; Wright, I.; Gonzalez-Vizcayno, C.; Vaquero, M.P. Effects of an Iron or Iron and Vitamin D-Fortified Flavored Skim Milk on Iron Metabolism: A Randomized Controlled Double-Blind Trial in Iron-Deficient Women. J. Am. Coll. Nutr. 2013, 32, 312–320. [Google Scholar] [CrossRef]
- Santoro, D.; Caccamo, D.; Lucisano, S.; Buemi, M.; Sebekova, K.; Teta, D.; De Nicola, L. Interplay of Vitamin D, Erythropoiesis, and the Renin-Angiotensin System. BioMed Res. Int. 2015, 2015, 145828. [Google Scholar] [CrossRef]
- Katsumata, S.; Katsumata, R.; Matsumoto, N.; Inoue, H.; Takahashi, N.; Uehara, M. Iron deficiency decreases renal 25-hydroxyvitamin D3-1α-hydroxylase activity and bone formation in rats. BMC Nutr. 2016, 2, 33. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; López-Frías, M.R.; Campos, M.S.; López-Frías, M.; Alférez, M.J.M.; Nestares, T.; Ojeda, M.L.; López-Aliaga, I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur. J. Nutr. 2012, 51, 241–247. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Plattner, A.; Jennings, D.; Stoecker, B. Bone Morphology, Strength and Density Are Compromised in Iron-Deficient Rats and Exacerbated by Calcium Restriction. J. Nutr. 2002, 132, 3135–3141. [Google Scholar] [CrossRef] [PubMed]
- Parelman, M.; Stoecker, B.; Baker, A.; Medeiros, D. Iron Restriction Negatively Affects Bone in Female Rats and Mineralization of hFOB Osteoblast Cells. Exp. Biol. Med. 2006, 231, 378–386. [Google Scholar] [CrossRef]
- Harris, M.M.; Houtkooper, L.B.; Stanford, V.A.; Parkhill, C.; Weber, J.L.; Flint-Wagner, H.; Weiss, L.; Going, S.B.; Lohman, T.G. Dietary Iron Is Associated with Bone Mineral Density in Healthy Postmenopausal Women. J. Nutr. 2003, 133, 3598–3602. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Pahor, M.; Lauretani, F.; Penninx, B.W.H.J.; Bartali, B.; Russo, R.; Cherubini, A.; Woodman, R.; Bandinelli, S.; Guralnik, J.M.; et al. Bone density and hemoglobin levels in older persons: Results from the InCHIANTI study. Osteoporos. Int. 2005, 16, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.; Blanco-Rojo, R.; Fernandez, M.; Toxqui, L.; Moreno, G.; Perez-Granados, A.; de la Piedra, C.; Remacha, F.; Vaquero, M.P. Bone remodelling is reduced by recovery from iron-deficiency anaemia in premenopausal women. J. Physiol. Biochem. 2013, 69, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Carboo, J.A.; Dolman-Macleod, R.C.; Uyoga, M.A.; Nienaber, A.; Lombard, M.J.; Malan, L. The relationship between serum 25-hydroxyvitamin D and iron status and anaemia in undernourished and non-undernourished children under five years in South Africa. Hum. Nutr. Metab. 2023, 34, 200224. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K.D. A systematic review of vitamin D status in southern European countries. Eur. J. Nutr. 2018, 57, 2001–2036. [Google Scholar] [CrossRef]
- García-Maldonado, E.; Zapatera, B.; Alcorta, A.; Vaquero, M.P. Metabolic and nutritional biomarkers in adults consuming lacto-ovo vegetarian, vegan and omnivorous diets in Spain. A cross-sectional study. Food Funct. 2023, 14, 1608–1616. [Google Scholar] [CrossRef]
- García-Maldonado, E.; Zapatera, B.; Alcorta, A.; Vaquero, M.P. A microalgae docosahexaenoic acid supplement does not modify the influence of gender and diet on iron status in Spanish vegetarians or omnivores. A randomized placebo-controlled crossover study. Nutrition 2023, 118, 112282. [Google Scholar] [CrossRef]
- Gallego-Narbón, A.; Zapatera, B.; Barrios, L.; Vaquero, M.P. Vitamin B12 and folate status in Spanish lacto-ovo vegetarians and vegans. J. Nutr. Sci. 2019, 8, e7. [Google Scholar] [CrossRef]
- Gallego-Narbón, A.; Zapatera, B.; Vaquero, M.P. Physiological and Dietary Determinants of Iron Status in Spanish Vegetarians. Nutrients 2019, 11, 1734. [Google Scholar] [CrossRef] [PubMed]
- García-Maldonado, E.; Gallego-Narbón, A.; Zapatera, B.; Alcorta, A.; Martínez-Suárez, M.; Vaquero, M.P. Bone Remodelling, Vitamin D Status, and Lifestyle Factors in Spanish Vegans, Lacto-Ovo Vegetarians, and Omnivores. Nutrients 2024, 16, 448. [Google Scholar] [CrossRef]
- Gómez-Alonso, C.; Naves-Díaz, M.L.; Fernández-Martín, J.L.; Díaz-López, J.B.; Fernández-Coto, M.T.; Cannata-Andía, J.B. Vitamin D status and secondary hyperparathyroidism: The importance of 25-hydroxyvitamin D cut-off levels. Kidney Int. 2003, 63, S44–S48. [Google Scholar] [CrossRef]
- López-Ramiro, E.; Rubert, M.; Mahillo, I.; de la Piedra, C. Hiperparatiroidismo secundario al déficit de vitamina D. Rev. Osteoporos. Metab. Miner. 2016, 8, 55–60. [Google Scholar] [CrossRef]
- García-Maldonado, E.; Alcorta, A.; Zapatera, B.; Vaquero, M.P. Changes in fatty acid levels after consumption of a novel docosahexaenoic supplement from algae: A crossover randomized controlled trial in omnivorous, lacto-ovo vegetarians and vegans. Eur. J. Nutr. 2022, 62, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products N and A (NDA). Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products N and A (NDA). Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015, 13, 4101. [Google Scholar]
- Blanco-Rojo, R.; Perez-Granados, A.; Toxqui, L.; Gonzalez-Vizcayno, C.; Delgado, M.; Vaquero, M.P. Efficacy of a microencapsulated iron pyrophosphate-fortified fruit juice: A randomised, double-blind, placebo-controlled study in Spanish iron-deficient women. Br. J. Nutr. 2011, 105, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/331505 (accessed on 25 June 2024).
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Wang, J.; Lee, C.H.; Sud, S.; et al. Erythropoietin Couples Hematopoiesis with Bone Formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Styczyński, J.; Słomka, A.; Łęcka, M.; Albrecht, K.; Romiszewski, M.; Pogorzała, M.; Kubicka, M.; Kuryło-Rafińska, B.; Tejza, B.; Gadomska, G.; et al. Soluble Hemojuvelin and Ferritin: Potential Prognostic Markers in Pediatric Hematopoietic Cell Transplantation. Cancers 2023, 15, 1041. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Kuśmierska, K.; Klemarczyk, W.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children after Vegetarian and Omnivorous Diets. Nutrients 2023, 15, 1376. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Khan, A.A.; Hanley, D.A.; Rizzoli, R.; Bollerslev, J.; Young, J.E.M.; Rejnmark, L.; Thakker, R.; D’amour, P.; Paul, T.; Van Uum, S.; et al. Primary hyperparathyroidism: Review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos. Int. 2017, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, W.Y.; Noltes, M.E.; van Ginhoven, T.M.; Kruijff, S. Secondary and Tertiary Hyperparathyroidism: A Narrative Review. Scand. J. Surg. 2020, 109, 271–278. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Toxqui, L.; Lopez-Parra, A.; Baeza-Richer, C.; Perez-Granados, A.; Arroyo-Pardo, E.; Vaquero, M.P. Influence of Diet, Menstruation and Genetic Factors on Iron Status: A Cross-Sectional Study in Spanish Women of Childbearing Age. Int. J. Mol. Sci. 2014, 15, 4077–4087. [Google Scholar] [CrossRef]
- Toxqui, L.; Perez-Granados, A.; Blanco-Rojo, R.; Wright, I.; Vaquero, M.P. A simple and feasible questionnaire to estimate menstrual blood loss: Relationship with hematological and gynecological parameters in young women. BMC Women’s Health 2014, 14, 71. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. ISO: Geneva, Switzerland, 2005.
 omnivores,
omnivores,  lacto-ovo vegetarians, and
lacto-ovo vegetarians, and  vegans. * Significantly different compared to omnivores (general linear model with Bonferroni correction for multiple comparisons).
vegans. * Significantly different compared to omnivores (general linear model with Bonferroni correction for multiple comparisons).
 omnivores,
omnivores,  lacto-ovo vegetarians, and
lacto-ovo vegetarians, and  vegans. * Significantly different compared to omnivores (general linear model with Bonferroni correction for multiple comparisons).
vegans. * Significantly different compared to omnivores (general linear model with Bonferroni correction for multiple comparisons).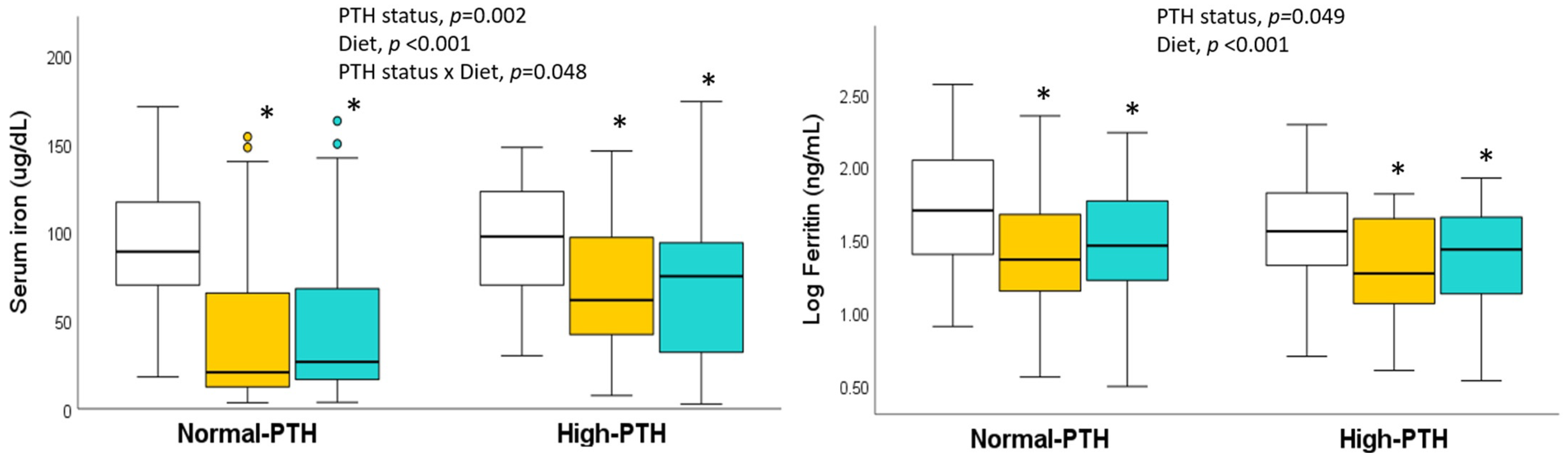
| Normal-PTH (≤65 pg/mL) | High-PTH (>65 pg/mL) | p | |
|---|---|---|---|
| n (%) | 228 (76.8) | 69 (23.2) | |
| Women, n (%) | 153 (67.1) | 46 (66.7) | 0.946 |
| Omnivore, n (%) | 71 (31.1) | 18 (26.1) | |
| Lacto-ovo vegetarian, n (%) | 77 (33.8) | 18 (26.1) | 0.158 |
| Vegan, n (%) | 80 (35.1) | 33 (47.8) | |
| Vitamin D sufficiency a, n (%) | 19 (8.3) | 1 (1.5) | |
| Vitamin D insufficient b, n (%) | 88 (38.6) | 12 (17.6) | <0.001 |
| Vitamin D deficiency c, n (%) | 121 (53.1) | 55 (80.9) | |
| Iron sufficiency d, n (%) | 112 (49.1) | 29 (42.0) | |
| Iron deficiency e, n (%) | 69 (30.3) | 20 (29.0) | 0.327 |
| Iron depletion and anemia f, n (%) | 47 (20.6) | 20 (28.9) | |
| Age (y) | 28 ± 7 | 27 ± 6 | 0.270 |
| Body weight (kg) | 62.6 ± 11.5 | 62.6 ± 10.4 | 0.870 |
| BMI (kg/m2) | 22.4 ± 3.3 | 22.2 ± 2.7 | 0.738 |
| Waist perimeter (cm) | 78.6 ± 9.2 | 76.7 ± 8.8 | 0.234 |
| Hip perimeter (cm) | 95.4 ± 6.8 | 94.9 ± 5.5 | 0.609 |
| Muscle mass (kg) | 46.7 ± 9.4 | 46.7 ± 9.3 | 0.858 |
| Fat mass (kg) | 21.9 ± 7.8 | 21.5 ± 7.5 | 0.625 |
| Bone mass (kg) | 2.5 ± 0.5 | 2.5 ± 0.4 | 0.970 |
| Normal-PTH (≤65 pg/mL) | High-PTH (>65 pg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Man n = 75 | Woman n = 153 | All n = 228 | Man n = 23 | Woman n = 46 | All n = 69 | p Sex | p PTH Status | |
| PTH (pg/mL) | 42.0 ± 13.5 | 42.2 ± 13.8 | 42.1 ± 13.7 | 79.4 ± 12.7 | 80.4 ± 11.3 | 80.1 ± 11.7 | 0.754 | <0.001 |
| 25-OHD (nmol/L) | 47.4 ± 16.8 | 50.2 ± 19.3 | 49.3 ± 18.5 | 37.7 ± 15.7 | 38.5 ± 13.4 | 38.2 ± 14.1 | 0.395 | <0.001 |
| BAP (µg/L) a | 18 (17, 20) | 14 (14, 15) | 15 (14, 17) | 21 (17, 27) | 16 (15, 18) | 17 (15, 19) | 0.004 | 0.070 |
| NTx (nmol/mmol creatinine) a | 76 (69, 86) | 69 (62, 69) | 68 (66, 74) | 100 (90, 114) | 93 (76, 109) | 94 (82, 108) | 0.468 | 0.005 |
| Normal-PTH (≤65 pg/mL) | High-PTH (>65 pg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Man n = 75 | Woman n = 153 | All n = 228 | Man n = 23 | Woman n = 46 | All n = 69 | p Sex | p PTH Status | |
| Erythrocytes (106/mm3) | 5.1 ± 0.4 | 4.5 ± 0.3 | 4.7 ± 0.4 | 5.1 ± 0.4 | 4.4 ± 0.3 | 4.6 ± 0.5 | <0.001 | 0.340 |
| Hemoglobin (g/dL) | 15.5 ± 1.0 | 13.5 ± 1.0 | 14.2 ± 1.4 | 15.2 ± 1.1 | 13.2 ± 1.1 | 13.9 ± 1.4 | <0.001 | 0.058 |
| Hematocrit (%) | 45.5 ± 2.8 | 40.6 ± 2.9 | 42.2 ± 3.7 | 44.9 ± 3.2 | 39.5 ± 3.1 | 41.3 ± 4.0 | <0.001 | 0.039 |
| Serum iron (µg/dL) | 79.6 ± 48.5 | 51.0 ± 44.1 | 60.4 ± 47.5 | 93.5 ± 39.8 | 67.4 ± 39.4 | 76.1 ± 41.2 | <0.001 | 0.020 |
| Transferrin (mg/dL) | 260 ± 41 | 300 ± 57 | 287 ± 56 | 279 ± 48 | 296 ± 58 | 290 ± 55 | <0.001 | 0.323 |
| Transferrin saturation (%) | 32.3 ± 11.8 | 23.1 ± 13.5 | 26.1 ± 13.6 | 28.3 ± 11.1 | 20.6 ± 11.3 | 23.2 ± 11.8 | <0.001 | 0.076 |
| Ferritin (ng/mL) a | 83 (64, 108) | 23 (20, 26) | 30 (26, 33) | 51 (36, 65) | 19 (15, 27) | 27 (20, 36) | <0.001 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaquero, M.P.; García-Maldonado, E.; Gallego-Narbón, A.; Zapatera, B.; Alcorta, A.; Martínez-Suárez, M. Iron Deficiency Is Associated with Elevated Parathormone Levels, Low Vitamin D Status, and Risk of Bone Loss in Omnivores and Plant-Based Diet Consumers. Int. J. Mol. Sci. 2024, 25, 10290. https://doi.org/10.3390/ijms251910290
Vaquero MP, García-Maldonado E, Gallego-Narbón A, Zapatera B, Alcorta A, Martínez-Suárez M. Iron Deficiency Is Associated with Elevated Parathormone Levels, Low Vitamin D Status, and Risk of Bone Loss in Omnivores and Plant-Based Diet Consumers. International Journal of Molecular Sciences. 2024; 25(19):10290. https://doi.org/10.3390/ijms251910290
Chicago/Turabian StyleVaquero, M. Pilar, Elena García-Maldonado, Angélica Gallego-Narbón, Belén Zapatera, Alexandra Alcorta, and Miriam Martínez-Suárez. 2024. "Iron Deficiency Is Associated with Elevated Parathormone Levels, Low Vitamin D Status, and Risk of Bone Loss in Omnivores and Plant-Based Diet Consumers" International Journal of Molecular Sciences 25, no. 19: 10290. https://doi.org/10.3390/ijms251910290







