New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol
Abstract
1. Introduction
2. Results
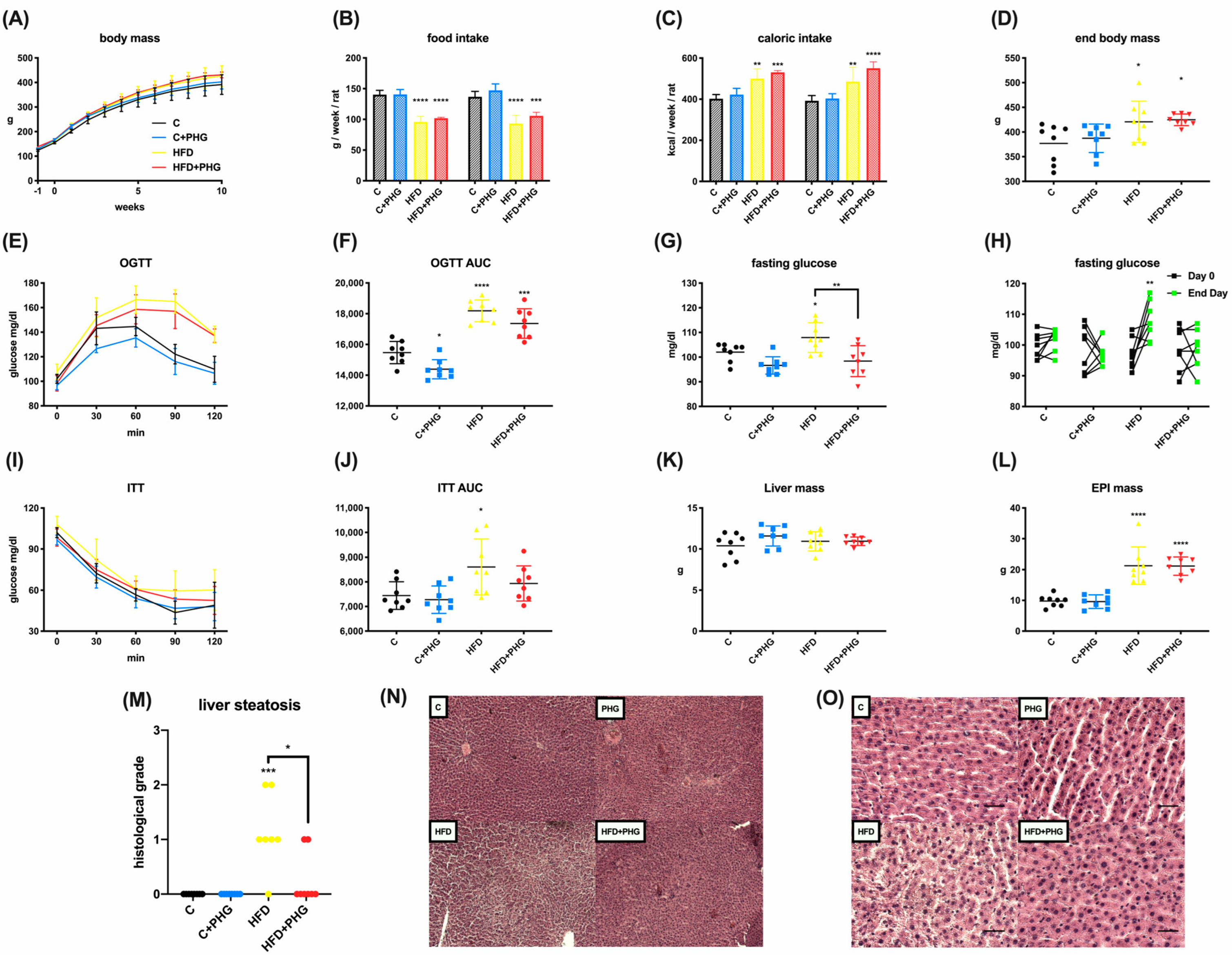
2.1. PHG Protects Wistar Rats from High-Fat-Diet-Induced NAFLD and Insulin Resistance
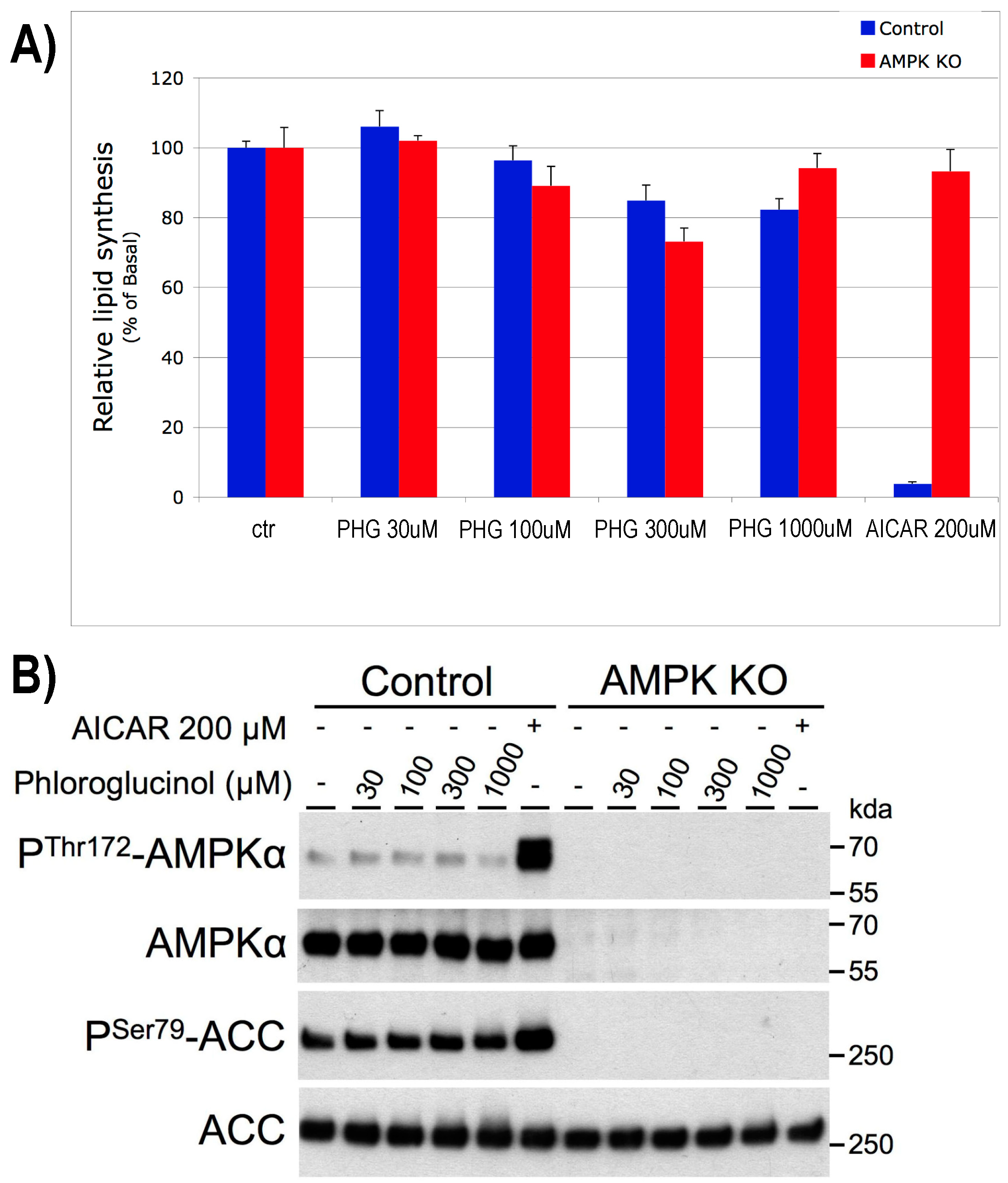
2.2. The Effect of PHG Is Not Related to Hepatic Lipogenesis or AMPK Mediated
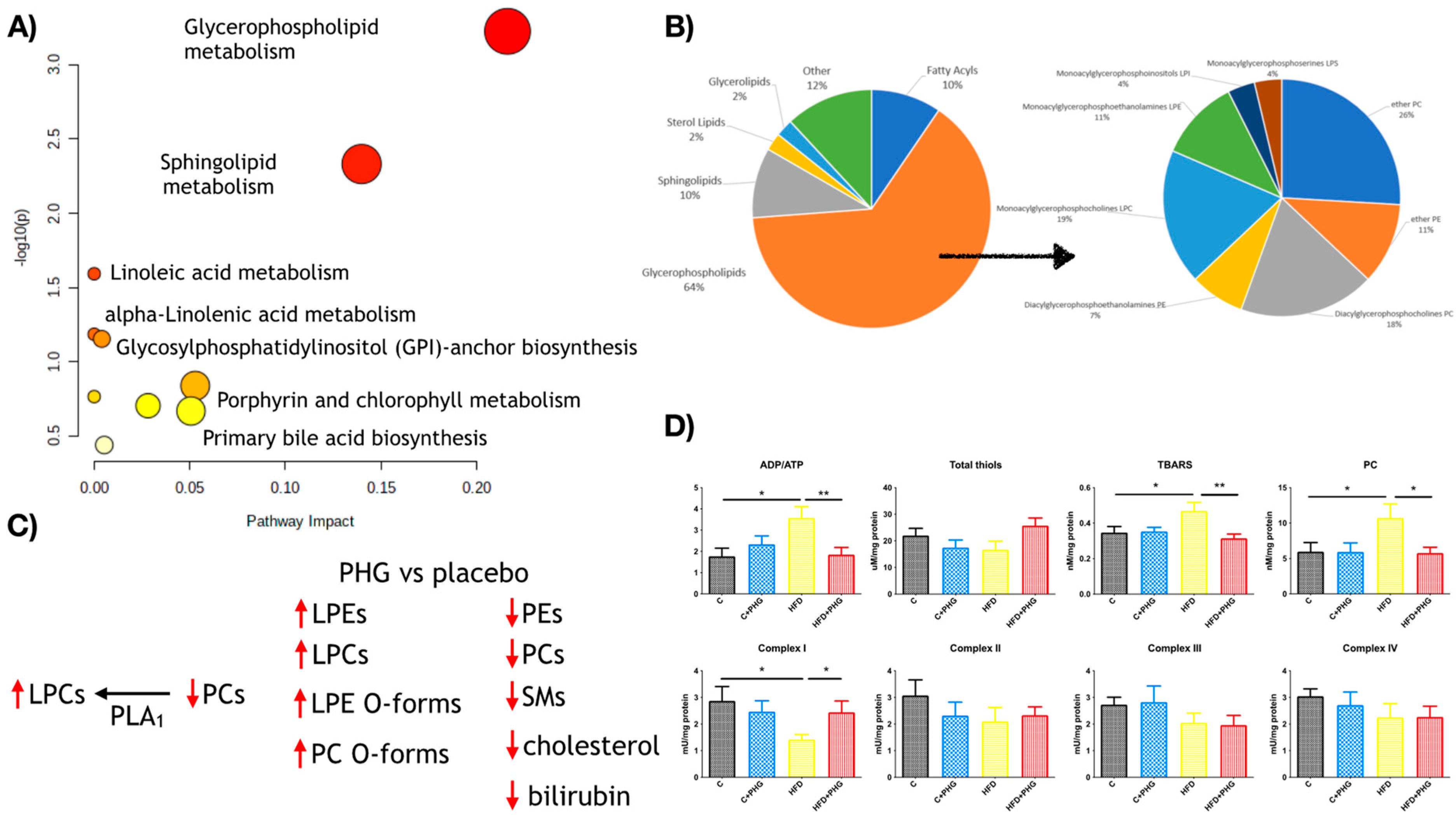
2.3. PHG Protects Mitochondria from Oxidative Stress
2.4. PHG Relaxes Gastric Smooth Muscles
2.5. PHG Ameliorates Postprandial Glucose Tolerance and Calorimetry
2.6. PHG Affects Postprandial Lipid Metabolism
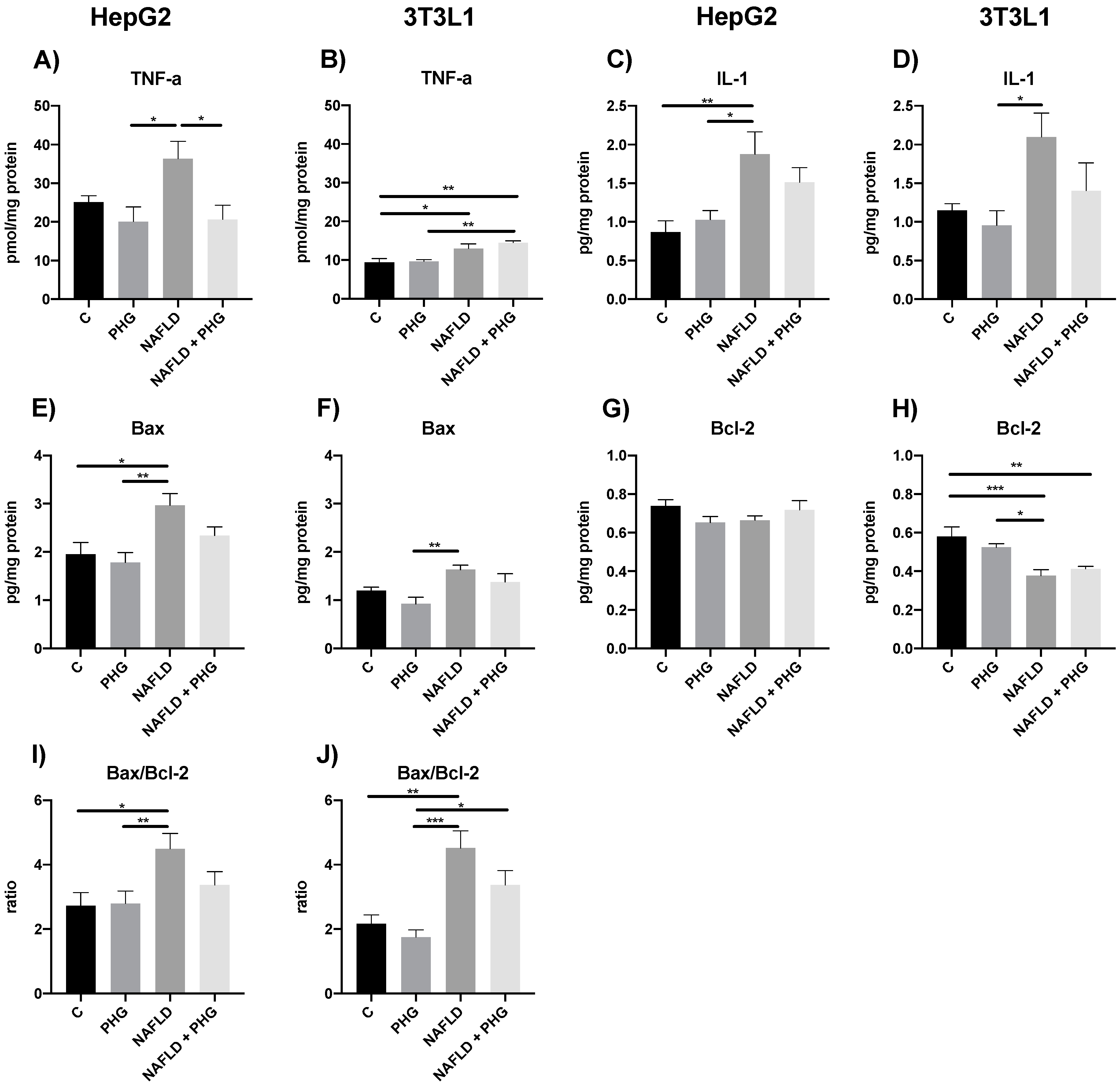
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. High-Fat Diet (HFD) Treatment
4.3. AMPK Knock-Out Hepatocytes and Lipid Synthesis Assay
4.4. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
4.5. Liver Histology
4.6. Cell Culture and Mitochondria Isolation
4.7. Muscle Relaxation Assay
4.7.1. Sample Processing
4.7.2. Experimental Protocol
4.7.3. Measurement of Contraction Parameters
4.8. Single Dose Crossed Intervention
4.8.1. IMP/Placebo
4.8.2. Biochemical Analysis
4.9. Untargeted Metabolomics
4.9.1. Chemical and Reagents
4.9.2. Sample Preparation
4.9.3. HPLC-MS Analysis
4.9.4. Metabolite Annotation
4.9.5. Metabolic Pathway Analysis
4.10. Molecular Assays
4.10.1. Mitochondrial Activity
4.10.2. Glutathione Metabolism
4.10.3. Nitrosative Stress
4.10.4. Oxidative Stress
4.10.5. ELISA
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef]
- Pérez-Carreras, M.; Del Hoyo, P.; Martín, M.A.; Rubio, J.C.; Martín, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef]
- Chavin, K.D.; Yang, S.Q.; Lin, H.Z.; Chatham, J.; Chacko, V.P.; Hock, J.B.; Walajtys-Rode, E.; Rashid, A.; Chen, C.H.; Huang, C.C.; et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J. Biol. Chem. 1999, 274, 5692–5700. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Beneficial effects of exercise: Shifting the focus from body weight to other markers of health. Br. J. Sports Med. 2009, 43, 924–927. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D’Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F.; et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14, 186. [Google Scholar] [CrossRef]
- GRADE Study Research Group. Glycemia Reduction in Type 2 Diabetes—Microvascular and Cardiovascular Outcomes. N. Engl. J. Med. 2022, 387, 1075–1088. [Google Scholar] [CrossRef]
- GRADE Study Research Group. Glycemia Reduction in Type 2 Diabetes—Glycemic Outcomes. N. Engl. J. Med. 2022, 387, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Sirtuins, Aging, and Medicine. N. Engl. J. Med. 2011, 364, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Drygalski, K.; Berk, K.; Charytoniuk, T.; Iłowska, N.; Łukaszuk, B.; Chabowski, A.; Konstantynowicz-Nowicka, K. Does the enterolactone (ENL) affect fatty acid transporters and lipid metabolism in liver? Nutr. Metab. 2017, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berk, K.; Drygalski, K.; Harasim-Symbor, E.; Charytoniuk, T.; Iłowska, N.; Łukaszuk, B.; Chabowski, A.; Konstantynowicz-Nowicka, K. The effect of enterolactone on liver lipid precursors of inflammation. Life Sci. 2019, 221, 341–347. [Google Scholar] [CrossRef]
- Ngu, M.H.; Norhayati, M.N.; Rosnani, Z.; Zulkifli, M.M. Curcumin as adjuvant treatment in patients with non-alcoholic fatty liver (NAFLD) disease: A systematic review and meta-analysis. Complement. Ther. Med. 2022, 68, 102843. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Lin, S.; Chen, T.; Chang, D.; Sun, Y.; Wang, C.; Liu, Y.; Lu, Y.; Song, J.; et al. Advanced effect of curcumin and resveratrol on mitigating hepatic steatosis in metabolic associated fatty liver disease via the PI3K/AKT/mTOR and HIF-1/VEGF cascade. Biomed. Pharmacother. 2023, 165, 115279. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Drygalski, K.; Siewko, K.; Chomentowski, A.; Odrzygóźdź, C.; Zalewska, A.; Krȩtowski, A.; MacIejczyk, M. Phloroglucinol Strengthens the Antioxidant Barrier and Reduces Oxidative/Nitrosative Stress in Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell. Longev. 2021, 2021, 8872702. [Google Scholar] [CrossRef]
- Drygalski, K.; Fereniec, E.; Zalewska, A.; Krętowski, A.; Żendzian-Piotrowska, M.; Maciejczyk, M. Phloroglucinol prevents albumin glycation as well as diminishes ROS production, glycooxidative damage, nitrosative stress and inflammation in hepatocytes treated with high glucose. Biomed. Pharmacother. 2021, 142, 111958. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Feng, Q.P.; Kerchberger, V.E.; Nelson, S.D.; Chen, Q.; Li, B.; Edwards, T.L.; Cox, N.J.; Phillips, E.J.; Stein, C.M.; et al. Integrating gene expression and clinical data to identify drug repurposing candidates for hyperlipidemia and hypertension. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Choi, H.; Jun, H.-S. The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production. Mar. Drugs 2017, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Pouchain, D.; Vanderkam, P.; Perault-Pochat, M.C.; Boussageon, R.; Vaillant-Roussel, H. Efficacy of phloroglucinol for treatment of abdominal pain: A systematic review of literature and meta-analysis of randomised controlled trials versus placebo. Eur. J. Clin. Pharmacol. 2018, 74, 541–548. [Google Scholar] [CrossRef]
- Annaházi, A.; Róka, R.; Rosztóczy, A.; Wittmann, T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 6031. [Google Scholar] [CrossRef]
- Shin, S.Y.; Cha, B.K.; Kim, W.S.; Park, J.Y.; Kim, J.W.; Choi, C.H. The effect of phloroglucinol in patients with diarrhea-predominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Neurogastroenterol. Motil. 2020, 26, 117–127. [Google Scholar] [CrossRef]
- Subissi, A.; Brunori, P.; Bachi, M. Effects of spasmolytics on K+-induced contraction of rat intestine in vivo. Eur. J. Pharmacol. 1983, 96, 295–301. [Google Scholar] [CrossRef]
- Chassany, O.; Bonaz, B.; Bruley Des Varannes, S.; Bueno, L.; Cargill, G.; Coffin, B.; Ducrotté, P.; Grangé, V. Acute exacerbation of pain in irritable bowel syndrome: Efficacy of phloroglucinol/trimethylphloroglucinol. A randomized, double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2007, 25, 1115–1123. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Kim, M.Y.; Bang, E.J.; Moon, S.K.; Yun, S.J.; Kim, W.J.; Noh, J.S.; Kim, G.Y.; Cho, S.; et al. Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS. Antioxidants 2022, 11, 2353. [Google Scholar] [CrossRef]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. AMPK Re-Activation Suppresses Hepatic Steatosis but Its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef]
- Foretz, M.; Viollet, B. Measurement of AMPK-Induced Inhibition of Lipid Synthesis Flux in Cultured Cells. Methods Mol. Biol. 2018, 1732, 363–371. [Google Scholar] [PubMed]
- Blouin, C.M.; Le Lay, S.; Lasnier, F.; Dugail, I.; Hajduch, E. Regulated Association of Caveolins to Lipid Droplets during Differentiation of 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2008, 376, 331–335. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Harasim-Symbor, E.; Polak, A.; Drygalski, K.; Berk, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. Influence of Resveratrol on Sphingolipid Metabolism in Hepatocellular Carcinoma Cells in Lipid Overload State. Anticancer. Agents Med. Chem. 2019, 19, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.J.M.; Trijbels, F.J.M.; Sengers, R.C.A.; Smeitink, J.A.M.; Van Den Heuvel, L.P.; Wintjes, L.T.M.; Stoltenborg-Hogenkamp, B.J.M.; Rodenburg, R.J.T. Spectrophotometric Assay for Complex I of the Respiratory Chain in Tissue Samples and Cultured Fibroblasts. Clin. Chem. 2007, 53, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Ziembicka, D.; Zendzian-Piotrowska, M.; MacIejczyk, M. The impact of high-fat diet on mitochondrial function, free radical production, and nitrosative stress in the salivary glands of wistar rats. Oxid. Med. Cell. Longev. 2019, 2019, 2606120. [Google Scholar] [CrossRef]
- Mantena, S.K.; Vaughn, D.P.; Andringa, K.K.; Eccleston, H.B.; King, A.L.; Abrams, G.A.; Doeller, J.E.; Kraus, D.W.; Darley-Usmar, V.M.; Bailey, S.M. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem. J. 2009, 417, 183–193. [Google Scholar] [CrossRef]
- Zalewska, A.; Ziȩba, S.; Kostecka-Sochoń, P.; Kossakowska, A.; Żendzian-Piotrowska, M.; Matczuk, J.; Maciejczyk, M. NAC Supplementation of Hyperglycemic Rats Prevents the Development of Insulin Resistance and Improves Antioxidant Status but only Alleviates General and Salivary Gland Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 8831855. [Google Scholar] [CrossRef]
- Wojciak, P.A.; Pawłuszewicz, P.; Diemieszczyk, I.; Komorowska-Wojtunik, E.; Czerniawski, M.; Krętowski, A.; Błachnio-Zabielska, A.; Dadan, J.; Ładny, J.R.; Hady, H.R. Laparoscopic sleeve gastrectomy: A study of efficiency in treatment of metabolic syndrome components, comorbidities and influence on certain biochemical markers. Videosurg. Other Miniinvasive Tech. 2020, 15, 136–147. [Google Scholar] [CrossRef]
- Diemieszczyk, I.; Woźniewska, P.; Gołaszewski, P.; Drygalski, K.; Nadolny, K.; Ładny, J.R.; Hady, H.R. Does weight loss after laparoscopic sleeve gastrectomy contribute to reduction in blood pressure? Polish Arch. Intern. Med. 2021, 131, 693–700. [Google Scholar] [CrossRef]
- Modzelewska, B.; Kleszczewski, T.; Kostrzewska, A. The effect of a selective inhibition of potassium channels on the relaxation induced by nitric oxide in the human pregnant myometrium. Cell. Mol. Biol. Lett. 2003, 8, 69–75. [Google Scholar]
- Gagnon, R.C.; Peterson, J.J. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J. Pharmacokinet. Biopharm. 1998, 26, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, K.W.; Gilijamse, P.W.; De Weijer, B.A.; Kilicarslan, M.; Ackermans, M.T.; Nederveen, A.J.; Nieuwdorp, M.; Romijn, J.A.; Serlie, M.J. Sexual dimorphism in hepatic, adipose tissue, and peripheral tissue insulin sensitivity in obese humans. Front. Endocrinol. 2015, 6, 167090. [Google Scholar] [CrossRef]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Daniluk, U.; Daniluk, J.; Kucharski, R.; Kowalczyk, T.; Pietrowska, K.; Samczuk, P.; Filimoniuk, A.; Kretowski, A.; Lebensztejn, D.; Ciborowski, M. Untargeted Metabolomics and Inflammatory Markers Profiling in Children with Crohn’s Disease and Ulcerative Colitis—A Preliminary Study. Inflamm. Bowel Dis. 2019, 25, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Godzien, J.; Alonso-Herranz, V.; Barbas, C.; Armitage, E.G. Controlling the quality of metabolomics data: New strategies to get the best out of the QC sample. Metabolomics 2015, 11, 518–528. [Google Scholar] [CrossRef]
- Gil-De-La-Fuente, A.; Godzien, J.; Saugar, S.; Garcia-Carmona, R.; Badran, H.; Wishart, D.S.; Barbas, C.; Otero, A. CEU Mass Mediator 3.0: A Metabolite Annotation Tool. J. Proteome Res. 2019, 18, 797–802. [Google Scholar] [CrossRef]
- Godzien, J.; Ciborowski, M.; Martínez-Alcázar, M.P.; Samczuk, P.; Kretowski, A.; Barbas, C. Rapid and Reliable Identification of Phospholipids for Untargeted Metabolomics with LC-ESI-QTOF-MS/MS. J. Proteome Res. 2015, 14, 3204–3216. [Google Scholar] [CrossRef]
- Lange, M.; Angelidou, G.; Ni, Z.; Criscuolo, A.; Schiller, J.; Blüher, M.; Fedorova, M. AdipoAtlas: A Reference Lipidome for Human White Adipose Tissue. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rustin, P.; Chretien, D.; Bourgeron, T.; Gérard, B.; Rötig, A.; Saudubray, J.M.; Munnich, A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 1994, 228, 35–51. [Google Scholar] [CrossRef]
- Wharton, D.C.; Tzagoloff, A. [45] Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 1967, 10, 245–250. [Google Scholar]
- Srere, P.A. [1] Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol. 1969, 13, 3–11. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2022, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef]
- Kalousová, M.; Škrha, J.; Zima, T. Advanced Glycation End-Products and Advanced Oxidation Protein Products in Patients with Diabetes Mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [CrossRef]
- Münch, G.; Keis, R.; Weβels, A.; Riederer, P.; Bahner, U.; Heidland, A.; Niwa, T.; Lemke, H.D.; Schinzel, R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 669–678. [Google Scholar] [CrossRef]
- Mil, K.M.; Gryciuk, M.E.; Pawlukianiec, C.; Żendzian-Piotrowska, M.; Ładny, J.R.; Zalewska, A.; Maciejczyk, M. Pleiotropic Properties of Valsartan: Do They Result from the Antiglycooxidant Activity? Literature Review and in Vitro Study. Oxid. Med. Cell. Longev. 2021, 2021, 5575545. [Google Scholar] [CrossRef]
- Bondy, S.C.; Guo, S.X. Effect of ethanol treatment on indices of cumulative oxidative stress. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994, 270, 349–355. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Matczuk, J.; Żendzian-Piotrowska, M.; Niklińska, W.; Fejfer, K.; Szarmach, I.; Ładny, J.R.; Zieniewska, I.; Zalewska, A. Eight-Week Consumption of High-Sucrose Diet Has a Pro-Oxidant Effect and Alters the Function of the Salivary Glands of Rats. Nutrients 2018, 10, 5575545. [Google Scholar] [CrossRef]




| logEC50 | p-Value | Emax | p-Value | |
|---|---|---|---|---|
| Phloroglucinol alone | −3.92 ± 0.14 | 42.27 ± 4.54 | ||
| Phloroglucinol after preincubation with | ||||
| L-NAME | −3.50 ± 0.60 * | 0.021 | 75.15 ± 7.92 *** | p < 0.001 |
| ODQ | −2.98 ± 1.17 * | 0.0106 | 84.64 ± 11.12 ** | 0.0082 |
| 4-AP | −2.94 ± 0.57 **** | p < 0.0001 | 70.95 ± 7.06 ** | 0.0049 |
| ChTX | −3.92 ± 0.11 | 0.472 | 48.11 ± 3.84 | 0.5887 |
| Apamin | −3.00 ± 0.22 **** | p < 0.0001 | 62.11 ± 3.64 ** | 0.0077 |
| Glibenclamide | −2.65 ± 0.82 **** | p < 0.0001 | 59.47 ± 6.87 * | 0.0408 |
| TEA | −4.01 ± 0.14 | 0.1001 | 37.49 ± 6.25 | 0.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drygalski, K.; Maciejczyk, M.; Miksza, U.; Ustymowicz, A.; Godzień, J.; Buczyńska, A.; Chomentowski, A.; Walczak, I.; Pietrowska, K.; Siemińska, J.; et al. New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol. Int. J. Mol. Sci. 2024, 25, 10291. https://doi.org/10.3390/ijms251910291
Drygalski K, Maciejczyk M, Miksza U, Ustymowicz A, Godzień J, Buczyńska A, Chomentowski A, Walczak I, Pietrowska K, Siemińska J, et al. New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol. International Journal of Molecular Sciences. 2024; 25(19):10291. https://doi.org/10.3390/ijms251910291
Chicago/Turabian StyleDrygalski, Krzysztof, Mateusz Maciejczyk, Urszula Miksza, Andrzej Ustymowicz, Joanna Godzień, Angelika Buczyńska, Andrzej Chomentowski, Iga Walczak, Karolina Pietrowska, Julia Siemińska, and et al. 2024. "New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol" International Journal of Molecular Sciences 25, no. 19: 10291. https://doi.org/10.3390/ijms251910291
APA StyleDrygalski, K., Maciejczyk, M., Miksza, U., Ustymowicz, A., Godzień, J., Buczyńska, A., Chomentowski, A., Walczak, I., Pietrowska, K., Siemińska, J., Pawlukianiec, C., Czajkowski, P., Fiedorczuk, J., Moroz, M., Modzelewska, B., Zalewska, A., Kutryb-Zając, B., Kleszczewski, T., Ciborowski, M., ... Adamska-Patruno, E. (2024). New Application of an Old Drug: Anti-Diabetic Properties of Phloroglucinol. International Journal of Molecular Sciences, 25(19), 10291. https://doi.org/10.3390/ijms251910291












