Melatonin Supplementation Alleviates Impaired Spatial Memory by Influencing Aβ1-42 Metabolism via γ-Secretase in the icvAβ1-42 Rat Model with Pinealectomy
Abstract
:1. Introduction
2. Results
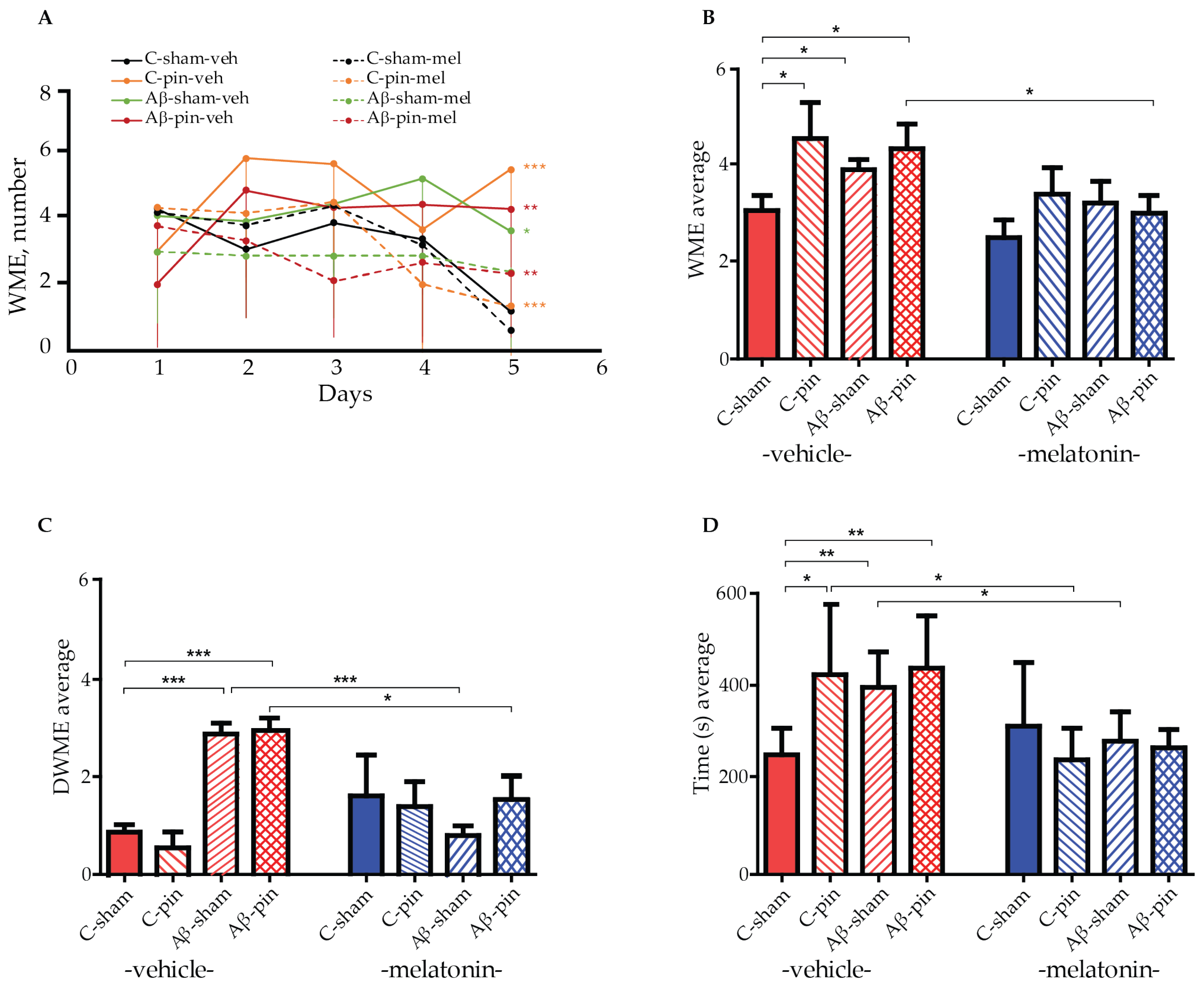
2.1. Chronic Melatonin Treatment Mitigated the Impaired Spatial Memory in the Aβ1-42 Rat Model with Pinealectomy
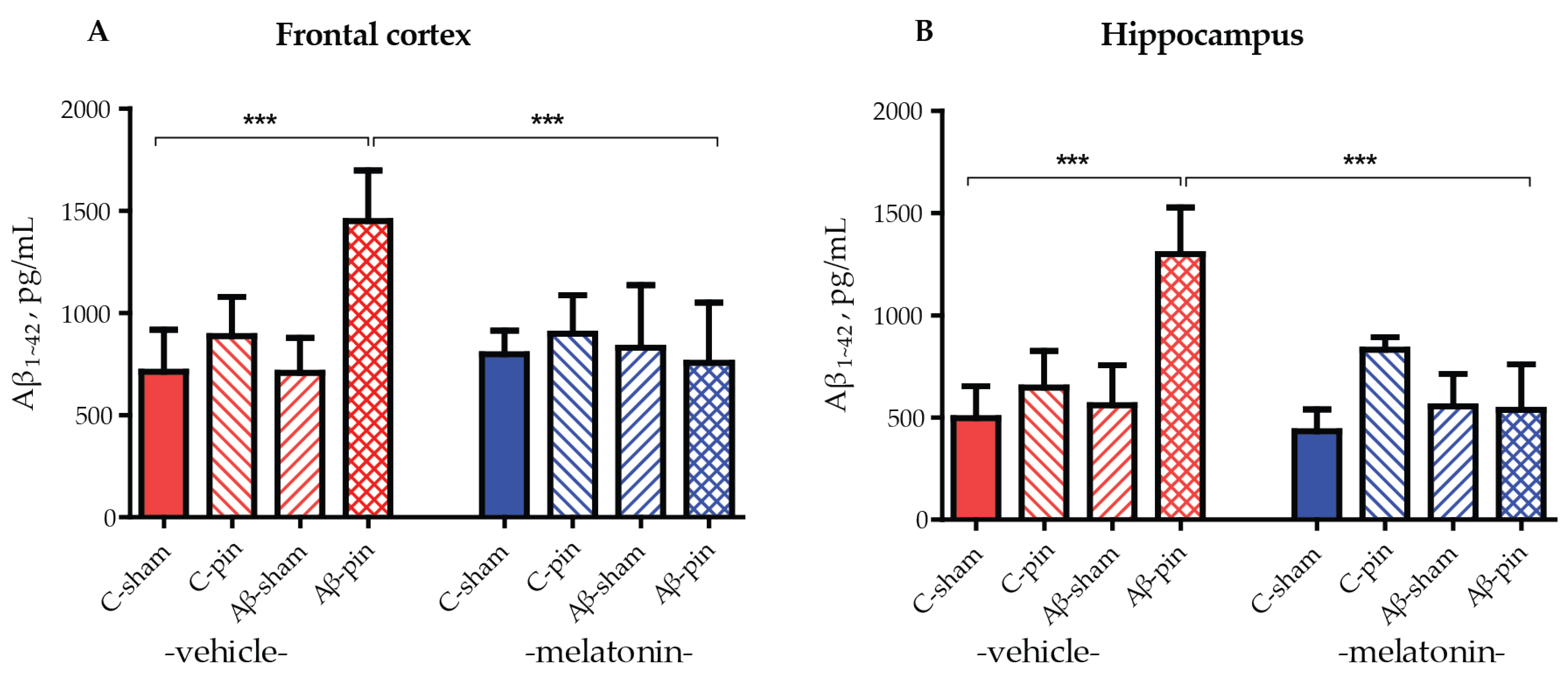
2.2. The Combined Treatment of icvAβ1-42 and Pinealectomy Increased Aβ1-42 Levels in the Frontal Cortex and Hippocampus
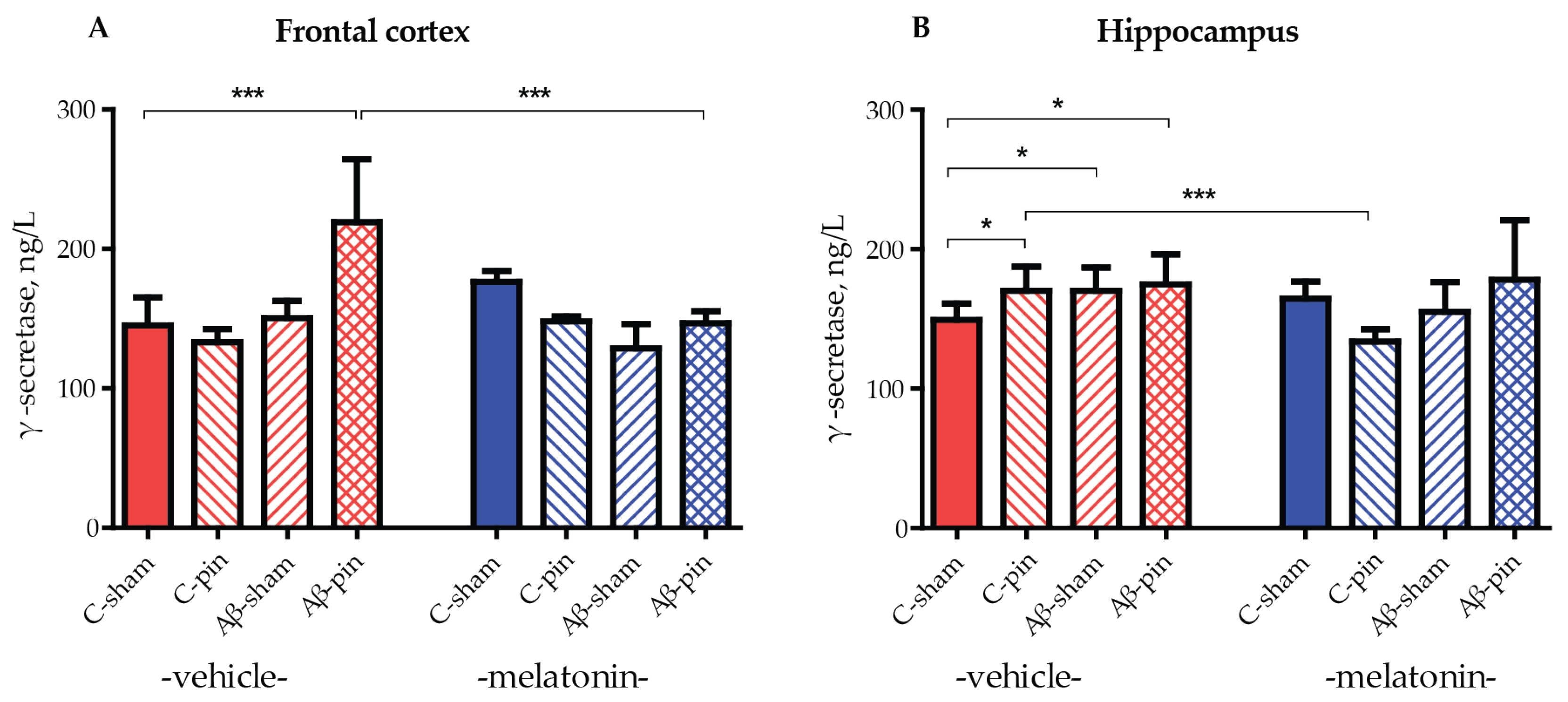
2.3. Melatonin Alleviated icvAβ1-42 + Pinealectomy-Induced an Increase in GS Levels in the Frontal Cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design and Treatment with Melatonin
4.3. Surgery and icv Injection of Aβ1-42
4.4. Radial Arm Maze Test
4.5. Detection of Biochemical Markers in the Homogenates from the Frontal Cortex and Hippocampus
4.5.1. Determination of Aβ1-42 Levels
4.5.2. Determination of GS Levels
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; Dekosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef]
- Götz, J.; Chen, F.; Van Dorpe, J.; Nitsch, R.M. Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Aβ42 Fibrils. Science 2001, 293, 1491–1495. [Google Scholar] [CrossRef]
- Lewis, J.; Dickson, D.W.; Lin, W.L.; Chisholm, L.; Corral, A.; Jones, G.; Yen, S.H.; Sahara, N.; Skipper, L.; Yager, D.; et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 2001, 293, 1487–1491. [Google Scholar] [CrossRef]
- Musiek, E.S.; Holtzman, D.M. Three dimensions of the amyloid hypothesis: Time, space and “wingmen”. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef]
- Whittaker, D.S.; Akhmetova, L.; Carlin, D.; Romero, H.; Welsh, D.K.; Colwell, C.S.; Desplats, P. Circadian modulation by time-restricted feeding rescues brain pathology and improves memory in mouse models of Alzheimer’s disease. Cell Metab. 2023, 35, 1704–1721.e6. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Nixon, R.A. Amyloid precursor protein and endosomal–lysosomal dysfunction in Alzheimer’s disease: Inseparable partners in a multifactorial disease. FASEB J. 2017, 31, 2729–2743. [Google Scholar] [CrossRef] [PubMed]
- Bursavich, M.G.; Harrison, B.A.; Blain, J.F. Gamma Secretase Modulators: New Alzheimer’s Drugs on the Horizon? J. Med. Chem. 2016, 59, 7389–7409. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Diehl, T.S.; Narayanan, S.; Funamoto, S.; Ihara, Y.; De Strooper, B.; Steiner, H.; Haass, C.; Wolfe, M.S. Active γ-Secretase Complexes Contain Only One of Each Component. J. Biol. Chem. 2007, 282, 33985–33993. [Google Scholar] [CrossRef] [PubMed]
- Nordvall, G.; Lundkvist, J.; Sandin, J. Gamma-secretase modulators: A promising route for the treatment of Alzheimer’s disease. Front. Mol. Neurosci. 2023, 16, 1279740. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.; Pickford, F.; Kim, J.; Onstead, L.; Eriksen, J.; Yu, C.; Skipper, L.; Murphy, M.P.; Beard, J.; Das, P.; et al. Aβ42 Is Essential for Parenchymal and Vascular Amyloid Deposition in Mice. Neuron 2005, 47, 191–199. [Google Scholar] [CrossRef]
- Szaruga, M.; Veugelen, S.; Benurwar, M.; Lismont, S.; Sepulveda-Falla, D.; Lleo, A.; Ryan, N.S.; Lashley, T.; Fox, N.C.; Murayama, S.; et al. Qualitative changes in human γ-secretase underlie familial Alzheimer’s disease. J. Exp. Med. 2015, 212, 2003–2013. [Google Scholar] [CrossRef]
- Kretner, B.; Fukumori, A.; Gutsmiedl, A.; Page, R.M.; Luebbers, T.; Galley, G.; Baumann, K.; Haass, C.; Steiner, H. Attenuated Aβ42 Responses to Low Potency γ-Secretase Modulators Can Be Overcome for Many Pathogenic Presenilin Mutants by Second-generation Compounds. J. Biol. Chem. 2011, 286, 15240–15251. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Rigat, L.; Ouk, K.; Kramer, A.; Priller, J. Dysfunction of circadian and sleep rhythms in the early stages of Alzheimer’s disease. Acta Physiol. 2023, 238, e13970. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, P.; Sarkar, J.; Izhaar, R. Circadian dysfunction and Alzheimer’s disease—An updated review. Aging Med. 2023, 6, 71–81. [Google Scholar] [CrossRef]
- Song, J. Pineal gland dysfunction in Alzheimer’s disease: Relationship with the immune-pineal axis, sleep disturbance, and neurogenesis. Mol. Neurodegener. 2019, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, B.; Adorni, F.; Musicco, M.; Appollonio, I.; Bonanni, E.; Caffarra, P.; Caltagirone, C.; Cerroni, G.; Concari, L.; Cosentino, F.I.I.; et al. Prevalence of Sleep Disturbances in Mild Cognitive Impairment and Dementing Disorders: A Multicenter Italian Clinical Cross-Sectional Study on 431 Patients. Dement. Geriatr. Cogn. Disord. 2012, 33, 50–58. [Google Scholar] [CrossRef]
- Mure, L.S. Intrinsically Photosensitive Retinal Ganglion Cells of the Human Retina. Front. Neurol. 2021, 12, 636330. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, P.; Bendlin, B.B.; Zetterberg, H.; De Felice, F.; Tan, X.; Benedict, C. Melatonin: A potential nighttime guardian against Alzheimer’s. Mol. Psychiatry 2024, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Xie, D.; Qin, T.; Zhong, Y.; Xu, Y.; Sun, T. Effect and Mechanism of Exogenous Melatonin on Cognitive Deficits in Animal Models of Alzheimer’s Disease: A Systematic Review and Meta-analysis. Neuroscience 2022, 505, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Tzoneva, R.; Georgieva, I.; Ivanova, N.; Uzunova, V.; Nenchovska, Z.; Apostolova, S.; Stoyanova, T.; Tchekalarova, J. The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvA β 1-42 Rat Model with Pinealectomy. Int. J. Mol. Sci. 2021, 22, 12763. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.H.; Wang, Y.; Fung, M.L.; Zhang, C.; Lim, L.W. Rodent Models of Amyloid-Beta Feature of Alzheimer’s Disease: Development and Potential Treatment Implications. Aging Dis. 2020, 11, 1235–1259. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.D.C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Llibre-Guerra, J.J.; Iaccarino, L.; Coble, D.; Edwards, L.; Li, Y.; Mcdade, E.; Strom, A.; Gordon, B.; Mundada, N.; Schindler, S.E.; et al. Longitudinal clinical, cognitive and biomarker profiles in dominantly inherited versus sporadic early-onset Alzheimer’s disease. Brain Commun. 2023, 5, fcad280. [Google Scholar] [CrossRef]
- Budni, J.; de Oliveira, J. Amyloid beta 1–42-induced animal model of dementia: A review. Genet. Neurol. Behav. Diet Dement. Neurosci. Dement. 2020, 2, 865–880. [Google Scholar] [CrossRef]
- Kasza, Á.; Penke, B.; Frank, Z.; Bozsó, Z.; Szegedi, V.; Hunya, Á.; Németh, K.; Kozma, G.; Fülöp, L. Studies for Improving a Rat Model of Alzheimer’s Disease: Icv Administration of Well-Characterized β-Amyloid 1-42 Oligomers Induce Dysfunction in Spatial Memory. Molecules 2017, 22, 2007. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Nenchovska, Z.; Atanasova, D.; Atanasova, M.; Kortenska, L.; Stefanova, M.; Alova, L.; Lazarov, N. Consequences of long-term treatment with agomelatine on depressive-like behavior and neurobiological abnormalities in pinealectomized rats. Behav. Brain Res. 2016, 302, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Garcez, M.L.; Mina, F.; Bellettini-Santos, T.; Carneiro, F.G.; Luz, A.P.; Schiavo, G.L.; Andrighetti, M.S.; Scheid, M.G.; Bolfe, R.P.; Budni, J. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid β (1-42) in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Feijó, D.P.; Batista-Silva, H.; Garcez, M.L.; Mina, F.; Belletini-Santos, T.; Krasilchik, L.R.; Luz, A.P.; Schiavo, G.L.; Quevedo, J. Lithium and memantine improve spatial memory impairment and neuroinflammation induced by β-amyloid 1-42 oligomers in rats. Neurobiol. Learn. Mem. 2017, 141, 84–92. [Google Scholar] [CrossRef]
- Petrasek, T.; Vojtechova, I.; Lobellova, V.; Popelikova, A.; Janikova, M.; Brozka, H.; Houdek, P.; Sladek, M.; Sumova, A.; Kristofikova, Z.; et al. The McGill Transgenic Rat Model of Alzheimer’s Disease Displays Cognitive and Motor Impairments, Changes in Anxiety and Social Behavior, and Altered Circadian Activity. Front. Aging Neurosci. 2018, 10, 376604. [Google Scholar] [CrossRef]
- Wallace, C.H.; Oliveros, G.; Xie, L.; Serrano, P.; Rockwell, P.; Figueiredo-Pereira, M. Potential Alzheimer’s early biomarkers in a transgenic rat model and benefits of diazoxide/dibenzoylmethane co-treatment on spatial memory and AD-pathology. Sci. Rep. 2024, 14, 3730. [Google Scholar] [CrossRef]
- Ilieva, K.; Atanasova, M.; Atanasova, D.; Kortenska, L.; Tchekalarova, J. Chronic agomelatine treatment alleviates icvAβ-induced anxiety and depressive-like behavior through affecting Aβ metabolism in the hippocampus in a rat model of Alzheimer’s disease. Physiol. Behav. 2021, 239, 113525. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J. Pineal Res. 2015, 59, 47–59. [Google Scholar] [CrossRef]
- Andrade, M.K.; Souza, L.C.; Azevedo, E.M.; Bail, E.L.; Zanata, S.M.; Andreatini, R.; Vital, M.A.B.F. Melatonin reduces β-amyloid accumulation and improves short-term memory in streptozotocin-induced sporadic Alzheimer’s disease model. IBRO Neurosci. Rep. 2023, 14, 264–272. [Google Scholar] [CrossRef]
- Xiao, Q.; Yan, P.; Ma, X.; Liu, H.; Perez, R.; Zhu, A.; Gonzales, E.; Burchett, J.M.; Schuler, D.R.; Cirrito, J.R.; et al. Enhancing astrocytic lysosome biogenesis facilitates Aβ clearance and attenuates amyloid plaque pathogenesis. J. Neurosci. 2014, 34, 9607–9620. [Google Scholar] [CrossRef]
- Li, M.; Pi, H.; Yang, Z.; Reiter, R.J.; Xu, S.; Chen, X.; Chen, C.; Zhang, L.; Yang, M.; Li, Y.; et al. Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 2016, 61, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Htoo, H.H.; Wintachai, P.; Hernandez, J.F.; Dubois, C.; Postina, R.; Xu, H.; Checler, F.; Smith, D.R.; Govitrapong, P.; et al. Melatonin stimulates the nonamyloidogenic processing of βAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J. Pineal Res. 2015, 58, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Kukar, T.L.; Ladd, T.B.; Bann, M.A.; Fraering, P.C.; Narlawar, R.; Maharvi, G.M.; Healy, B.; Chapman, R.; Welzel, A.T.; Price, R.W.; et al. Substrate-targeting γ-secretase modulators. Nature 2008, 453, 925–929. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Ivanova, P.; Krushovlieva, D.; Kortenska, L.; Angelova, V.T. Protective Effect of the Novel Melatonin Analogue Containing Donepezil Fragment on Memory Impairment via MT/ERK/CREB Signaling in the Hippocampus in a Rat Model of Pinealectomy and Subsequent Aβ1-42 Infusion. Int. J. Mol. Sci. 2024, 25, 1867. [Google Scholar] [CrossRef]
- Rudnitskaya, E.A.; Muraleva, N.A.; Maksimova, K.Y.; Kiseleva, E.; Kolosova, N.G.; Stefanova, N.A. Melatonin Attenuates Memory Impairment, Amyloid-β Accumulation, and Neurodegeneration in a Rat Model of Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 47, 103–116. [Google Scholar] [CrossRef]
- Liu, R.-Y.; Zhou, J.-N.; van Heerikhuize, J.; Hofman, M.A.; Swaab, D.F. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J. Clin. Endocrinol. Metab. 1999, 84, 323–327. [Google Scholar] [CrossRef]
- Poeggeler, B.; Miravalle, L.; Zagorski, M.G.; Wisniewski, T.; Chyan, Y.J.; Zhang, Y.; Shao, H.; Bryant-Thomas, T.; Vidal, R.; Frangione, B.; et al. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry 2001, 40, 14995–15001. [Google Scholar] [CrossRef]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: A 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition, 7th ed.; Elsevier Science: Amsterdam, The Netherlands, 2013; ISBN 9780124157521. [Google Scholar]
- Tchekalarova, J.; Krushovlieva, D.; Ivanova, P.; Nenchovska, Z.; Toteva, G.; Atanasova, M. The role of melatonin deficiency induced by pinealectomy on motor activity and anxiety responses in young adult, middle-aged and old rats. Behav. Brain Funct. 2024, 20, 3. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, I.; Tchekalarova, J.; Nenchovska, Z.; Kortenska, L.; Tzoneva, R. Melatonin Supplementation Alleviates Impaired Spatial Memory by Influencing Aβ1-42 Metabolism via γ-Secretase in the icvAβ1-42 Rat Model with Pinealectomy. Int. J. Mol. Sci. 2024, 25, 10294. https://doi.org/10.3390/ijms251910294
Georgieva I, Tchekalarova J, Nenchovska Z, Kortenska L, Tzoneva R. Melatonin Supplementation Alleviates Impaired Spatial Memory by Influencing Aβ1-42 Metabolism via γ-Secretase in the icvAβ1-42 Rat Model with Pinealectomy. International Journal of Molecular Sciences. 2024; 25(19):10294. https://doi.org/10.3390/ijms251910294
Chicago/Turabian StyleGeorgieva, Irina, Jana Tchekalarova, Zlatina Nenchovska, Lidia Kortenska, and Rumiana Tzoneva. 2024. "Melatonin Supplementation Alleviates Impaired Spatial Memory by Influencing Aβ1-42 Metabolism via γ-Secretase in the icvAβ1-42 Rat Model with Pinealectomy" International Journal of Molecular Sciences 25, no. 19: 10294. https://doi.org/10.3390/ijms251910294
APA StyleGeorgieva, I., Tchekalarova, J., Nenchovska, Z., Kortenska, L., & Tzoneva, R. (2024). Melatonin Supplementation Alleviates Impaired Spatial Memory by Influencing Aβ1-42 Metabolism via γ-Secretase in the icvAβ1-42 Rat Model with Pinealectomy. International Journal of Molecular Sciences, 25(19), 10294. https://doi.org/10.3390/ijms251910294







