Are Methylation Patterns in the KALRN Gene Associated with Cognitive and Depressive Symptoms? Findings from the Moli-sani Cohort
Abstract
:1. Introduction
2. Results
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Subjects and Samples
4.2. Cognitive and Psychiatric Assessment
4.3. Statistical Analysis
4.3.1. Analysis of Pyrosequencing Data
4.3.2. Analysis of Array Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parnell, E.; Shapiro, L.P.; Voorn, R.A.; Forrest, M.P.; Jalloul, H.A.; Loizzo, D.D.; Penzes, P. KALRN: A central regulator of synaptic function and synaptopathies. Gene 2021, 768, 145306. [Google Scholar] [CrossRef]
- Marcora, E.; Carlisle, H.J.; Kennedy, M.B. 4.32-The Role of the Postsynaptic Density and the Spine Cytoskeleton in Synaptic Plasticity. In Learning and Memory: A Comprehensive Reference; Byrne, J.H., Ed.; Academic Press: Oxford, UK, 2008; pp. 649–673. [Google Scholar] [CrossRef]
- Paskus, J.D.; Herring, B.E.; Roche, K.W. Kalirin and Trio: RhoGEFs in Synaptic Transmission, Plasticity, and Complex Brain Disorders. Trends Neurosci. 2020, 43, 505–518. [Google Scholar] [CrossRef]
- Penzes, P.; Jones, K.A. Dendritic spine dynamics–A key role for kalirin-7. Trends Neurosci. 2008, 31, 419–427. [Google Scholar] [CrossRef]
- Russell, T.A.; Grubisha, M.J.; Remmers, C.L.; Kang, S.K.; Forrest, M.P.; Smith, K.R.; Kopeikina, K.J.; Gao, R.; Sweet, R.A.; Penzes, P. A Schizophrenia-Linked KALRN Coding Variant Alters Neuron Morphology, Protein Function, and Transcript Stability. Biol. Psychiatry 2018, 83, 499–508. [Google Scholar] [CrossRef]
- Russell, T.A.; Blizinsky, K.D.; Cobia, D.J.; Cahill, M.E.; Xie, Z.; Sweet, R.A.; Duan, J.; Gejman, P.V.; Wang, L.; Csernansky, J.G.; et al. A sequence variant in human KALRN impairs protein function and coincides with reduced cortical thickness. Nat. Commun. 2014, 5, 4858. [Google Scholar] [CrossRef]
- McClory, H.; Wang, X.; Sapp, E.; Gatune, L.W.; Iuliano, M.; Wu, C.-Y.; Nathwani, G.; Kegel-Gleason, K.B.; DiFiglia, M.; Li, X. The COOH-terminal domain of huntingtin interacts with RhoGEF kalirin and modulates cell survival. Sci. Rep. 2018, 8, 8000. [Google Scholar] [CrossRef]
- Singh, H.N.; Swarup, V.; Dubey, N.K.; Jha, N.K.; Singh, A.K.; Lo, W.-C.; Kumar, S. Differential Transcriptome Profiling Unveils Novel Deregulated Gene Signatures Involved in Pathogenesis of Alzheimer’s Disease. Biomedicines 2022, 10, 611. [Google Scholar] [CrossRef]
- Tirozzi, A.; Quiccione, M.S.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Gialluisi, A. A Multi-Trait Association Analysis of Brain Disorders and Platelet Traits Identifies Novel Susceptibility Loci for Major Depression, Alzheimer’s and Parkinson’s Disease. Cells 2023, 12, 245. [Google Scholar] [CrossRef]
- Puigdellívol, M.; Saavedra, A.; Pérez-Navarro, E. Cognitive dysfunction in Huntington’s disease: Mechanisms and therapeutic strategies beyond BDNF. Brain Pathol. (Zur. Switz.) 2016, 26, 752–771. [Google Scholar] [CrossRef]
- Xie, Z.; Shapiro, L.P.; Cahill, M.E.; Russell, T.A.; Lacor, P.N.; Klein, W.L.; Penzes, P. Kalirin-7 prevents dendritic spine dysgenesis induced by amyloid beta-derived oligomers. Eur. J. Neurosci. 2019, 49, 1091–1101. [Google Scholar] [CrossRef]
- Tirozzi, A.; Izzi, B.; Noro, F.; Marotta, A.; Gianfagna, F.; Hoylaerts, M.F.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Assessing Genetic Overlap between Platelet Parameters and Neurodegenerative Disorders. Front Immunol. 2020, 11, 02127. [Google Scholar] [CrossRef]
- Gialluisi, A.; Reccia, M.G.; Tirozzi, A.; Nutile, T.; Lombardi, A.; De Sanctis, C.; Varanese, S.; Pietracupa, S.; Modugno, N.; Simeone, A.; et al. Whole Exome Sequencing Study of Parkinson Disease and Related Endophenotypes in the Italian Population. Front. Neurol. 2019, 10, 1362. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Riess, O.; Soehn, A.S.; Nguyen, H.P. The Guanine nucleotide exchange factor kalirin-7 is a novel synphilin-1 interacting protein and modifies synphilin-1 aggregate transport and formation. PLoS ONE 2012, 7, e51999. [Google Scholar] [CrossRef]
- Remmers, C.; Sweet, R.A.; Penzes, P. Abnormal kalirin signaling in neuropsychiatric disorders. Brain Res. Bull. 2014, 103, 29–38. [Google Scholar] [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Collins, K.A.; Eng, G.K.; Tural, Ü.; Irvin, M.K.; Iosifescu, D.V.; Stern, E.R. Affective and somatic symptom clusters in depression and their relationship to treatment outcomes in the STAR*D sample. J. Affect. Disord. 2022, 300, 469–473. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, J.; Feng, Y.; Feng, L.; Xiao, L.; Chen, X.; Feng, Z.; Yang, J.; Wang, G. The novel subtype of major depressive disorder characterized by somatic symptoms is associated with poor treatment efficacy and prognosis: A data-driven cluster analysis of a prospective cohort in China. J. Affect Disord. 2024, 347, 576–583. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, L.; Wang, Z.; Zhou, X.; Fan, X.; Li, Y.; Xu, J.; Hu, S.; Niu, M.; Song, X.; et al. EWASdb: Epigenome-wide association study database. Nucleic Acids Res. 2019, 47, D989–D993. [Google Scholar] [CrossRef]
- Battram, T.; Yousefi, P.; Crawford, G.; Prince, C.; Sheikhali Babaei, M.; Sharp, G.; Hatcher, C.; Vega-Salas, M.J.; Khodabakhsh, S.; Whitehurst, O.; et al. The EWAS Catalog: A database of epigenome-wide association studies. Wellcome Open Res 2022, 7, 41. [Google Scholar] [CrossRef]
- Hernandez Cordero, A.I.; Yang, C.X.; Obeidat, M.; Yang, J.; MacIsaac, J.; McEwen, L.; Lin, D.; Kobor, M.; Novak, R.; Hudson, F.; et al. DNA methylation is associated with airflow obstruction in patients living with HIV. Thorax 2021, 76, 448–455. [Google Scholar] [CrossRef]
- Qiao, H.; An, S.-C.; Ren, W.; Ma, X.-M. Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav. Brain Res. 2014, 275, 191–200. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef]
- Hill, J.J.; Hashimoto, T.; Lewis, D.A. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2006, 11, 557–566. [Google Scholar] [CrossRef]
- Rubio, M.D.; Haroutunian, V.; Meador-Woodruff, J.H. Abnormalities of the Duo/Ras-related C3 botulinum toxin substrate 1/p21-activated kinase 1 pathway drive myosin light chain phosphorylation in frontal cortex in schizophrenia. Biol. Psychiatry 2012, 71, 906–914. [Google Scholar] [CrossRef]
- Deo, A.J.; Cahill, M.E.; Li, S.; Goldszer, I.; Henteleff, R.; Vanleeuwen, J.E.; Rafalovich, I.; Gao, R.; Stachowski, E.K.; Sampson, A.R.; et al. Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: Its role in dendritic pathology. Neurobiol. Dis. 2012, 45, 796–803. [Google Scholar] [CrossRef]
- Youn, H.; Jeoung, M.; Koo, Y.; Ji, H.; Markesbery, W.R.; Ji, I.; Ji, T.H. Kalirin is under-expressed in Alzheimer’s disease hippocampus. J. Alzheimer’s Dis. JAD 2007, 11, 385–397. [Google Scholar] [CrossRef]
- Murray, P.S.; Kirkwood, C.M.; Gray, M.C.; Ikonomovic, M.D.; Paljug, W.R.; Abrahamson, E.E.; Henteleff, R.A.; Hamilton, R.L.; Kofler, J.K.; Klunk, W.E.; et al. β-Amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol. Aging 2012, 33, 2807–2816. [Google Scholar] [CrossRef]
- Rizzo, H.E.; Escaname, E.N.; Alana, N.B.; Lavender, E.; Gelfond, J.; Fernandez, R.; Hibbs, M.A.; King, J.M.; Carr, N.R.; Blanco, C.L. Maternal diabetes and obesity influence the fetal epigenome in a largely Hispanic population. Clin. Epigenet. 2020, 12, 34. [Google Scholar] [CrossRef]
- Poisel, E.; Zillich, L.; Streit, F.; Frank, J.; Friske, M.M.; Foo, J.C.; Mechawar, N.; Turecki, G.; Hansson, A.C.; Nöthen, M.M.; et al. DNA methylation in cocaine use disorder-An epigenome-wide approach in the human prefrontal cortex. Front Psychiatry 2023, 14, 1075250. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; Olivieri, M.; de Curtis, A.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; The MOLI-SANI Project Investigators. Distribution of short and lifetime risks for cardiovascular disease in Italians. Eur. J. Prev. Cardiol. 2012, 19, 723–730. [Google Scholar] [CrossRef]
- Iacoviello, L.; Bonanni, A.; Costanzo, S.; Curtis, A.; Castelnuovo, A.; Olivieri, M. The Moli-Sani Project, a randomized, prospective cohort study in the Molise region in Italy; design, rationale and objectives. Ital. J. Public Health 2007, 4, 110–118. [Google Scholar] [CrossRef]
- Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Esposito, S.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Incremental monounsaturated to saturated fat ratio and fibre consumption is associated with a reduction in a composite score of modifiable cardiovascular risk factors: Prospective results from the Moli-sani study. Eur. J. Clin. Nutr. 2022, 76, 1697–1704. [Google Scholar] [CrossRef]
- Tian, Y.; Morris, T.J.; Webster, A.P.; Yang, Z.; Beck, S.; Feber, A.; Teschendorff, A.E. ChAMP: Updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics (Oxf. Engl.) 2017, 33, 3982–3984. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Gialluisi, A.; Santonastaso, F.; Bonaccio, M.; Bracone, F.; Shivappa, N.; Hebert, J.R.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Circulating Inflammation Markers Partly Explain the Link between the Dietary Inflammatory Index and Depressive Symptoms. J. Inflamm. Res. 2021, 14, 4955–4968. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Rasmussen, J.; Langerman, H. Alzheimer’s Disease-Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Davis, D.H.; Creavin, S.T.; Yip, J.L.; Noel-Storr, A.H.; Brayne, C.; Cullum, S. Montreal Cognitive Assessment for the detection of dementia. Cochrane Database Syst. Rev. 2021, 7, Cd010775. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/.
- Ahmed, A.; Muhammad, R. A Beginners Review of Jamovi Statistical Software for Economic Research. Dutse Int. J. Soc. Econ. Res. 2021, 6, 109–118. [Google Scholar]
- Kowarik, A.; Templ, M. Imputation with the R package VIM. J. Stat. Softw. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer-Verlag: Berlin, Germany, 2002. [Google Scholar] [CrossRef]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S152. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Palli, D.; Berrino, F.; Vineis, P.; Tumino, R.; Panico, S.; Masala, G.; Saieva, C.; Salvini, S.; Cerati, M.; Pala, V.; et al. A Molecular Epidemiology Project on Diet and Cancer: The Epic-Italy Prospective Study. Design and Baseline Characteristics of Participants. Tumori J. 2003, 89, 586–593. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Ruggiero, E.; Costanzo, S.; Grosso, G.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Joint association of food nutritional profile by Nutri-Score front-of-pack label and ultra-processed food intake with mortality: Moli-sani prospective cohort study. BMJ 2022, 378, e070688. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Byrd, D.A.; Judd, S.E.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Bostick, R.M. Development and Validation of Novel Dietary and Lifestyle Inflammation Scores. J. Nutr. 2019, 149, 2206–2218. [Google Scholar] [CrossRef]
- Washburn, R.A.; Goldfield, S.R.; Smith, K.W.; McKinlay, J.B. The validity of self-reported exercise-induced sweating as a measure of physical activity. Am. J. Epidemiol. 1990, 132, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.A.; LAdams, L.; Haile, G.T. Physical activity assessment for epidemiologic research: The utility of two simplified approaches. Prev. Med. 1987, 16, 636–646. [Google Scholar] [CrossRef]
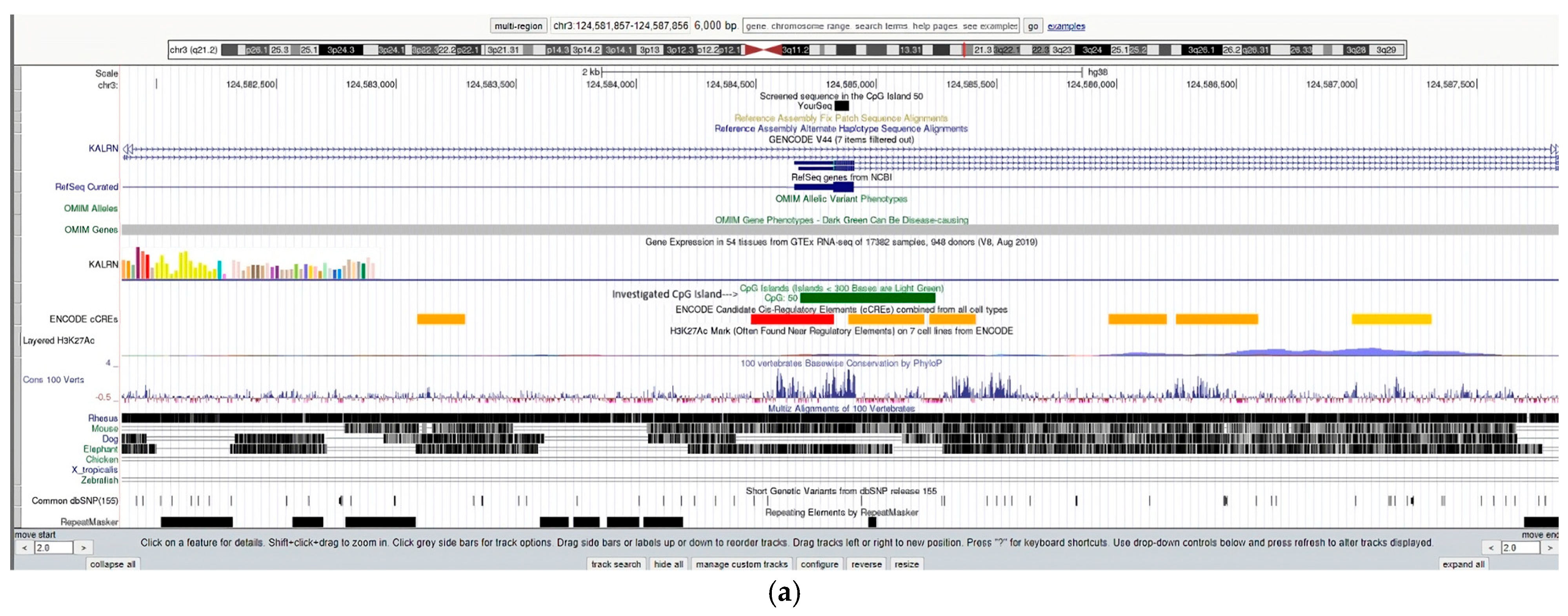
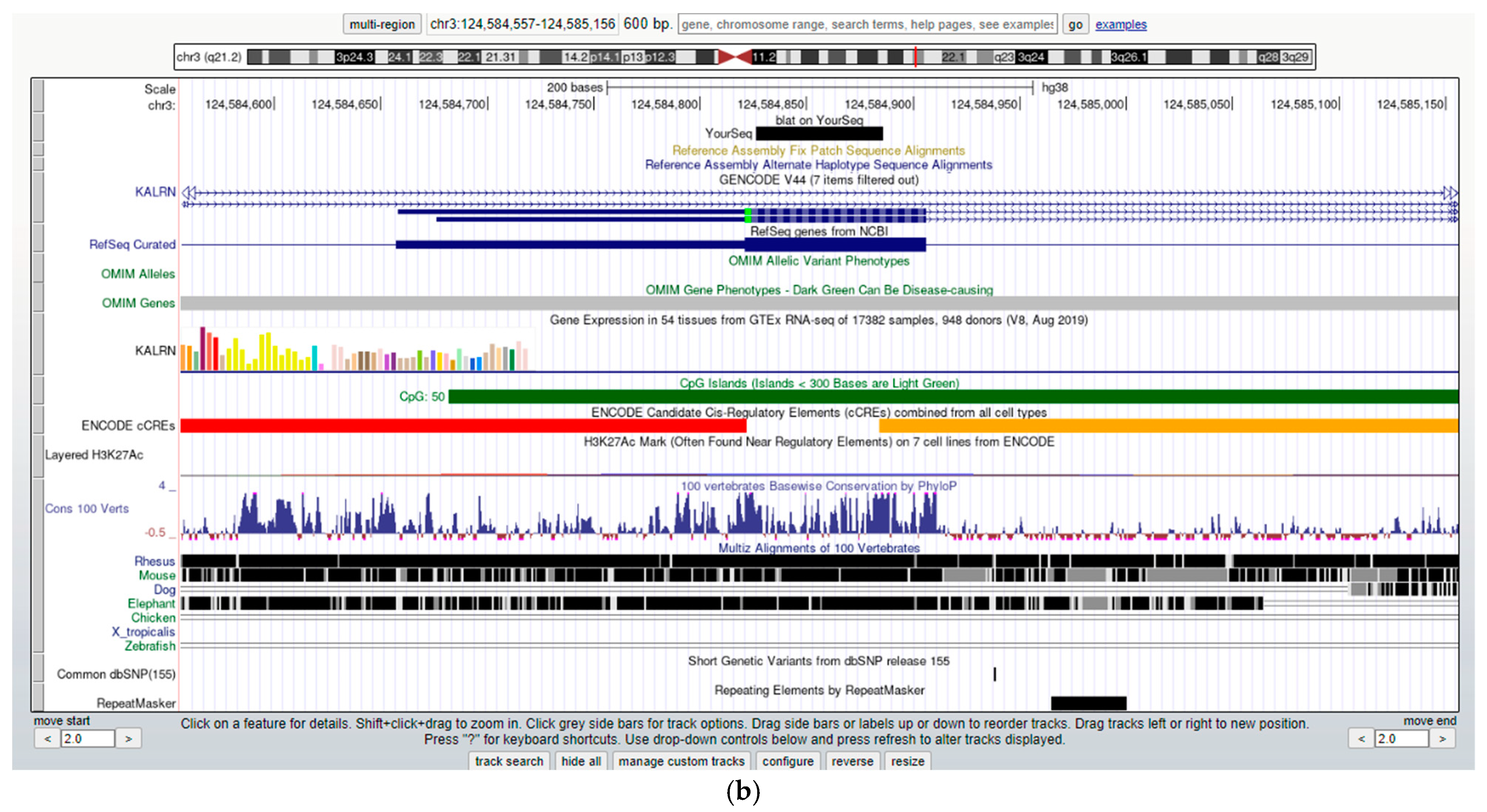
| (a) | |||
| Regression Model | CpG1 β (SE), p | CpG2 β (SE), p | CpG3 β (SE), p |
| Model 1 | −0.050 (0.163), 0.76 | −0.084 (0.160), 0.60 | 0.016 (0.136), 0.91 |
| Model 2 | 0.064 (0.151), 0.67 | 0.003 (0.149), 0.99 | 0.069 (0.127), 0.59 |
| Model 3 | 0.065 (0.151), 0.67 | 0.011 (0.150), 0.94 | 0.073 (0.127), 0.56 |
| Model 4 | 0.068 (0.151), 0.65 | 0.011(0.149), 0.94 | 0.079 (0.127), 0.53 |
| Model 5 | 0.072 (0.151), 0.64 | 0.018 (0.149), 0.91 | 0.085 (0.127), 0.50 |
| (b) | |||
| Regression Model | CpG1 β (SE), p | CpG2 β (SE), p | CpG3 β (SE), p |
| Model 1 | 0.209 (0.216), 0.33 | 0.357 (0.212), 0.09 | 0.191 (0.181), 0.29 |
| Model 2 | 0.206 (0.216), 0.34 | 0.359 (0.213), 0.09 | 0.207 (0.182), 0.25 |
| Model 3 | 0.206 (0.215), 0.34 | 0.338 (0.213), 0.11 | 0.210 (0.181), 0.25 |
| Model 4 | 0.165 (0.215), 0.44 | 0.313 (0.212), 0.14 | 0.203 (0.181), 0.26 |
| Model 5 | 0.169 (0.215), 0.43 | 0.313 (0.212), 0.14 | 0.207 (0.181), 0.25 |
| (a) | ||||
| CpG | Position (GRCh38) | β (SE) | Raw p-Values | Bonferroni p-Values |
| cg10103145 | 124488112 | −0.18 (0.06) | 0.0016 | 0.08 |
| cg05720721 | 124084281 | −0.25 (0.08) | 0.0023 | 0.11 |
| cg22376833 | 124269069 | 0.19 (0.07) | 0.0042 | 0.20 |
| cg05633656 | 124197837 | 0.17 (0.07) | 0.011 | 0.53 |
| cg11148677 | 124157868 | 0.12 (0.05) | 0.013 | 0.64 |
| cg11688731 | 124657745 | 0.16 (0.06) | 0.013 | 0.64 |
| cg00086941 | 124315253 | 0.16 (0.07) | 0.021 | 1 |
| cg21274175 | 124659599 | −0.15 (0.07) | 0.024 | 1 |
| cg00259083 | 124298882 | 0.22 (0.1) | 0.03 | 1 |
| cg16395286 | 124674322 | −0.12 (0.05) | 0.03 | 1 |
| cg23475018 | 124558791 | 0.19 (0.09) | 0.03 | 1 |
| cg16100687 | 124347786 | 0.14 (0.06) | 0.031 | 1 |
| cg24323597 | 124094657 | −0.13 (0.06) | 0.039 | 1 |
| cg12144803 | 124094536 | −0.1 (0.05) | 0.043 | 1 |
| cg24175649 | 124585314 | 0.07 (0.04) | 0.05 | 1 |
| cg01734829 | 124621210 | 0.09 (0.05) | 0.05 | 1 |
| cg04807106 | 124384713 | −0.15 (0.08) | 0.06 | 1 |
| cg07483657 | 124468583 | 0.09 (0.05) | 0.06 | 1 |
| cg07600936 | 124504032 | −0.06 (0.03) | 0.07 | 1 |
| cg19918027 | 124268798 | −0.1 (0.05) | 0.07 | 1 |
| cg24252694 | 124351590 | −0.07 (0.04) | 0.08 | 1 |
| cg20570458 | 124351086 | −0.09 (0.05) | 0.11 | 1 |
| cg23981549 | 124583877 | −0.07 (0.05) | 0.16 | 1 |
| (b) | ||||
| CpG | Position (GRCh38) | β (SE) | Raw p-Values | Bonferroni p-Values |
| cg13549966 | 124106738 | 0.28 (0.08) | 0.0005 | 0.025 |
| cg26510634 | 124158957 | −0.21 (0.07) | 0.0053 | 0.25 |
| cg18981420 | 124584694 | 0.19 (0.07) | 0.0068 | 0.33 |
| cg23837547 | 124584561 | −0.18 (0.07) | 0.0071 | 0.34 |
| cg09990141 | 124563137 | 0.21 (0.08) | 0.010 | 0.50 |
| cg21167201 | 124559065 | 0.13 (0.06) | 0.016 | 0.76 |
| cg01718380 | 124266674 | 0.21 (0.09) | 0.016 | 0.77 |
| cg24323597 | 124094657 | −0.16 (0.07) | 0.024 | 1 |
| cg07600936 | 124504032 | −0.09 (0.04) | 0.028 | 1 |
| cg09751451 | 124521183 | 0.1 (0.05) | 0.048 | 1 |
| cg16919675 | 124518463 | −0.1 (0.05) | 0.05 | 1 |
| cg24829557 | 124094618 | 0.17 (0.09) | 0.06 | 1 |
| cg02754399 | 124584717 | −0.13 (0.07) | 0.06 | 1 |
| cg10908929 | 124208194 | −0.15 (0.08) | 0.06 | 1 |
| cg12777296 | 124624268 | −0.1 (0.05) | 0.06 | 1 |
| cg23440058 | 124384849 | −0.13 (0.07) | 0.07 | 1 |
| cg24175649 | 124585314 | −0.09 (0.05) | 0.09 | 1 |
| cg21618455 | 124231171 | 0.15 (0.09) | 0.09 | 1 |
| cg13779109 | 124407647 | 0.08 (0.05) | 0.1 | 1 |
| cg04516112 | 124584557 | 0.11 (0.07) | 0.1 | 1 |
| cg16100687 | 124347786 | −0.12 (0.07) | 0.1 | 1 |
| cg16395286 | 124674322 | −0.1 (0.06) | 0.11 | 1 |
| cg16278716 | 124094740 | 0.1 (0.06) | 0.11 | 1 |
| cg11148677 | 124157868 | −0.08 (0.06) | 0.13 | 1 |
| cg10430690 | 124094749 | 0.08 (0.06) | 0.15 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiccione, M.S.; Tirozzi, A.; Cassioli, G.; Morelli, M.; Costanzo, S.; Pepe, A.; Bracone, F.; Magnacca, S.; Cerletti, C.; Licastro, D.; et al. Are Methylation Patterns in the KALRN Gene Associated with Cognitive and Depressive Symptoms? Findings from the Moli-sani Cohort. Int. J. Mol. Sci. 2024, 25, 10317. https://doi.org/10.3390/ijms251910317
Quiccione MS, Tirozzi A, Cassioli G, Morelli M, Costanzo S, Pepe A, Bracone F, Magnacca S, Cerletti C, Licastro D, et al. Are Methylation Patterns in the KALRN Gene Associated with Cognitive and Depressive Symptoms? Findings from the Moli-sani Cohort. International Journal of Molecular Sciences. 2024; 25(19):10317. https://doi.org/10.3390/ijms251910317
Chicago/Turabian StyleQuiccione, Miriam Shasa, Alfonsina Tirozzi, Giulia Cassioli, Martina Morelli, Simona Costanzo, Antonietta Pepe, Francesca Bracone, Sara Magnacca, Chiara Cerletti, Danilo Licastro, and et al. 2024. "Are Methylation Patterns in the KALRN Gene Associated with Cognitive and Depressive Symptoms? Findings from the Moli-sani Cohort" International Journal of Molecular Sciences 25, no. 19: 10317. https://doi.org/10.3390/ijms251910317






