MicroRNA-195-5p Inhibits Intracerebral Hemorrhage-Induced Inflammatory Response and Neuron Cell Apoptosis
Abstract
:1. Introduction
2. Results
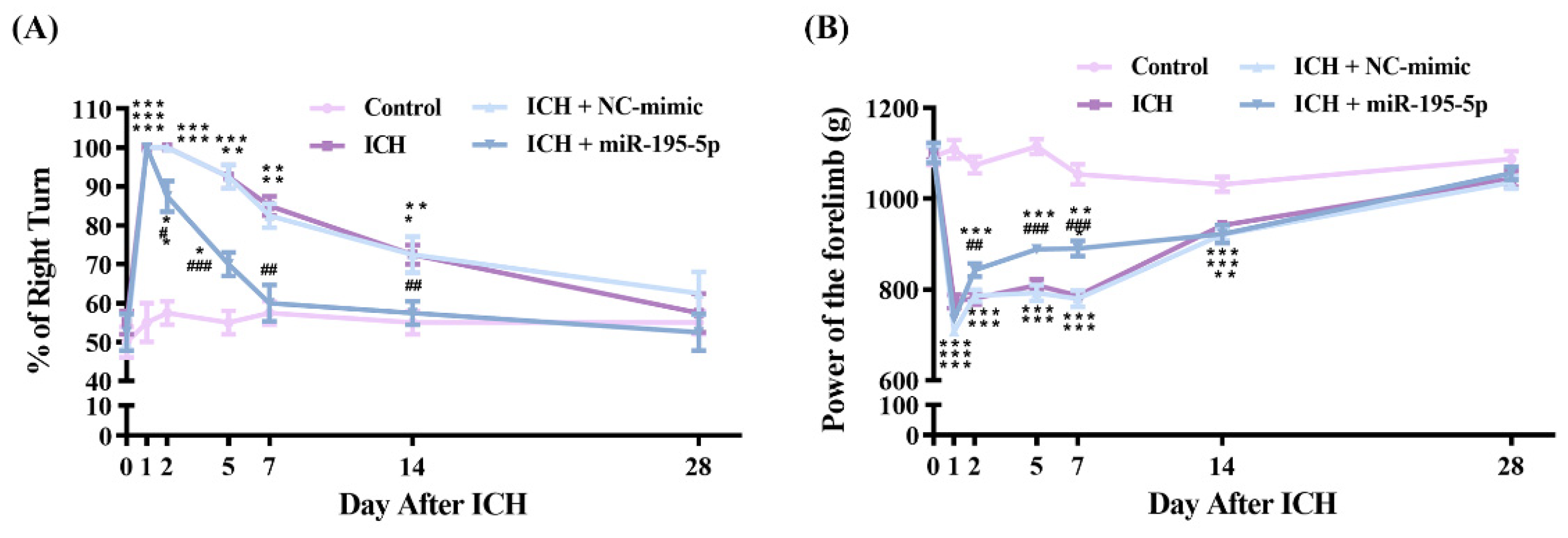
2.1. miR-195-5p Prevents Neurological Dysfunction Following Intracerebral Hemorrhage in Rat Models
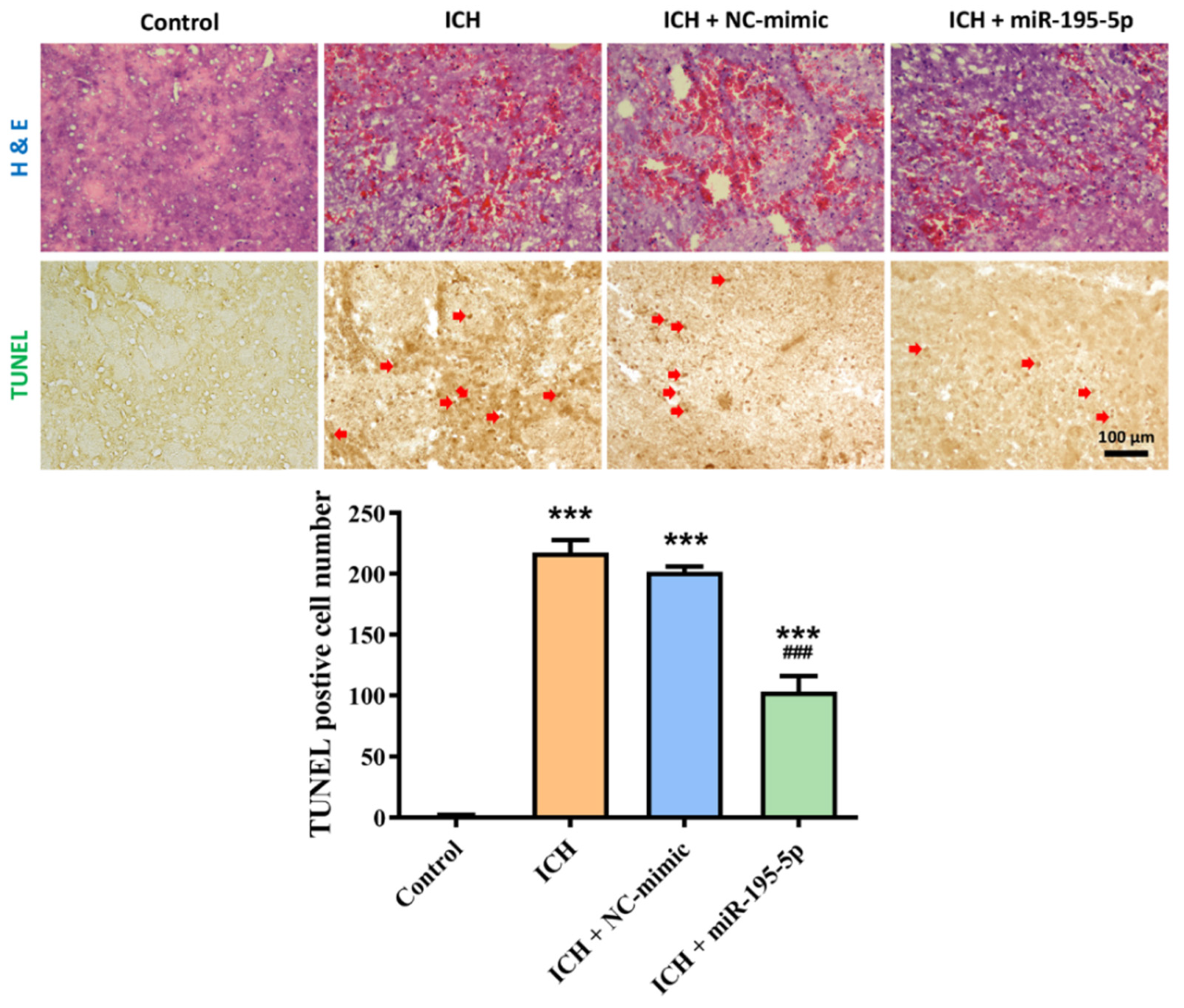
2.2. Effect of miR-195-5p on Tissue Damage and Apoptosis Following Intracerebral Hemorrhage (ICH)
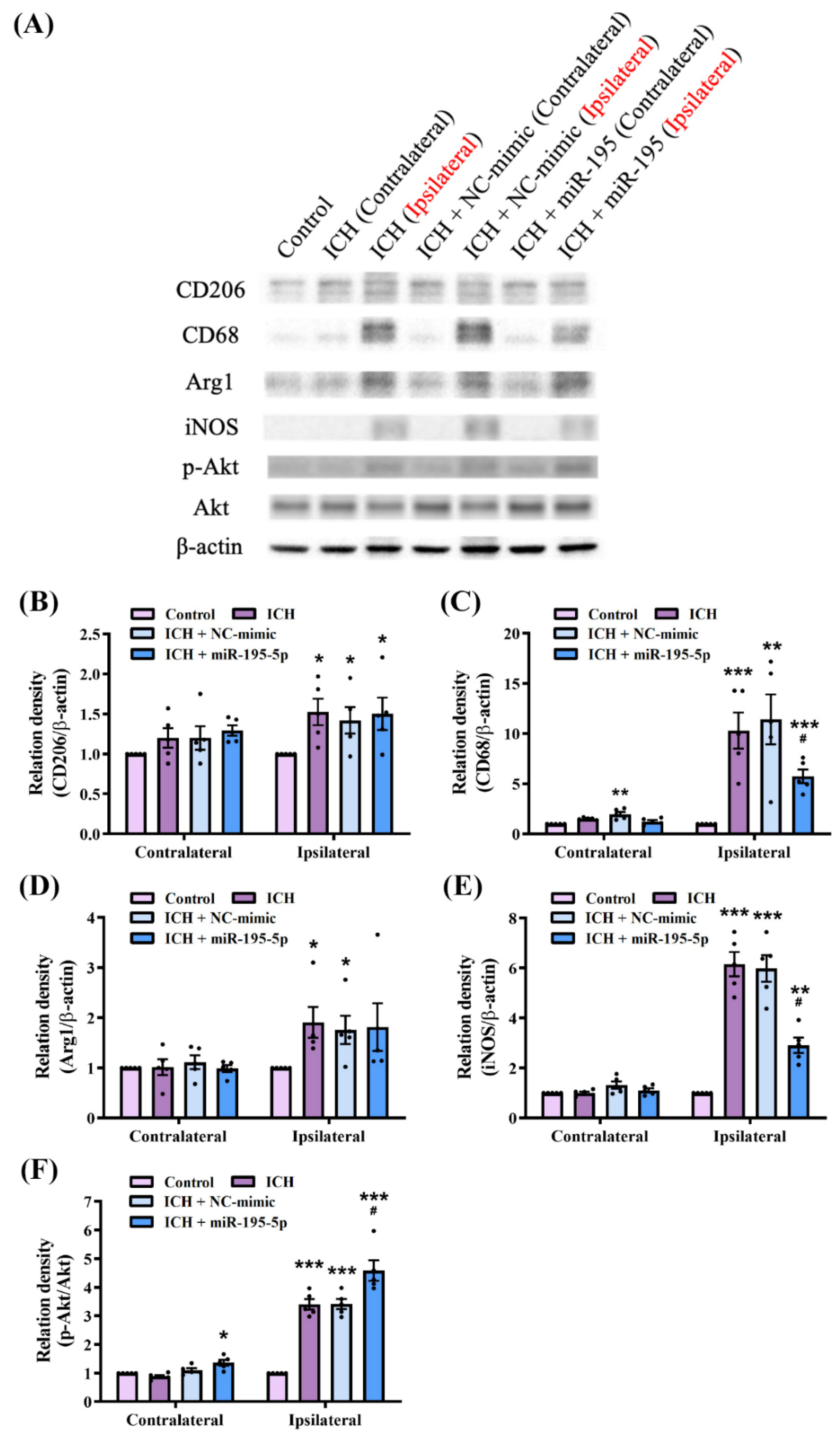
2.3. miR-195-5p Modulates Macrophage Polarization in Intracerebral Hemorrhage
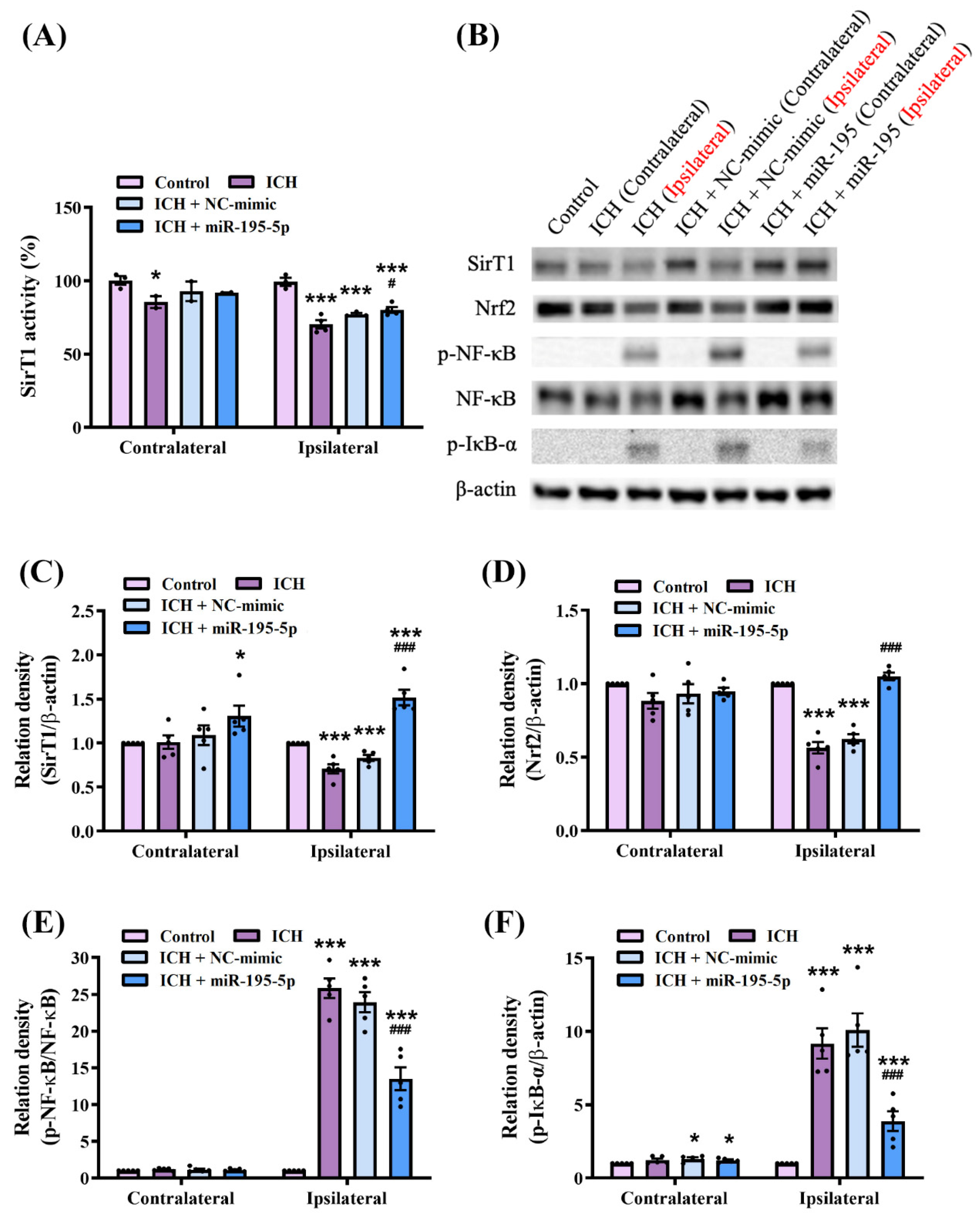
2.4. miR-195-5p Modulates Oxidative Stress and Inflammatory Pathways Following Intracerebral Hemorrhage
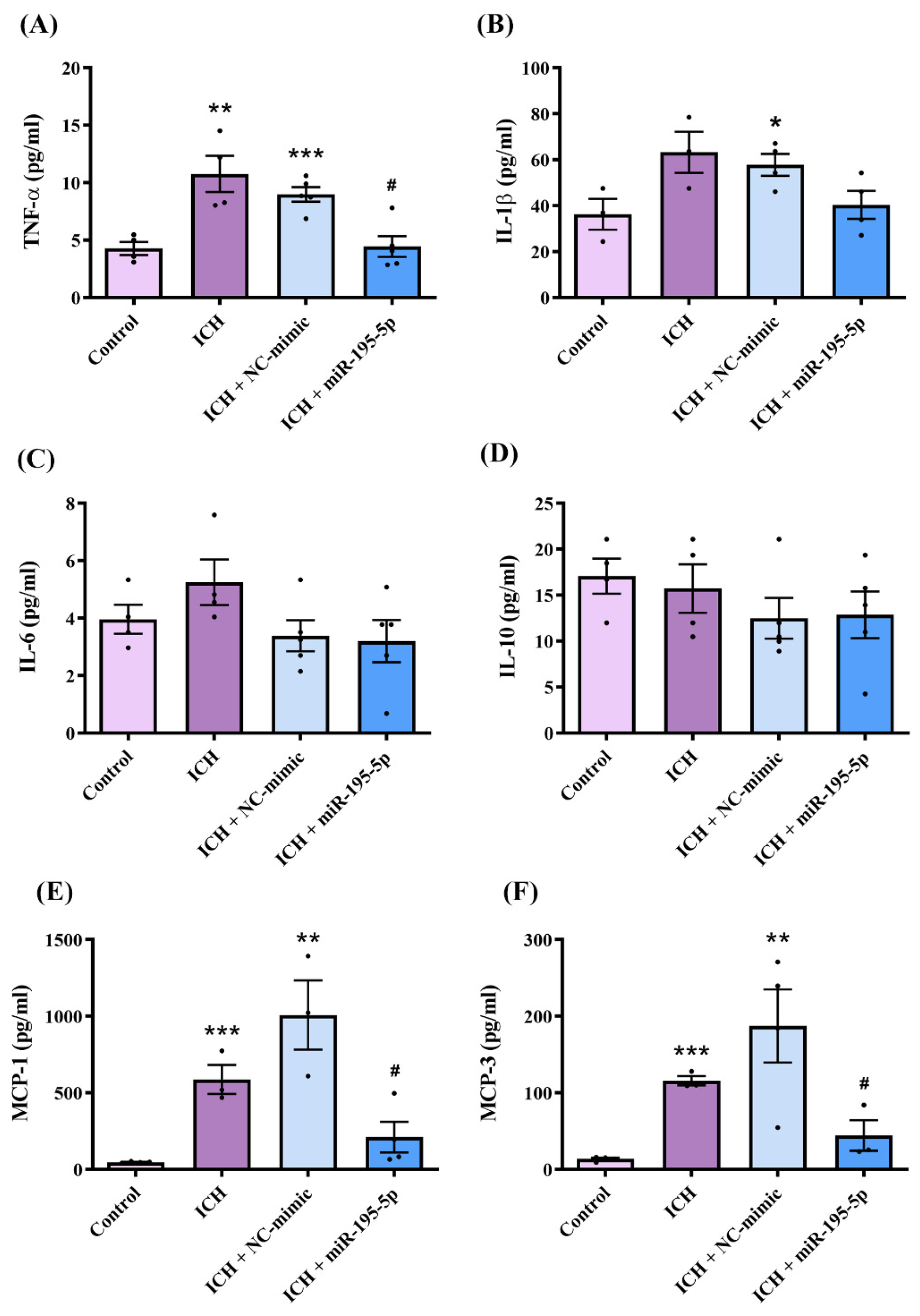
2.5. miR-195-5p Attenuated Intracerebral Hemorrhage-Induced Inflammation Following Intracerebral Hemorrhage Induction
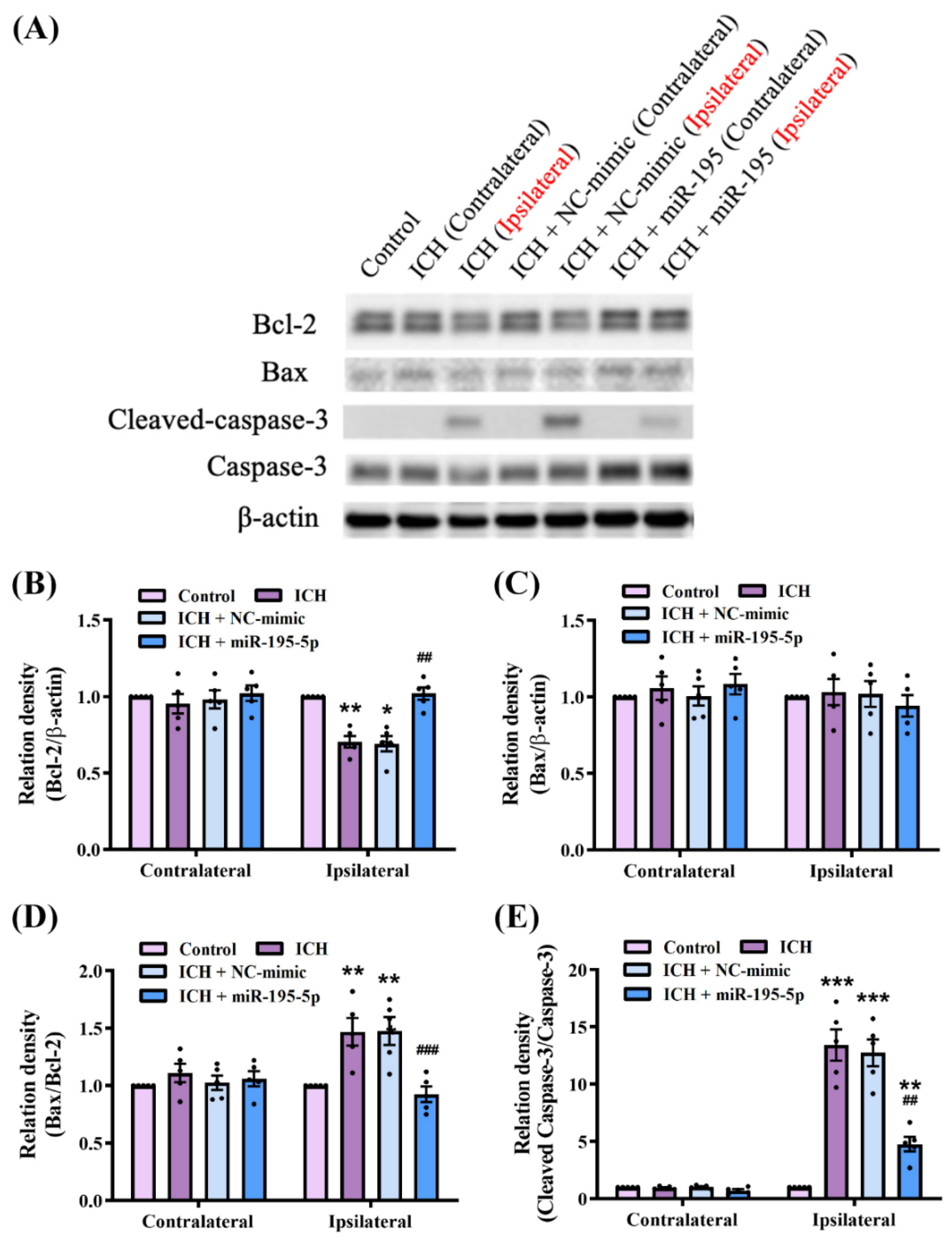
2.6. miR-195-5p Modulates the Apoptosis Mechanism in Intracerebral Hemorrhage
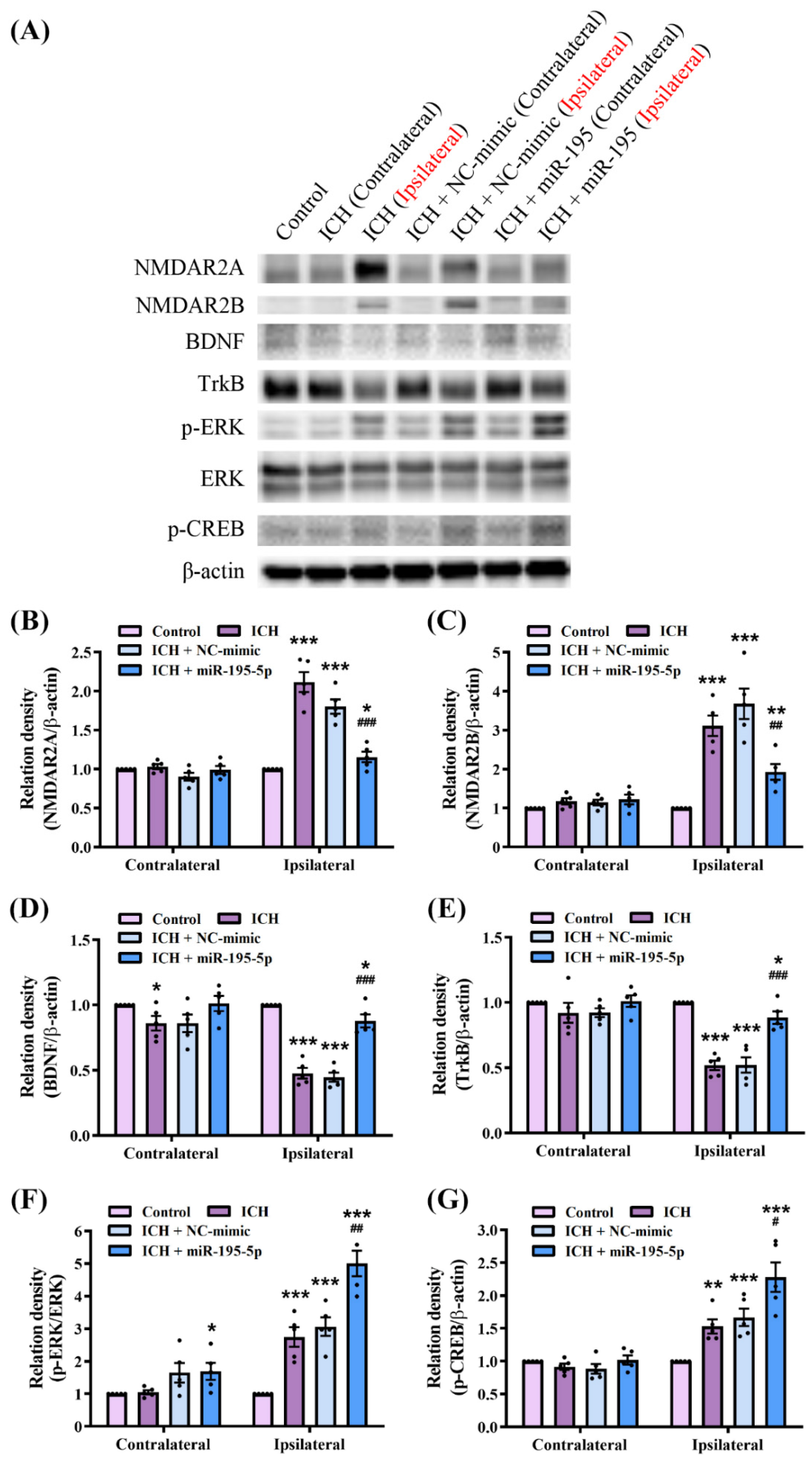
2.7. miR-195-5p Modulates Synaptic Plasticity Pathways in Response to Intracerebral Hemorrhage
3. Discussion
4. Materials and Methods
4.1. Design, Model, and Surgical Preparations: Induction of Intracerebral Hemorrhage
4.2. Neurological Tests
4.2.1. Corner Turn Test
4.2.2. Forelimb Grip Strength Test
4.3. TUNEL and H&E Stain
4.4. Western Blot Analysis
4.5. ELISA
4.6. SirT1 Enzymatic Activity Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markus, H.S. Stroke genetics: Prospects for personalized medicine. BMC Med. 2012, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, G.R.; Auer, R.N. Primary intracerebral hemorrhage. J. Clin. Neurosci. 2006, 13, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, J.; Ge, L.; Xiao, H.; Huang, Y.; Zeng, L.; Jiang, Z.; Lu, M.; Hu, Z. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res. Ther. 2021, 12, 413. [Google Scholar] [CrossRef]
- Zhao, W.B.; Wu, C.J.; Stone, C.; Ding, Y.C.; Ji, X.M. Treatment of intracerebral hemorrhage: Current approaches and future directions. J. Neurol. Sci. 2020, 416, 117020. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef]
- An, S.J.; Kim, T.J.; Yoon, B.W. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef]
- Fiorella, D.; Zuckerman, S.L.; Khan, I.S.; Ganesh Kumar, N.; Mocco, J. Intracerebral Hemorrhage: A Common and Devastating Disease in Need of Better Treatment. World Neurosurg. 2015, 84, 1136–1141. [Google Scholar] [CrossRef]
- Unwin, D.H.; Batjer, H.H.; Greenlee, R.G., Jr. Management controversy. Medical versus surgical therapy for spontaneous intracerebral hemorrhage. Neurosurg. Clin. N. Am. 1992, 3, 533–537. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X.R. Molecular Pathophysiology of Cerebral Hemorrhage Secondary Brain Injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- Zhao, X.; Ting, S.M.; Liu, C.H.; Sun, G.; Kruzel, M.; Roy-O’Reilly, M.; Aronowski, J. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat. Commun. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Peng, C.; Bian, C.; Ocak, P.E.; Zhang, J.H.; Yang, Y.; Zhou, D.; Chen, G.; Luo, Y. Targeting Oxidative Stress and Inflammatory Response for Blood-Brain Barrier Protection in Intracerebral Hemorrhage. Antioxid. Redox Signal. 2022, 37, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.; Ostrow, P.T.; Kim, S.H.; Ali, Z.; Shatla, A.A.; Guterman, L.R.; Hopkins, L.N. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 2003, 52, 1041–1047; discussion 1047–1048. [Google Scholar]
- Tian, Q.; Liu, S.; Han, S.M.; Zhang, W.; Qin, X.Y.; Chen, J.H.; Liu, C.L.; Guo, Y.J.; Li, M.C. The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage. Neural Regen. Res. 2023, 18, 244–252. [Google Scholar] [CrossRef]
- Patil, V.S.; Zhou, R.; Rana, T.M. Gene regulation by non-coding RNAs. Crit. Rev. Biochem. Mol. 2014, 49, 16–32. [Google Scholar] [CrossRef]
- Liu, H.Y.; Lei, C.; He, Q.; Pan, Z.; Xiao, D.S.; Tao, Y.G. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol. Cancer 2018, 17, 64. [Google Scholar] [CrossRef]
- Gomes, A.Q.; Nolasco, S.; Soares, H. Non-Coding RNAs: Multi-Tasking Molecules in the Cell. Int. J. Mol. Sci. 2013, 14, 16010–16039. [Google Scholar] [CrossRef]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Shivdasani, R.A. MicroRNAs: Regulators of gene expression and cell differentiation. Blood 2006, 108, 3646–3653. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Sheng, N.; Zhang, S.; Cao, Y.; Fu, Y. Predicting miRNA-disease associations based on multi-view information fusion. Front. Genet. 2022, 13, 979815. [Google Scholar] [CrossRef] [PubMed]
- Kashif, H.; Shah, D.; Sukumari-Ramesh, S. Dysregulation of microRNA and Intracerebral Hemorrhage: Roles in Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 8115. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.R.; Acuna, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Gong, Y.H.; Hao, S.L.; Wang, B.C. Mesenchymal Stem Cells Transplantation in Intracerebral Hemorrhage: Application and Challenges. Front. Cell. Neurosci. 2021, 15, 653367. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, G.; Sze, J.; Liu, Y.; Lin, S.; Yao, H.; Zhang, J.; Xie, D.; Liu, Q.; Kung, H.F.; et al. Plasma miR-124 Is a Promising Candidate Biomarker for Human Intracerebral Hemorrhage Stroke. Mol. Neurobiol. 2018, 55, 5879–5888. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, G.; Ye, J.; Jiang, J.; Xu, Q. Protective Effect of miR-340-5p against Brain Injury after Intracerebral Hemorrhage by Targeting PDCD4. Cerebrovasc. Dis. 2020, 49, 593–600. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, X.; Hao, Y.; Tang, L.; He, Z. MicroRNA-26a-5p alleviates neuronal apoptosis and brain injury in intracerebral hemorrhage by targeting RAN binding protein 9. Acta Histochem. 2020, 122, 151571. [Google Scholar] [CrossRef]
- Jolana, L.; Kamil, D. The Role of microRNA in Ischemic and Hemorrhagic Stroke. Curr. Drug Deliv. 2017, 14, 816–831. [Google Scholar] [CrossRef]
- Vasudeva, K.; Munshi, A. miRNA dysregulation in ischaemic stroke: Focus on diagnosis, prognosis, therapeutic and protective biomarkers. Eur. J. Neurosci. 2020, 52, 3610–3627. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, X.; Liu, Y.; Liu, H.; Cheng, X.; Gu, Y.; Xu, Y.; Zhu, L. MiR-195-5p facilitates the proliferation, migration, and invasion of human trophoblast cells by targeting FGF2. J. Obstet. Gynaecol. Res. 2022, 48, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Scalavino, V.; Piccinno, E.; Lacalamita, A.; Tafaro, A.; Armentano, R.; Giannelli, G.; Serino, G. miR-195-5p Regulates Tight Junctions Expression via Claudin-2 Downregulation in Ulcerative Colitis. Biomedicines 2022, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, Z.; Guo, C. MiR-195-5p Ameliorates Cerebral Ischemia-Reperfusion Injury by Regulating the PTEN-AKT Signaling Pathway. Neuropsychiatr. Dis. Treat. 2021, 17, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, E.; Scalavino, V.; Labarile, N.; De Marinis, L.; Armentano, R.; Giannelli, G.; Serino, G. Identification of a Novel miR-195-5p/PNN Axis in Colorectal Cancer. Int. J. Mol. Sci. 2024, 11, 5980. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.Y.; Zhou, X.B.; Lai, R.L. Poor expression of miR-195-5p can assist the diagnosis of cerebral vasospasm after subarachnoid hemorrhage and predict adverse outcomes. Brain Behav. 2022, 12, e2766. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.F.; Zhang, L.J.; Guo, F.; Wu, X.Y.; Liu, Y. MiR-195-5p inhibits proliferation and invasion of nerve cells in Hirschsprung disease by targeting GFRA4. Mol. Cell. Biochem. 2021, 476, 2061–2073. [Google Scholar] [CrossRef]
- Xia, H.X.; Zhao, H.; Yang, W.Z.; Luo, X.M.; Wei, J.; Xia, H. MiR-195-5p represses inflammation, apoptosis, oxidative stress, and endoplasmic reticulum stress in sepsis-induced myocardial injury by targeting activating transcription factor 6. Cell Biol. Int. 2022, 46, 243–254. [Google Scholar] [CrossRef]
- Scalavino, V.; Piccinno, E.; Bianco, G.; Schena, N.; Armentano, R.; Giannelli, G.; Serino, G. The Increase of miR-195-5p Reduces Intestinal Permeability in Ulcerative Colitis, Modulating Tight Junctions’ Expression. Int. J. Mol. Sci. 2022, 23, 5840. [Google Scholar] [CrossRef]
- Li, L.; Feng, T.N.; Zhang, W.T.; Gao, S.M.; Wang, R.Y.; Lv, W.W.; Zhu, T.T.; Yu, H.; Qian, B.Y. MicroRNA Biomarker hsa-miR-195-5p for Detecting the Risk of Lung Cancer. Int. J. Genom. 2020, 2020, 7415909. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Li, Q.; Zhang, L.S.; Zhong, L.M.; Gu, M.; He, B.; Qu, Q.; Lao, Y.L.; Gu, K.L.; Zheng, B.R.; et al. Serum miR-195-5p Exhibits Clinical Significance in the Diagnosis of Essential Hypertension with Type 2 Diabetes Mellitus DRD1. Clinics 2021, 76, e2502. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chang, C.H.; Chong, Y.B.; Wu, C.H.; Tsai, H.P.; Cheng, T.L.; Lin, C.L. MicroRNA-195-5p Attenuates Intracerebral-Hemorrhage-Induced Brain Damage by Inhibiting MMP-9/MMP-2 Expression. Biomedicines 2024, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.J.; Takanohashi, A.; Bell, M. Microglia and inflammation: Impact on developmental brain injuries. Ment. Retard. Dev. Disabil. Res. Rev. 2006, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhao, L.; Huang, X.; Ma, C.; Wang, Y.; Zhang, J.; Xuan, D. Enhanced Activity of the Macrophage M1/M2 Phenotypes and Phenotypic Switch to M1 in Periodontal Infection. J. Periodontol. 2016, 87, 1092–1102. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Jaerve, A.; Muller, H.W. Chemokines in CNS injury and repair. Cell Tissue Res. 2012, 349, 229–248. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. Tickets to the brain: Role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J. Neuroimmunol. 2010, 224, 80–84. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Yu, J.; Yang, X.; He, F.; Liu, Z.; Che, F.; Chen, X.; Ren, H.; Hong, M.; et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 2019, 178, 101610. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Yao, Z.; Bai, Q.; Wang, G. Mechanisms of Oxidative Stress and Therapeutic Targets following Intracerebral Hemorrhage. Oxid. Med. Cell. Longev. 2021, 2021, 8815441. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Cui, C.; Zheng, J.; Zhang, H.; Xing, Z. Pterostilbene ameliorates oxidative stress and neuronal apoptosis after intracerebral hemorrhage via the sirtuin 1-mediated Nrf2 pathway in vivo and in vitro. J. Stroke Cerebrovasc. Dis. 2024, 33, 107950. [Google Scholar] [CrossRef]
- Yang, B.; Xu, B.; Zhao, H.; Wang, Y.B.; Zhang, J.; Li, C.W.; Wu, Q.; Cao, Y.K.; Li, Y.; Cao, F. Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways. Mol. Med. Rep. 2018, 18, 973–980. [Google Scholar] [CrossRef]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011, 406, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, L.; Wang, X.; Wen, T.; Xing, J.; Zhao, J. Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radic. Res. 2008, 42, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, T. Inflammatory cause of metabolic syndrome via brain stress and NF-κB. Aging 2012, 4, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.H.; Ge, J.B.; Li, M.; Wu, F.; Zhang, W.; Qin, Z.H. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur. J. Pharm. Sci. 2012, 47, 652–660. [Google Scholar] [CrossRef]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef]
- Alam, A.; Thelin, E.P.; Tajsic, T.; Khan, D.Z.; Khellaf, A.; Patani, R.; Helmy, A. Cellular infiltration in traumatic brain injury. J. Neuroinflamm. 2020, 17, 328. [Google Scholar] [CrossRef]
- Thapa, K.; Shivam, K.; Khan, H.; Kaur, A.; Dua, K.; Singh, S.; Singh, T.G. Emerging Targets for Modulation of Immune Response and Inflammation in Stroke. Neurochem. Res. 2023, 48, 1663–1690. [Google Scholar] [CrossRef]
- Sachana, M.; Rolaki, A.; Bal-Price, A. Development of the Adverse Outcome Pathway (AOP): Chronic binding of antagonist to N-methyl-d-aspartate receptors (NMDARs) during brain development induces impairment of learning and memory abilities of children. Toxicol. Appl. Pharmacol. 2018, 354, 153–175. [Google Scholar] [CrossRef]
- Marini, A.M.; Jiang, X.; Wu, X.; Tian, F.; Zhu, D.; Okagaki, P.; Lipsky, R.H. Role of brain-derived neurotrophic factor and NF-κB in neuronal plasticity and survival: From genes to phenotype. Restor. Neurol. Neurosci. 2004, 22, 121–130. [Google Scholar]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, J.; Wang, Z.; Xu, L.; Liu, A.; Du, G. DL0410 attenuates oxidative stress and neuroinflammation via BDNF/TrkB/ERK/CREB and Nrf2/HO-1 activation. Int. Immunopharmacol. 2020, 86, 106729. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Chu, K.; Jung, K.H.; Kim, S.U.; Kim, M.; Roh, J.K. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 2003, 34, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Jeong, S.W.; Han, S.Y.; Lee, S.T.; Kim, J.Y.; Kim, M.; Roh, J.K. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke 2004, 35, 1744–1749. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Chang, C.-H.; Chong, Y.B.; Wu, C.-H.; Tsai, H.-P.; Cheng, T.-L.; Lin, C.-L. MicroRNA-195-5p Inhibits Intracerebral Hemorrhage-Induced Inflammatory Response and Neuron Cell Apoptosis. Int. J. Mol. Sci. 2024, 25, 10321. https://doi.org/10.3390/ijms251910321
Tsai Y-C, Chang C-H, Chong YB, Wu C-H, Tsai H-P, Cheng T-L, Lin C-L. MicroRNA-195-5p Inhibits Intracerebral Hemorrhage-Induced Inflammatory Response and Neuron Cell Apoptosis. International Journal of Molecular Sciences. 2024; 25(19):10321. https://doi.org/10.3390/ijms251910321
Chicago/Turabian StyleTsai, Yi-Cheng, Chih-Hui Chang, Yoon Bin Chong, Chieh-Hsin Wu, Hung-Pei Tsai, Tian-Lu Cheng, and Chih-Lung Lin. 2024. "MicroRNA-195-5p Inhibits Intracerebral Hemorrhage-Induced Inflammatory Response and Neuron Cell Apoptosis" International Journal of Molecular Sciences 25, no. 19: 10321. https://doi.org/10.3390/ijms251910321






