Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies
Abstract
1. Introduction
2. Expression of B7-H6
2.1. Cancer
2.1.1. Liquid Cancer
Acute Myeloid Leukemia (AML)
Chronic Myeloid Leukemia (CML)
T-Lymphoblastic Lymphoma
Non-Hodgkin Lymphoma (NHL)
2.1.2. Solid Cancer
Oral Squamous Cell Carcinoma
Hepatocellular Carcinoma
Small-Cell Lung Cancer (SCLC)
Non-Small-Cell Lung Cancer (NSCLC)
Cervical Squamous Cell Carcinoma
Ovarian Cancer
Pancreatic Cancer (PC)
Esophageal Squamous Cell Carcinoma
Breast Cancer
Gastric Carcinoma/Colorectal Cancer
High-Risk Neuroblastoma
Astrocytoma
Glioma
2.2. CD14+CD16+ Pro-Inflammatory Monocytes and Neutrophils
2.3. Activated T Cell
3. Upstream of B7-H6
4. Intrinsic Pathways of B7-H6
5. Extrinsic Pathway of B7-H6
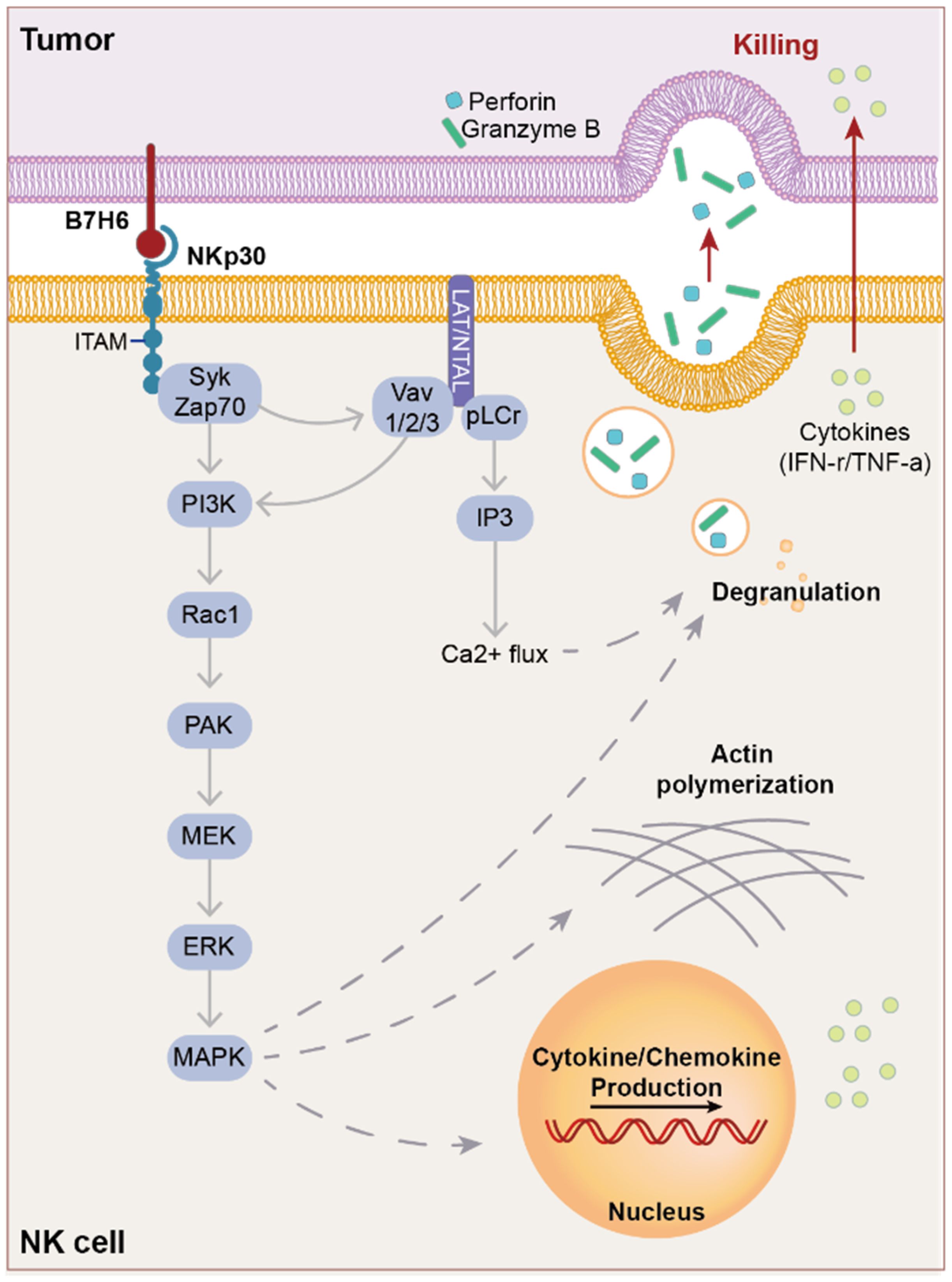
5.1. NKp30
5.2. Ligands of NKp30
5.3. Structure of NKp30
5.4. NK Lysis through NKp30
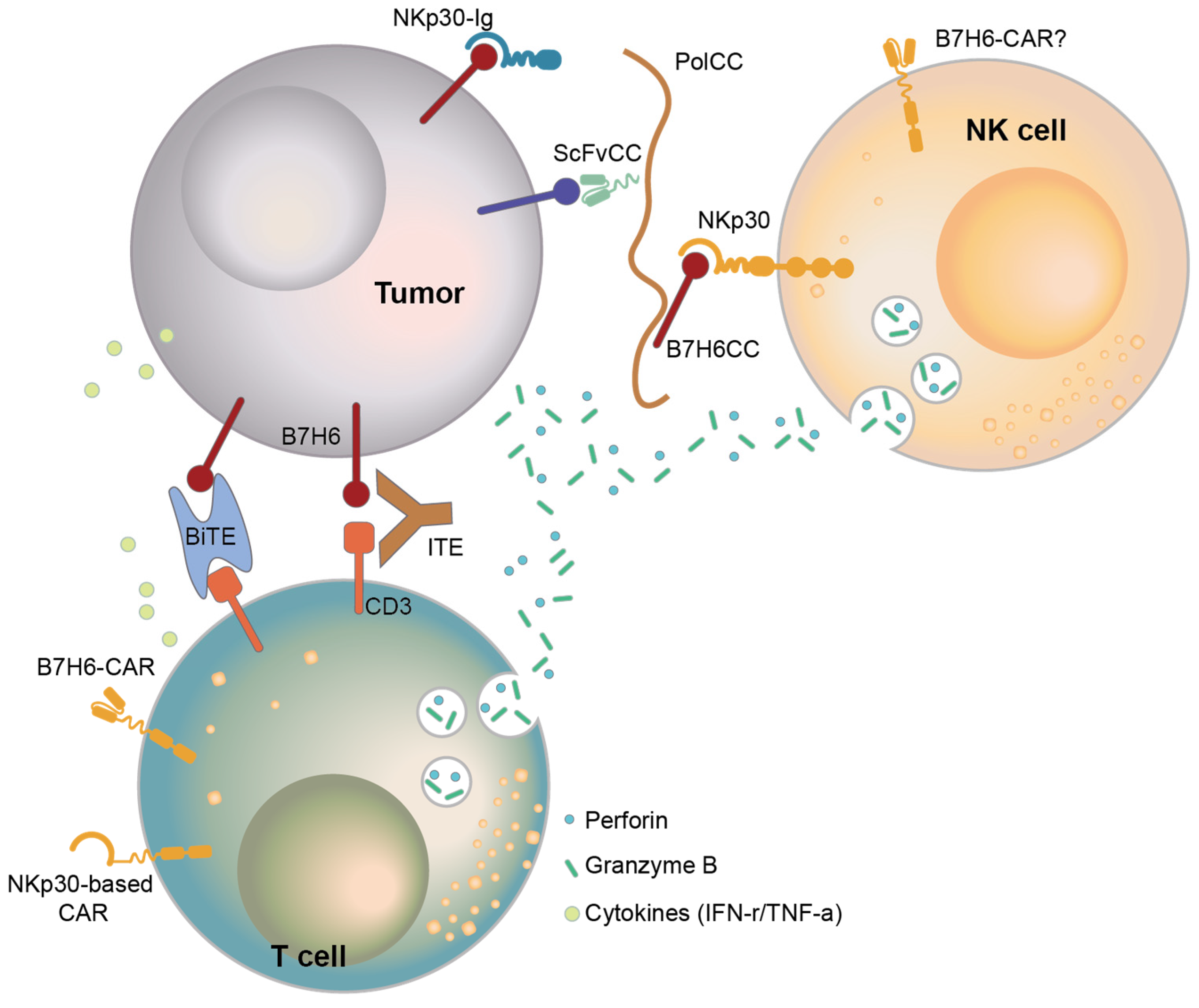
6. Therapy Targeting B7-H6
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Min, H.-Y.; Lee, H.-Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Suh, W.-K. The CD28-B7 Family in Anti-Tumor Immunity: Emerging Concepts in Cancer Immunotherapy. Immune Netw. 2014, 14, 265–276. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, M.; Ghosh, P.; Ganguly, K.K.; Basu, M.; Ghosh, M.K. Targeting PD-1/PD-L1 in cancer immunotherapy: An effective strategy for treatment of triple-negative breast cancer (TNBC) patients. Genes. Dis. 2023, 10, 1318–1350. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’bYrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, J.; Jia, X.; He, Y. The B7 Family Member B7-H6: A New Bane of Tumor. Pathol. Oncol. Res. 2017, 24, 717–721. [Google Scholar] [CrossRef]
- Bolandi, N.; Derakhshani, A.; Hemmat, N.; Baghbanzadeh, A.; Asadzadeh, Z.; Nour, M.A.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Baradaran, B. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10719. [Google Scholar] [CrossRef]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. OncoImmunology 2015, 4, e1001224. [Google Scholar] [CrossRef]
- Raneros, A.B.; Rodriguez, R.M.; Flórez, A.B.; Palomo, P.; Colado, E.; Minguela, A.; Suarez-Alvarez, B.; López-Larrea, C. Bromodomain protein BRD4 is an epigenetic activator of B7-H6 expression in acute myeloid leukemia. OncoImmunology 2021, 10, 1897294. [Google Scholar] [CrossRef] [PubMed]
- Haznedaroglu, I.C. Monitoring the Response to Tyrosine Kinase Inhibitor (TKI) Treatment in Chronic Myeloid Leukemia (CML). Mediterr. J. Hematol. Infect. Dis. 2013, 6, e2014009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, Y.; Huo, L.; Zhou, L.; Yang, J.; Weng, Z.; Yang, X.; Cen, J.; He, Y. Expression of B7-H6 in chronic myeloid leukemia and its clinical significance. Int. J. Clin. Exp. Pathol. 2019, 12, 568–575. [Google Scholar] [PubMed]
- Yuan, L.; Sun, L.; Yang, S.; Chen, X.; Wang, J.; Jing, H.; Zhao, Y.; Ke, X. B7-H6 is a new potential biomarker and therapeutic target of T-lymphoblastic lymphoma. Ann. Transl. Med. 2021, 9, 328. [Google Scholar] [CrossRef]
- Cao, G.; Wang, J.; Zheng, X.; Wei, H.; Tian, Z.; Sun, R. Tumor Therapeutics Work as Stress Inducers to Enhance Tumor Sensitivity to Natural Killer (NK) Cell Cytolysis by Up-regulating NKp30 Ligand B7-H. J. Biol. Chem. 2015, 290, 29964–29973. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Ke, X. Knockdown of B7-H6 inhibits tumor progression and enhances chemosensitivity in B-cell non-Hodgkin lymphoma. Int. J. Oncol. 2016, 48, 1561–1570. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, L.; Wang, Y.; Zhu, M.; Wang, J.; Ke, X. B7-H6 Promotes Cell Proliferation, Migration and Invasion of Non-Hodgkin Lymphoma via Ras/MEK/ERK Pathway Based on Quantitative Phosphoproteomics Data. OncoTargets Ther. 2020, 13, 5795–5805. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Liu, J.; Zhao, K.; Xu, H.; Wen, J.; Jiang, L.; Zeng, X.; Li, J.; Chen, Q. The prognostic value of B7-H6 protein expression in human oral squamous cell carcinoma. J. Oral Pathol. Med. 2017, 46, 766–772. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, S.; Sun, Z.; Wang, J. Dual role of B7-H6 as a novel prognostic marker in hepatocellular carcinoma. APMIS 2020, 129, 105–117. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Xu, B.; Zhou, Y.; Zheng, X.; Wu, C.; Jiang, J. Expression of B7-H6 expression in human hepatocellular carcinoma and its clinical significance. Cancer Cell Int. 2018, 18, 126. [Google Scholar] [CrossRef]
- Li, Y.-M.; Liu, Z.-Y.; Li, Z.-C.; Wang, J.-C.; Yu, J.-M.; Yang, H.-J.; Chen, Z.-N.; Tang, J. Alterations of the Immunologic Co-Stimulator B7 and TNFR Families Correlate with Hepatocellular Carcinoma Prognosis and Metastasis by Inactivating STAT. Int. J. Mol. Sci. 2019, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, W.; Wang, Z.; Song, S.; Qin, Y.; Zhang, F.; Chen, F.; Cai, L. Expression of a novel immune checkpoint B7-H6 ligand in human small cell lung cancer. Ann. Transl. Med. 2020, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.L.; Groves, S.M.; Zhang, Y.-K.; Li, J.; Gonzalez-Ericsson, P.; Sivagnanam, S.; Betts, C.B.; Chen, H.-C.; Liu, Q.; Lowe, C.; et al. Beyond Programmed Death-Ligand 1: B7-H6 Emerges as a Potential Immunotherapy Target in SCLC. J. Thorac. Oncol. 2021, 16, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Qin, Y.; Bai, R.; Huang, J. B7-H6 expression in non-small cell lung cancers. Int. J. Clin. Exp. Pathol. 2014, 7, 6936–6942. [Google Scholar] [PubMed]
- Guo, R.; Liu, G.; Li, C.; Liu, X.; Xu, Y.; Yang, W.; Wang, F. B7 homolog 6 promotes the progression of cervical cancer. Exp. Ther. Med. 2021, 22, 774. [Google Scholar] [CrossRef]
- Liu, X.; Guo, R.; Xu, Y. B7-H6 as a Diagnostic Biomarker for Cervical Squamous Cell Carcinoma. Genet. Test. Mol. Biomarkers 2021, 25, 463–470. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Chen, L.; Xu, B.; Wu, C.; Jiang, J. B7-H6 expression correlates with cancer progression and patient’s survival in hu-man ovarian cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9428–9433. [Google Scholar]
- Zhu, Z.; Teng, K.-Y.; Zhou, J.; Xu, Y.; Zhang, L.; Zhao, H.; Zhang, X.; Tian, L.; Li, Z.; Lu, T.; et al. B7H6 Serves as a Negative Prognostic Marker and an Immune Modulator in Human Pancreatic Cancer. Front. Oncol. 2022, 12, 814312. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Auguste, A.; Auguste, A.; Liao, X.; Liao, X.; Walterskirchen, C.; Walterskirchen, C.; Bauer, K.; Bauer, K.; et al. A Novel B7-H6-Targeted IgG-Like T Cell-Engaging Antibody for the Treatment of Gastrointestinal Tumors. Clin. Cancer Res. 2022, 28, 5190–5201. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, J.; Guo, L.; Wang, X.; Wang, K.; Cai, X.; Yang, S. The prognostic value of B7-H6 in esophageal squamous cell carcinoma. Sci. Rep. 2019, 9, 18122. [Google Scholar] [CrossRef]
- Sun, J.; Tao, H.; Li, X.; Wang, L.; Yang, J.; Wu, P.; Zhang, Y.; Guo, Y. Clinical significance of novel costimulatory molecule B7-H6 in human breast cancer. Oncol. Lett. 2017, 14, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra55. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-G.; Guo, C.-C.; He, Z.-Q.; Liu, Z.-G.; Wang, Y.; Mou, Y.-G. Clinical significance of B7-H6 protein expression in astrocytoma. OncoTargets Ther. 2016, 9, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Che, F.; Xie, X.; Wang, L.; Su, Q.; Jia, F.; Ye, Y.; Zang, L.; Wang, J.; Li, H.; Quan, Y.; et al. B7-H6 expression is induced by lipopolysaccharide and facilitates cancer invasion and metastasis in human gliomas. Int. Immunopharmacol. 2018, 59, 318–327. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, W.; Zhang, H.; Zhang, X.; Zhang, D.; Wang, Q.; Huang, L.; Wang, Y.; Hang, C. High expression of B7-H6 in human glioma tissues promotes tumor progression. Oncotarget 2017, 8, 37435–37447. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Y.; Sun, J.; Dong, J.; Bao, Q.; Zhang, X.; Fu, F. Preferential Expression of B7-H6 in Glioma Stem-Like Cells Enhances Tumor Cell Proliferation via the c-Myc/RNMT Axis. J. Immunol. Res. 2020, 2020, 2328675. [Google Scholar] [CrossRef]
- Matta, J.; Baratin, M.; Chiche, L.; Forel, J.-M.; Cognet, C.; Thomas, G.; Farnarier, C.; Piperoglou, C.; Papazian, L.; Chaussabel, D.; et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 2013, 122, 394–404. [Google Scholar] [CrossRef]
- Kilian, M.; Friedrich, M.J.; Lu, K.H.-N.; Vonhören, D.; Jansky, S.; Michel, J.; Keib, A.; Stange, S.; Hackert, N.; Kehl, N.; et al. The immunoglobulin superfamily ligand B7H6 subjects T cell responses to NK cell surveillance. Sci. Immunol. 2024, 9, eadj7970. [Google Scholar] [CrossRef]
- Obiedat, A.; Charpak-Amikam, Y.; Tai-Schmiedel, J.; Seidel, E.; Mahameed, M.; Avril, T.; Stern-Ginossar, N.; Springuel, L.; Bolsée, J.; Gilham, D.E.; et al. The integrated stress response promotes B7H6 expression. J. Mol. Med. 2019, 98, 135–148. [Google Scholar] [CrossRef]
- Fiegler, N.; Textor, S.; Arnold, A.; Rölle, A.; Oehme, I.; Breuhahn, K.; Moldenhauer, G.; Witzens-Harig, M.; Cerwenka, A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 2013, 122, 684–693. [Google Scholar] [CrossRef]
- Textor, S.; Bossler, F.; Henrich, K.-O.; Gartlgruber, M.; Pollmann, J.; Fiegler, N.; Arnold, A.; Westermann, F.; Waldburger, N.; Breuhahn, K.; et al. The proto-oncogene Myc drives expression of the NK cell-activating NKp30 ligand B7-H6 in tumor cells. OncoImmunology 2016, 5, e1116674. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas-Prieto, L.; Flores-Campos, R.; Silver, A.; de Álava, E.; Hajji, N.; García-Domínguez, D.J. Synergistic Enhancement of Cancer Therapy Using HDAC Inhibitors: Opportunity for Clinical Trials. Front. Genet. 2020, 11, 578011. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.; Shen, Y.; Bhandari, A.; Zhou, X.; Wang, Y.; Yang, F.; Wang, O. Long non-coding RNA LINC00673 promotes breast cancer proliferation and metastasis through regulating B7-H6 and epithelial-mesenchymal transition. Am. J. Cancer Res. 2018, 8, 1273–1287. [Google Scholar] [PubMed]
- Khiabani, N.A.; Doustvandi, M.A.; Mohammadnejad, F.; Kohal, E.S.H.; Boushehri, N.; Jafarlou, M.; Baradaran, B. Combination of B7H6-siRNA and temozolomide synergistically reduces stemness and migration properties of glioblastoma cancer cells. Exp. Cell Res. 2023, 429, 113667. [Google Scholar] [CrossRef]
- Mohammadi, A.; Najafi, S.; Amini, M.; Mansoori, B.; Baghbanzadeh, A.; Hoheisel, J.D.; Baradaran, B. The potential of B7-H6 as a therapeutic target in cancer immunotherapy. Life Sci. 2022, 304, 120709. [Google Scholar] [CrossRef]
- Hazar, B.; Polat, G.; Seyrek, E.; Baǧdatoǧlǧlu, Ö.; Kanik, A.; Tiftik, N. Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in Hodgkin’s and non-Hodgkin’s lymphoma. Int. J. Clin. Pract. 2004, 58, 139–143. [Google Scholar] [CrossRef]
- Suminoe, A.; Matsuzaki, A.; Hattori, H.; Koga, Y.; Ishii, E.; Hara, T. Expression of matrix metalloproteinase (MMP) and tissue inhibitor of MMP (TIMP) genes in blasts of infant acute lymphoblastic leukemia with organ involvement. Leuk. Res. 2007, 31, 1437–1440. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.-B.; Gan, W.-J.; Xiong, F.; Li, Z.; Zhao, H.; Zhu, D.-M.; Zhang, B.; Zhang, X.-G.; Li, D.-C. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol. Lett. 2013, 5, 805–812. [Google Scholar] [CrossRef]
- Tamm, I.; Wang, Y.; Sausville, E.D.; Scudiero, D.A.; Vigna, N.; Oltersdorf, T.; Reed, J.C. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998, 58, 5315–5320. [Google Scholar]
- Brinkmann, K.; Kashkar, H. Targeting the mitochondrial apoptotic pathway: A preferred approach in hematologic malignancies? Cell Death Dis. 2014, 5, e1098. [Google Scholar] [CrossRef]
- Liang, R.; Chen, X.; Chen, L.; Wan, F.; Chen, K.; Sun, Y.; Zhu, X. STAT3 signaling in ovarian cancer: A potential therapeutic target. J. Cancer 2020, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, J.; Yao, X.; Li, J.; Tu, Y.; Yao, F.; Sun, S. Knockdown of B7H6 inhibits tumor progression in triple-negative breast cancer. Oncol. Lett. 2018, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, J.; Ma, L.; Sun, X.; Ge, J.; Yu, Y.; Ma, J.; Zhang, M. B7-H6 as an efficient target for T cell-induced cytotoxicity in haematologic malignant cells. Investig. New Drugs 2021, 39, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural Cytotoxicity Receptors in Health and Disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Parolini, S.; Pessino, A.; Sivori, S.; Augugliaro, R.; Morelli, L.; Marcenaro, E.; Accame, L.; Malaspina, A.; Biassoni, R.; et al. Identification and Molecular Characterization of Nkp30, a Novel Triggering Receptor Involved in Natural Cytotoxicity Mediated by Human Natural Killer Cells. J. Exp. Med. 1999, 190, 1505–1516. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Justino, G.C.; Marques, M.M. NKp30—A prospective target for new cancer immunotherapy strategies. Br. J. Pharmacol. 2020, 177, 4563–4580. [Google Scholar] [CrossRef]
- Correia, D.V.; Fogli, M.; Hudspeth, K.; da Silva, M.G.; Mavilio, D.; Silva-Santos, B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011, 118, 992–1001. [Google Scholar] [CrossRef]
- Tang, Q.; Grzywacz, B.; Wang, H.; Kataria, N.; Cao, Q.; Wagner, J.E.; Blazar, B.R.; Miller, J.S.; Verneris, M.R. Umbilical Cord Blood T Cells Express Multiple Natural Cytotoxicity Receptors after IL-15 Stimulation, but Only NKp30 Is Functional. J. Immunol. 2008, 181, 4507–4515. [Google Scholar] [CrossRef]
- Correia, M.P.; Stojanovic, A.; Bauer, K.; Juraeva, D.; Tykocinski, L.-O.; Lorenz, H.-M.; Brors, B.; Cerwenka, A. Distinct human circulating NKp30(+)FcepsilonRIgamma(+)CD8(+) T cell population exhibiting high natural killer-like antitumor potential. Proc. Natl. Acad. Sci. USA 2018, 115, E5980–E5989. [Google Scholar] [CrossRef]
- von Strandmann, E.P.; Shatnyeva, O.; Hansen, H.P. NKp30 and its ligands: Emerging players in tumor immune evasion from natural killer cells. Ann. Transl. Med. 2015, 3, 13. [Google Scholar] [CrossRef]
- Hecht, M.-L.; Rosental, B.; Horlacher, T.; Hershkovitz, O.; De Paz, J.L.; Noti, C.; Schauer, S.; Porgador, A.; Seeberger, P.H. Natural Cytotoxicity Receptors NKp30, NKp44 and NKp46 Bind to Different Heparan Sulfate/Heparin Sequences. J. Proteome Res. 2009, 8, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, O.; Jarahian, M.; Zilka, A.; Bar-Ilan, A.; Landau, G.; Jivov, S.; Tekoah, Y.; Glicklis, R.; Gallagher, J.T.; Hoffmann, S.C.; et al. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: A lesson for the use of recombinant immunoreceptors as an immunological tool. Glycobiology 2007, 18, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Warren, H.S.; Jones, A.L.; Freeman, C.; Bettadapura, J.; Parish, C.R. Evidence That the Cellular Ligand for the Human NK Cell Activation Receptor NKp30 Is Not a Heparan Sulfate Glycosaminoglycan. J. Immunol. 2005, 175, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Mariuzza, R.A. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H. J. Exp. Med. 2011, 208, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Ogbomo, H.; Mansour, M.K.; Xiang, R.F.; Szabo, L.; Munro, F.; Mukherjee, P.; Mariuzza, R.A.; Amrein, M.; Vyas, J.M.; et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat. Commun. 2018, 9, 751. [Google Scholar] [CrossRef]
- Xiang, R.F.; Li, S.; Ogbomo, H.; Stack, D.; Mody, C.H. β1 Integrins Are Required To Mediate NK Cell Killing of Cryptococcus neoformans. J. Immunol. 2018, 201, 2369–2376. [Google Scholar] [CrossRef]
- von Strandmann, E.P.; Simhadri, V.R.; von Tresckow, B.; Sasse, S.; Reiners, K.S.; Hansen, H.P.; Rothe, A.; Böll, B.; Simhadri, V.L.; Borchmann, P.; et al. Human Leukocyte Antigen-B-Associated Transcript 3 Is Released from Tumor Cells and Engages the NKp30 Receptor on Natural Killer Cells. Immunity 2007, 27, 965–974. [Google Scholar] [CrossRef]
- Jarahian, M.; Fiedler, M.; Cohnen, A.; Djandji, D.; Hämmerling, G.J.; Gati, C.; Cerwenka, A.; Turner, P.C.; Moyer, R.W.; Watzl, C.; et al. Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin. PLOS Pathog. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Arnon, T.I.; Achdout, H.; Levi, O.; Markel, G.; Saleh, N.; Katz, G.; Gazit, R.; Gonen-Gross, T.; Hanna, J.; Nahari, E.; et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 2005, 6, 515–523. [Google Scholar] [CrossRef]
- Veschi, V.; Petroni, M.; Bartolazzi, A.; Altavista, P.; Dominici, C.; Capalbo, C.; Boldrini, R.; Castellano, A.; McDowell, H.P.; Pizer, B.; et al. Galectin-3 is a marker of favorable prognosis and a biologically relevant molecule in neuroblastic tumors. Cell Death Dis. 2014, 5, e1100. [Google Scholar] [CrossRef][Green Version]
- Alter, G.; Jost, S.; Rihn, S.; Reyor, L.L.; Nolan, B.E.; Ghebremichael, M.; Bosch, R.; Altfeld, M.; Lauer, G.M. Reduced frequencies of NKp30+NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. J. Hepatol. 2011, 55, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Memmer, S.; Weil, S.; Beyer, S.; Zöller, T.; Peters, E.; Hartmann, J.; Steinle, A.; Koch, J. The Stalk Domain of NKp30 Contributes to Ligand Binding and Signaling of a Preassembled NKp30-CD3ζ Complex. J. Biol. Chem. 2016, 291, 25427–25438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, D.; Churov, A.; Fu, R. Research Progress on NK Cell Receptors and Their Signaling Pathways. Mediat. Inflamm. 2020, 2020, 6437057. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 2008, 227, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Fujikawa, K.; Tassi, I.; Kim, S.; Latinis, K.; Nishi, S.; Yokoyama, W.; Colonna, M.; Swat, W. Differential Requirements for Vav Proteins in DAP10- and ITAM-mediated NK Cell Cytotoxicity. J. Exp. Med. 2004, 200, 817–823. [Google Scholar] [CrossRef]
- Tassi, I.; Colonna, M. The Cytotoxicity Receptor CRACC (CS-1) Recruits EAT-2 and Activates the PI3K and Phospholipase Cγ Signaling Pathways in Human NK Cells. J. Immunol. 2005, 175, 7996–8002. [Google Scholar] [CrossRef]
- Ting, A.; Schoon, R.; Abraham, R.; Leibson, P. Interaction between protein kinase C-dependent and G protein-dependent pathways in the regulation of natural killer cell granule exocytosis. J. Biol. Chem. 1992, 267, 23957–23962. [Google Scholar] [CrossRef]
- Upshaw, J.L.; Schoon, R.A.; Dick, C.J.; Billadeau, D.D.; Leibson, P.J. The Isoforms of Phospholipase C-γ Are Differentially Used by Distinct Human NK Activating Receptors. J. Immunol. 2005, 175, 213–218. [Google Scholar] [CrossRef]
- Arnon, T.I.; Markel, G.; Bar-Ilan, A.; Hanna, J.; Fima, E.; Benchetrit, F.; Galili, R.; Cerwenka, A.; Benharroch, D.; Sion-Vardy, N.; et al. Harnessing Soluble NK Cell Killer Receptors for the Generation of Novel Cancer Immune Therapy. PLoS ONE 2008, 3, e2150. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef]
- Wu, M.-R.; Zhang, T.; Gacerez, A.T.; Coupet, T.A.; DeMars, L.R.; Sentman, C.L. B7H6-Specific Bispecific T Cell Engagers Lead to Tumor Elimination and Host Antitumor Immunity. J. Immunol. 2015, 194, 5305–5311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, M.-R.; Sentman, C.L. An NKp30-Based Chimeric Antigen Receptor Promotes T Cell Effector Functions and Antitumor Efficacy In Vivo. J. Immunol. 2012, 189, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-R.; Zhang, T.; DeMars, L.R.; Sentman, C.L. B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity. Gene Ther. 2015, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.K.; Gacerez, A.T.; Sentman, C.L.; Ackerman, M.E. Development of unique cytotoxic chimeric antigen receptors based on human scFv targeting B7H. Protein Eng. Des. Sel. 2017, 30, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Gacerez, A.T.; Hua, C.K.; Ackerman, M.E.; Sentman, C.L. Chimeric antigen receptors with human scFvs preferentially induce T cell anti-tumor activity against tumors with high B7H6 expression. Cancer Immunol. Immunother. 2018, 67, 749–759. [Google Scholar] [CrossRef]
- Kalousková, B.; Skořepa, O.; Cmunt, D.; Abreu, C.; Krejčová, K.; Bláha, J.; Sieglová, I.; Král, V.; Fábry, M.; Pola, R.; et al. Tumor Marker B7-H6 Bound to the Coiled Coil Peptide-Polymer Conjugate Enables Targeted Therapy by Activating Human Natural Killer Cells. Biomedicines 2021, 9, 1597. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, J.H.; Jang, I.-H.; Jo, S.; Lee, S.Y.; Oh, S.-C.; Kim, S.-M.; Kong, L.; Ko, J.; Kim, T.-D. Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 10326. https://doi.org/10.3390/ijms251910326
Lee S, Kim JH, Jang I-H, Jo S, Lee SY, Oh S-C, Kim S-M, Kong L, Ko J, Kim T-D. Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies. International Journal of Molecular Sciences. 2024; 25(19):10326. https://doi.org/10.3390/ijms251910326
Chicago/Turabian StyleLee, Sunyoung, Ji Hyun Kim, In-Hwan Jang, Seona Jo, Soo Yun Lee, Se-Chan Oh, Seok-Min Kim, Lingzu Kong, Jesang Ko, and Tae-Don Kim. 2024. "Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies" International Journal of Molecular Sciences 25, no. 19: 10326. https://doi.org/10.3390/ijms251910326
APA StyleLee, S., Kim, J. H., Jang, I.-H., Jo, S., Lee, S. Y., Oh, S.-C., Kim, S.-M., Kong, L., Ko, J., & Kim, T.-D. (2024). Harnessing B7-H6 for Anticancer Immunotherapy: Expression, Pathways, and Therapeutic Strategies. International Journal of Molecular Sciences, 25(19), 10326. https://doi.org/10.3390/ijms251910326






