Application of H2N-Fe3O4 Nanoparticles for Prostate Cancer Magnetic Resonance Imaging in an Animal Model
Abstract
:1. Introduction;
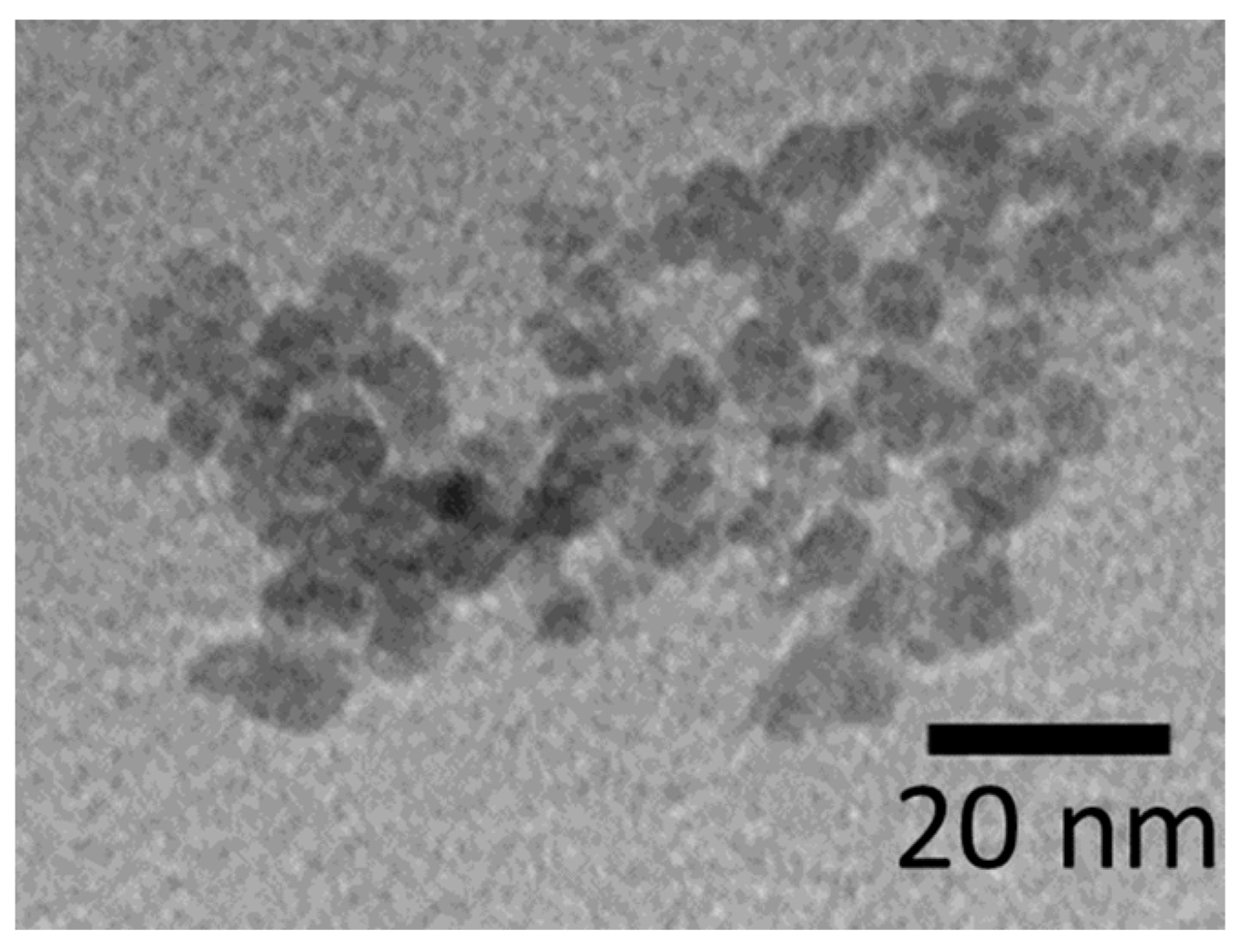
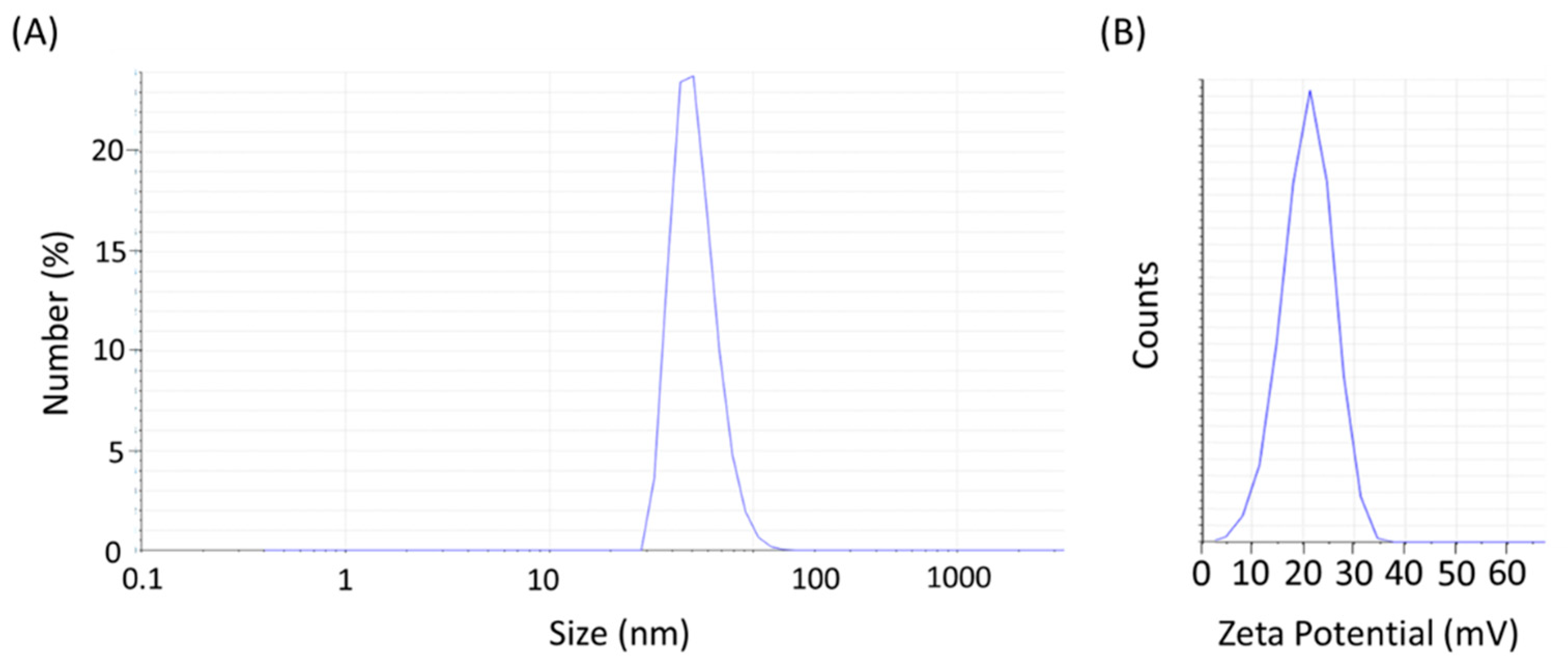
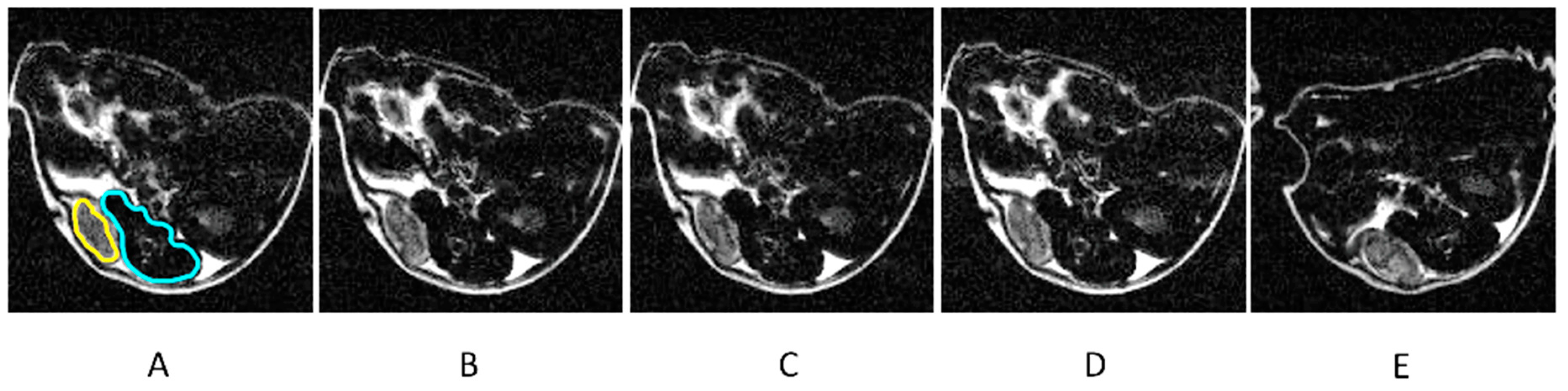
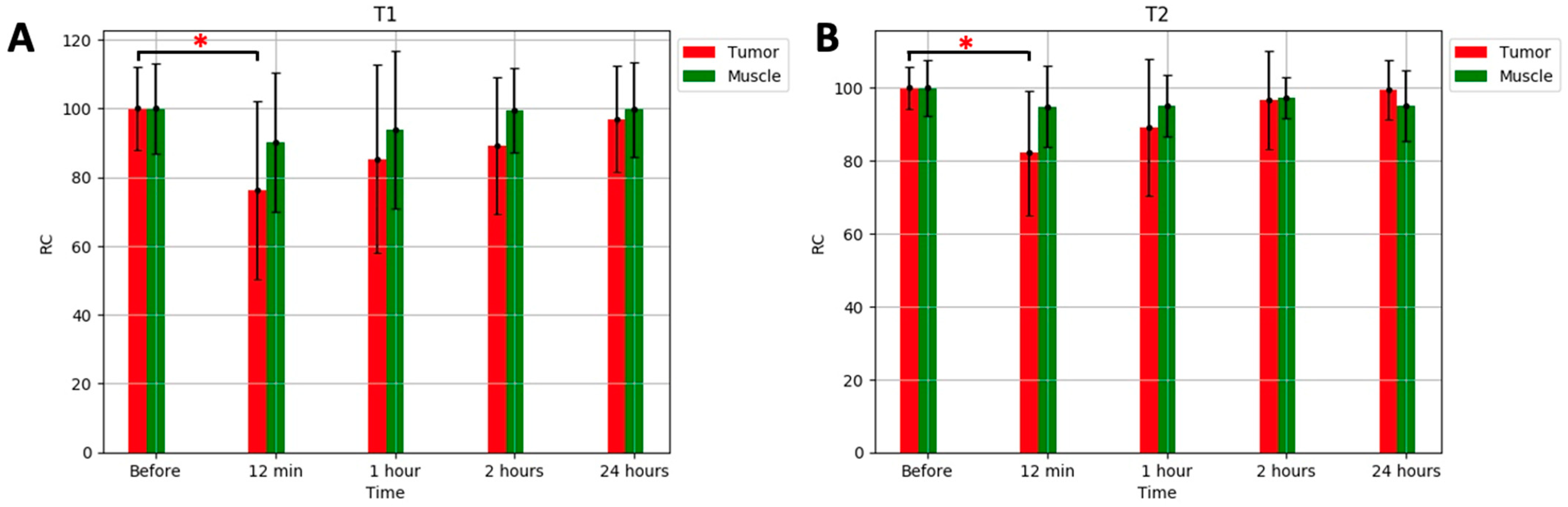
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of H2N-Fe3O4 Nanoparticles
4.2. Animal Model
4.3. MRI Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.L.A.; Scardino, P.T.; Flanigan, R.C.; DeKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol. 2017, 197, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L. Active surveillance with selective delayed intervention using PSA doubling time for good risk prostate cancer. Eur. Urol. 2005, 47, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Partin, A.W.; Brawer, M.K.; Albertsen, P.C.; Chan, P.T.; Scardino, P.T.; Kupelian, J.M.; Thompson, I.M.; Carroll, P.; Ries, G.; et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J. Natl. Cancer Inst. 2006, 98, 1521–1527. [Google Scholar] [CrossRef]
- Carter, H.B. Prostate cancers in men with low PSA levels—Must we find them? N. Engl. J. Med. 2004, 350, 2292–2294. [Google Scholar] [CrossRef]
- Welch, H.G.; Elmore, J.G.; Brown, M.L. Prostate-specific antigen levels in the United States: Implications of various definitions for abnormal. J. Natl. Cancer Inst. 2005, 97, 1132–1137. [Google Scholar] [CrossRef]
- Stephenson, R.A.; Stanford, J.L. Population-based prostate cancer trends in the United States: Patterns of change in the era of prostate-specific antigen. World J. Urol. 1997, 15, 331–335. [Google Scholar] [CrossRef]
- Schmid, D.T.; Klass, T.M. Fluorocholine PET/CT in patients with prostate cancer: Initial experience. Radiology 2005, 235, 623–628. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Zufall, P.E.; Coakley, J.J.; Males, T.P.; Swanson, A.P.; Nelson, M.P.; Carroll, P.R.; Hricak, J.K.; James, J.K. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J. Magn. Reson. Imaging 2002, 16, 451–463. [Google Scholar] [CrossRef]
- Hricak, H.; James, J.K.; Scardino, P.T.; Karakiewicz, J.F.; Hricak, H.; Taneja, S.S.; Zelefsky, M.J.; Hricak, H. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal—Pelvic phased-array coils. Radiology 1994, 193, 703–709. [Google Scholar] [CrossRef]
- Schnall, M.D.; Malley, P.C.; Connelly, J.S.; Beaver, B.J.; Reiger, S.B.; Rubens, M.L.; Scardino, P.T. Prostate: MR imaging with an endorectal surface coil. Radiology 1989, 172, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Hanks, G.E.; Rosenthal, S.A.; Rengan, R.; Lawton, C.A.F.; Dicker, A.P.; Mauer, A.M.; Zietman, A.; Lawton, C.A.F.; Dicker, A.P.; Roach, M.; et al. Comparison of pathologic and clinical evaluation of lymph nodes in prostate cancer: Implications of RTOG data for patient management and trial design and stratification. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Hazhirkarzar, B.; Dublin, A.B. Gadolinium Magnetic Resonance Imaging; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kader, A.; Mangarova, D.B.; Moeckel, J.; Brangsch, J.; Adams, L.C.; Zhao, J.; Reimann, C.; Saatz, J.; Traub, H.; Buchholz, R.; et al. Iron Oxide Nanoparticles for Visualization of Prostate Cancer in MRI. Cancers 2022, 14, 2909. [Google Scholar] [CrossRef]
- Dash, T.; Blasiak, B.; Tomanek, B.; Banerjee, A.; Trudel, S.; Latta, P.; van Veggel, F. Colloidally Stable Monodisperse Fe Nanoparticles as T2 Contrast Agents for High-Field Clinical and Preclinical Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2021, 4, 1235–1242. [Google Scholar] [CrossRef]
- Nagesh, P.K.; Johnson, N.R.; Boya, V.K.N.; Chowdhury, P.; Othman, S.F.; Khalilzad-Sharghi, V.; Hafeez, B.B.; Ganju, A.; Khan, S.; Behrman, S.W.; et al. PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surf. B Biointerfaces 2016, 144, 8–20. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Tse, B.W.; Cowin, G.J.; Soekmadji, C.; Jovanovic, L.; Vasireddy, R.S.; Pedersen, J.S.; Ling, M.T.; Khatri, A.; Liu, T.; Thierry, B. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine 2014, 10, 375–386. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Chen, Y.; Liu, W.; Jiang, J.; Guan, W.; Duan, Y. In Vivo Molecular MRI Imaging of Prostate Cancer by Targeting PSMA with Nanoparticles. Int. J. Mol. Sci. 2015, 16, 9573–9587. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.; Kaul, M.; Hansen, E.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Nitin, N.; LaConte, L.E.W.; Zurkiya, O.; Hu, X.; Bao, G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J. Biol. Inorg. Chem. 2004, 9, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Artemov, D.; Mori, N.; Okollie, B.; Bhujwalla, Z.M. Molecular magnetic resonance imaging with targeted contrast agents. J. Cell. Biochem. 2003, 90, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Takatsu, Y.; Noguchi, T.; Ohkawa, S.; Yoshioka, H.; Ohsawa, I.; Nakamura, T.; Shimizu, Y.; Yanagisawa, T.; Nakamura, T.; et al. Enhancement effects and relaxivities of gadolinium-DTPA at 1.5 versus 3 Tesla: A phantom study. Magn. Reson. Med. Sci. 2005, 4, 145–149. [Google Scholar] [CrossRef]
- Pu, F.; Salarian, M.; Xue, S.; Qiao, J.; Feng, J.; Tan, S.; Yang, J.J. Prostate-specific membrane antigen targeted protein contrast agents for molecular imaging of prostate cancer by MRI. Nanoscale 2016, 8, 12668–12682. [Google Scholar] [CrossRef]
- Hagberg, E.; Scheffler, K. Effect of r1 and r2 relaxivity of gadolinium-based contrast agents on the T1-weighted MR signal at increasing magnetic field strengths. CMMI 2013, 8, 456–465. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar]
- Ta, H.; Li, Z.; Wu, Y.; Cowin, G.; Zhang, S.; Yago, A.; Xu, Z. Effects of magnetic field strength and particle aggregation on relaxivity of ultra-small dual contrast iron oxide nanoparticles. Mater. Res. Express 2017, 4, 116105. [Google Scholar] [CrossRef]
- Dash, T.; Blasiak, B.; Tomanek, B.; Latta, P.; van Veggel, F. Target-Specific Magnetic Resonance Imaging of Human Prostate Adenocarcinoma Using NaDyF4–NaGdF4 Core–Shell Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 24345–24355. [Google Scholar] [CrossRef]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2019, 2019, 1845637. [Google Scholar] [CrossRef]
- Shieh, D.-B.; Cheng, F.Y.; Su, C.H.; Yeh, C.S.; Wu, M.T.; Wu, Y.N.; Tsai, C.Y.; Wu, C.L.; Chen, D.H.; Chou, C.H. Aqueous dispersions of magnetite nanoparticles with NH3+ surfaces for magnetic manipulations of biomolecules and MRI contrast agents. Biomaterials 2005, 26, 7183–7191. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, J.; Ma, Q.; Xu, S.; Wang, L. Single-Atom Gd Nanoprobes for Self-Confirmative MRI with Robust Stability. Small 2023, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, A.; MacDonald, D.; Blasiak, B.; Kasparek, A.; Tomanek, B.; Cheng, F.; Kruk, D. 1H Spin-Lattice Relaxation Processes in Soultions of H2N-Fe3O4 Nanoparticles: Insights from NMR Relaxometry. J. Phys. Chem. B 2024, 128, 1520–6106. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T 1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.E.; Schleicher, R.L.; Laguna, S.; Billot, B.; Schaefer, P.; McKaig, B.; Goldstein, J.N.; Sheth, K.N.; Rosen, M.S.; Kimberly, W.T. Quantitative Brain Morphometry of Portable Low-Field-Strength MRI Using Super-Resolution Machine Learning. Radiology 2022, 306, e220522. [Google Scholar] [CrossRef]
- Arnold, T.C.; Tu, D.; Okar, S.V.; Nair, G.; By, S.; Kawatra, K.D.; Robert-Fitzgerald, T.E.; Desiderio, L.M.; Schindler, M.K.; Shinohara, R.T.; et al. Sensitivity of portable low-field magnetic resonance imaging for multiple sclerosis lesions. Neuroimage Clin. 2022, 35, 103101. [Google Scholar] [CrossRef]
- Beaton, L.; Bandula, S.; Gaze, M.N.; Sharma, R.A. How rapid advances in imaging are defining the future of precision radiation oncology. Br. J. Cancer 2019, 120, 779–790. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Ma, L.; Liu, G.; Wang, Z. The Renal Clearable Magnetic Resonance Imaging Contrast Agents: State of the Art and Recent Advances. Molecules 2020, 25, 5072. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idée, J.M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef]
- Heesakkers, R.A.; Fütterer, J.J.; Hövels, A.M.; van den Bosch, H.C.; Scheenen, T.W.; Hoogeveen, Y.L.; Barentsz, J.O. Prostate cancer evaluated with ferumoxtran-10-enhanced T2*-weighted MR Imaging at 1.5 and 3.0 T: Early experience. Radiology 2006, 239, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.R.; Ngen, E.J.; Rotz, M.W.; Kakkad, S.; Lisok, A.; Pracitto, R.; Pomper, M.G. Synthesis and Evaluation of GdIII-Based Magnetic Resonance Contrast Agents for Molecular Imaging of Prostate-Specific Membrane Antigen. Angew. Chem. Int. Ed. 2015, 54, 10778–10782. [Google Scholar] [CrossRef] [PubMed]
- Ngen, E.J.; Behnam Azad, B.; Boinapally, S.; Lisok, A.; Brummet, M.; Jacob, D.; Banerjee, S.R. MRI Assessment of Prostate-Specific Membrane Antigen (PSMA) Targeting by a PSMA-Targeted Magnetic Nanoparticle: Potential for Image-Guided Therapy. Mol. Pharm. 2019, 16, 2060–2068. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]


| Contrast Agent | r1 [mM−1s−1] | r2 [mM−1s−1] | Field Strength [T] | Ref. |
|---|---|---|---|---|
| Gd-CP027 bound to albumin | 48 | 0.5 | [28] | |
| Gd-CP027 bound to albumin | 31 | 1.5 | [28] | |
| Gd-CP027 bound to albumin | 9.4 | 9.4 | [28] | |
| Gd-DTPA | 4.79 | 5.14 | 1.5 | [25] |
| Gd-DTPA | 4.05 | 5.09 | 3 | [25] |
| Gadolinium-based | 18.6 | 94 | 7 | [26] |
| Gd-based | 18 | 21.6 | 9.4 | [27] |
| SPIONS | 5.6 | 72 | 9.4 | [29] |
| Dy/Gd | 20.2 | 32.3 | 3 | [30] |
| Dy/Gd | 9.4 | 144.7 | 9.4 | [30] |
| H2N-Fe3O4 | 6.31 | 8.33 | 9.4 | [32] |
| H2N-Fe3O4 | 6.77 | 33.56 | 1.5 | [32] |
| Resovit® | 1.2 | 280 | 9.4 | [32] |
| Resovit® | 6.7 | 82 | 1.5 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasiak, B.; MacDonald, D.; Jasiński, K.; Cheng, F.-Y.; Tomanek, B. Application of H2N-Fe3O4 Nanoparticles for Prostate Cancer Magnetic Resonance Imaging in an Animal Model. Int. J. Mol. Sci. 2024, 25, 10334. https://doi.org/10.3390/ijms251910334
Blasiak B, MacDonald D, Jasiński K, Cheng F-Y, Tomanek B. Application of H2N-Fe3O4 Nanoparticles for Prostate Cancer Magnetic Resonance Imaging in an Animal Model. International Journal of Molecular Sciences. 2024; 25(19):10334. https://doi.org/10.3390/ijms251910334
Chicago/Turabian StyleBlasiak, Barbara, David MacDonald, Krzysztof Jasiński, Fong-Yu Cheng, and Boguslaw Tomanek. 2024. "Application of H2N-Fe3O4 Nanoparticles for Prostate Cancer Magnetic Resonance Imaging in an Animal Model" International Journal of Molecular Sciences 25, no. 19: 10334. https://doi.org/10.3390/ijms251910334






