Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis
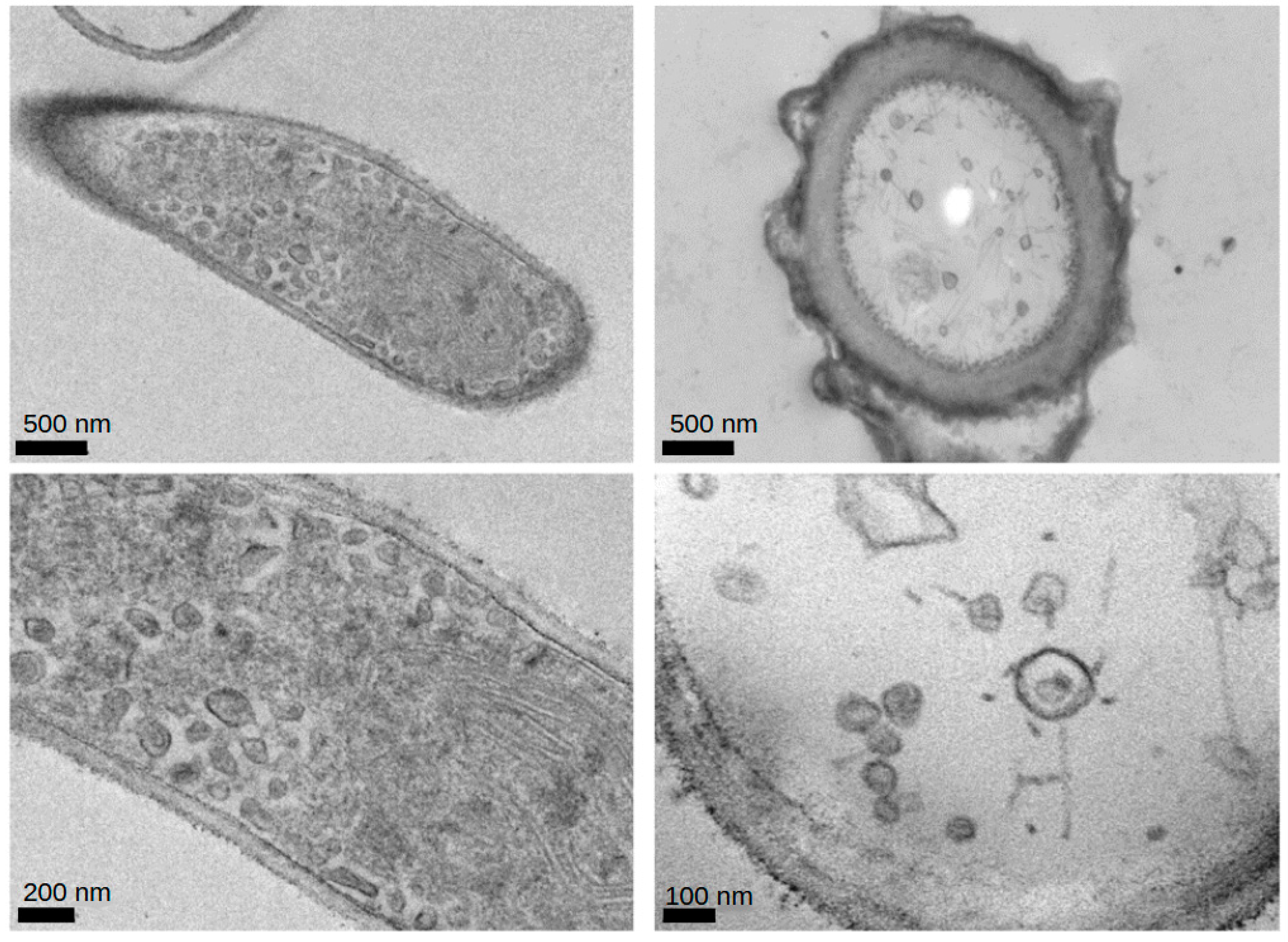
2.2. Electron Microscopy
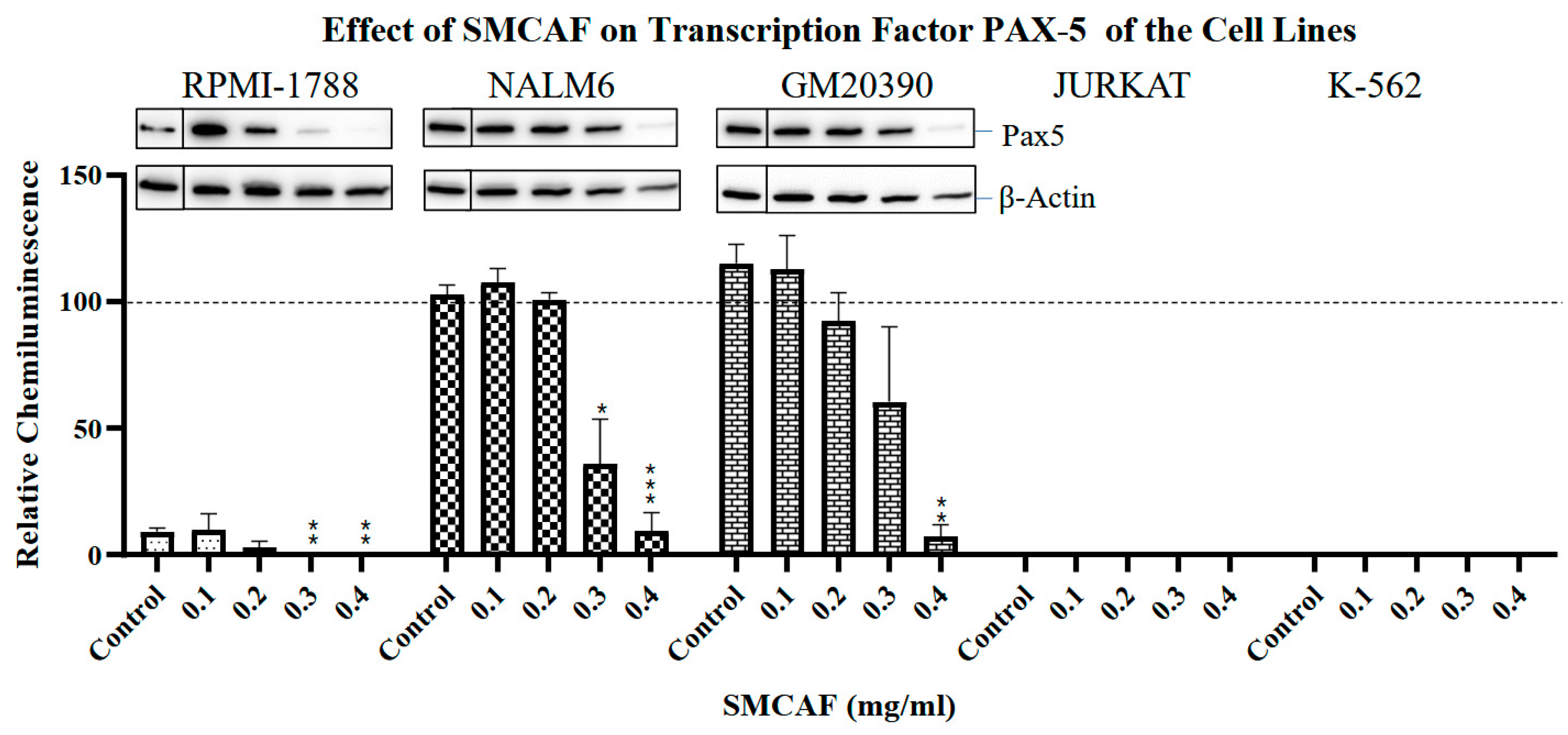
2.3. Transcription Factors
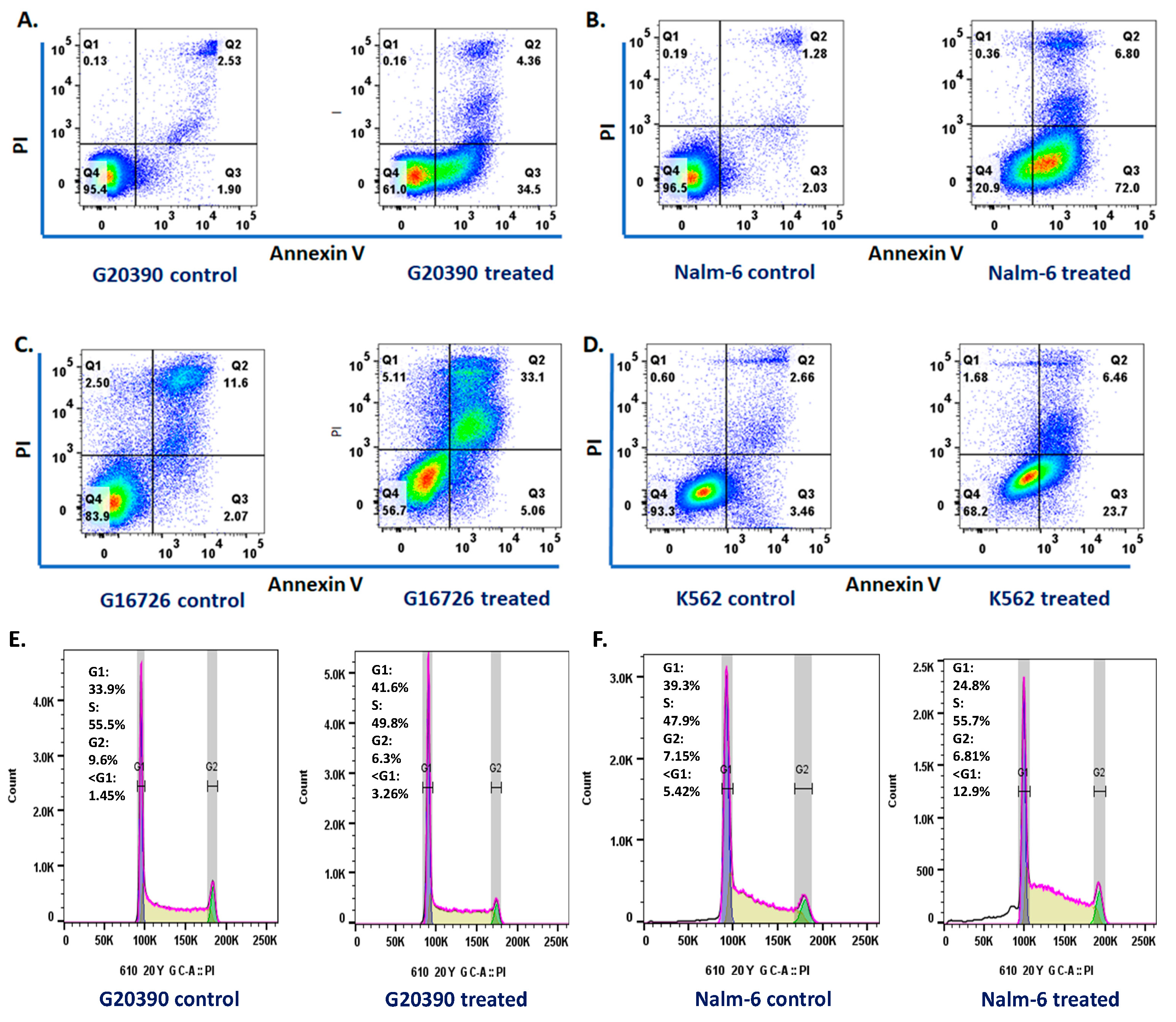
2.4. Apoptosis and Cell Cycle
3. Discussion
4. Materials and Methods
4.1. Mycovirus-Containing Aspergills flavus
4.2. Electron Microscopy
4.3. Cell Cycle Studies
4.4. Annexin V/PI Analysis
4.5. Analysis for Detection of Aflatoxin
4.6. Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Gocho, Y.; Yang, J.J. Genetic defects in hematopoietic transcription factors and predisposition to acute lymphoblastic leuke-mia. Blood 2019, 134, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Kee, B.L. The Transcriptional Regulation of B Cell Lineage Commitment. Immunity 2007, 26, 715–725, Erratum in Immunity 2017, 27, 361. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Leukaemia Firsts’ in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef]
- Wagener, R.; Elitzur, S.; Brozou, T.; Borkhardt, A. Functional damaging germline variants in ETV6, IKZF1, PAX5 and RUNX1 predisposing to B-cell precursor acute lymphoblastic leukemia. Eur. J. Med. Genet. 2023, 66, 104725. [Google Scholar] [CrossRef]
- Somasundaram, R.; Prasad, M.A.J.; Ungerbäck, J.; Sigvardsson, M. Transcription factor networks in B-cell differentiation link development to acute lymphoid leukemia. Blood 2015, 126, 144–152. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Goorha, S.; Radtke, I.; Miller, C.B.; Coustan-Smith, E.; Dalton, J.D.; Girtman, K.; Mathew, S.; Ma, J.; Pounds, S.B.; et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007, 446, 758–764. [Google Scholar] [CrossRef]
- Kordes, U.; Krappmann, D.; Heissmeyer, V.; Ludwig, W.D.; Scheidereit, C. Transcription factor NF-κB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia 2000, 14, 399–402. [Google Scholar] [CrossRef]
- Schinnerl, D.; Fortschegger, K.; Kauer, M.; Marchante, J.R.M.; Kofler, R.; Boer, M.L.D.; Strehl, S. The role of the Janus-faced transcription factor PAX5-JAK2 in acute lymphoblastic leukemia. Blood 2015, 125, 1282–1291. [Google Scholar] [CrossRef]
- Lee, T.I.; Young, R.A. Transcriptional Regulation and Its Misregulation in Disease. Cell 2013, 152, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Rosenbauer, F.; Wagner, K.; Kutok, J.L.; Iwasaki, H.; Le Beau, M.M.; Okuno, Y.; Akashi, K.; Fiering, S.; Tenen, D.G. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU. Nat. Genet. 2004, 36, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Heltemes-Harris, L.M.; Willette, M.J.; Ramsey, L.B.; Qiu, Y.H.; Neeley, E.S.; Zhang, N.; Thomas, D.A.; Koeuth, T.; Baechler, E.C.; Kornblau, S.M.; et al. Ebf1 or Pax5 haploinsufficiency synergizes with STAT5 activation to initiate acute lymphoblastic leukemia. J. Exp. Med. 2011, 208, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- de Smith, A.J.; Lavoie, G.; Walsh, K.M.; Aujla, S.; Evans, E.; Hansen, H.M.; Smirnov, I.; Kang, A.Y.; Zenker, M.; Ceremsak, J.J.; et al. Predisposing germline mutations in high hyperdiploid acute lymphoblastic leukemia in children. Genes Chromosomes Cancer 2019, 58, 723–730. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Hosking, F.J.; Vijayakrishnan, J.; Price, A.; Olver, B.; Sheridan, E.; E Kinsey, S.; Lightfoot, T.; Roman, E.; Irving, J.A.; et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat. Genet. 2009, 41, 1006–1010. [Google Scholar] [CrossRef]
- Sherborne, A.L.; Hosking, F.J.; Prasad, R.B.; Kumar, R.; Koehler, R.; Vijayakrishnan, J.; Papaemmanuil, E.; Bartram, C.R.; Stanulla, M.; Schrappe, M.; et al. Variation in CDKN2A at 9p21.3 influences childhood acute lymphoblastic leukemia risk. Nat. Genet. 2010, 42, 492–494. [Google Scholar] [CrossRef]
- Migliorini, G.; Fiege, B.; Hosking, F.J.; Ma, Y.; Kumar, R.; Sherborne, A.L.; da Silva Filho, M.I.; Vijayakrishnan, J.; Koehler, R.; Thomsen, H.; et al. Variation at 10p12.2 and 10p14 influences risk of childhood B-cell acute lymphoblastic leukemia and phe-notype. Blood 2013, 122, 3298–3307. [Google Scholar] [CrossRef]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Perez-Garcia, A.; Ambesi-Impiombato, A.; Hadler, M.; Rigo, I.; LeDuc, C.A.; Kelly, K.; Jalas, C.; Paietta, E.; Racevskis, J.; Rowe, J.M.; et al. Genetic loss of SH2B3 in acute lymphoblastic leukemia. Blood 2013, 122, 2425–2432. [Google Scholar] [CrossRef]
- Shah, S.; A Schrader, K.; Waanders, E.; E Timms, A.; Vijai, J.; Miething, C.; Wechsler, J.; Yang, J.; Hayes, J.; Klein, R.J.; et al. A recurrent germline PAX5 mutation confers susceptibility to pre-B cell acute lymphoblastic leukemia. Nat. Genet. 2013, 45, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Metzger, M.L.; Wu, G.; Nishii, R.; Qian, M.; Devidas, M.; Yang, W.; Cheng, C.; Cao, X.; Quinn, E.; et al. Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukemia: A systematic genetic study. Lancet Oncol. 2015, 16, 1659–1666. [Google Scholar] [CrossRef]
- Noetzli, L.; Lo, R.W.; Lee-Sherick, A.B.; Callaghan, M.; Noris, P.; Savoia, A.; Rajpurkar, M.; Jones, K.; Gowan, K.; Balduini, C.L.; et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat. Genet. 2015, 47, 535–538. [Google Scholar] [CrossRef]
- Zhang, M.Y.; E Churpek, J.; Keel, S.B.; Walsh, T.; Lee, M.K.; Loeb, K.R.; Gulsuner, S.; Pritchard, C.C.; Sanchez-Bonilla, M.; Delrow, J.J.; et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat. Genet. 2015, 47, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Topka, S.; Vijai, J.; Walsh, M.F.; Jacobs, L.; Maria, A.; Villano, D.; Gaddam, P.; Wu, G.; McGee, R.B.; Quinn, E.; et al. Germline ETV6 Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia. PLoS Genet. 2015, 11, e1005262. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Sakaguchi, H.; Muramatsu, H.; Okuno, Y.; Song, C.; Dovat, S.; Shimada, A.; Ozeki, M.; Ohnishi, H.; Teramoto, T.; et al. Germline IKAROS mutation associated with primary immunodeficiency that progressed to T-cell acute lymphoblastic leukemia. Leukemia 2017, 31, 1221–1223. [Google Scholar] [CrossRef]
- Kastner, P.; Chan, S. Role of Ikaros in T-cell acute lymphoblastic leukemia. World J. Biol. Chem. 2011, 2, 108–114. [Google Scholar] [CrossRef]
- Wiemels, J. Perspectives on the causes of childhood leukemia. Chem. Interact. 2012, 196, 59–67. [Google Scholar] [CrossRef]
- Greaves, M.F. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia 1988, 2, 120–125. [Google Scholar]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukemia. Nat. Rev. Cancer 2018, 18, 471–484, Correction in Nat. Rev. Cancer 2018, 18, 526. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can we prevent childhood Leukaemia? Leukemia 2021, 35, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qian, M.; Goodings, C.; Zhang, Y.; Yang, W.; Wang, P.; Xu, B.; Tian, C.; Pui, C.-H.; Hunger, S.P.; et al. Molecular Mechanisms of ARID5B-Mediated Genetic Susceptibility to Acute Lymphoblastic Leukemia. JNCI J. Natl. Cancer Inst. 2022, 114, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Martín-Lorenzo, A.; Hauer, J.; Vicente-Dueñas, C.; Auer, F.; González-Herrero, I.; García-Ramírez, I.; Ginzel, S.; Thiele, R.; Constantinescu, S.N.; Bartenhagen, C.; et al. Infection Exposure Is a Causal Factor in B-cell Precursor Acute Lymphoblastic Leukemia as a Result of Pax5-Inherited Susceptibility. Cancer Discov. 2015, 5, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K.; Badiga, A.; Sahakian, E.; Arora, A.I.; Nair, S.; Powers, J.J.B.; Achille, A.N.B.; Jaglal, M.V.; Patel, S.; Migone, F. Plasma of Acute Lymphoblastic Leukemia Patients React to the Culture of a Mycovirus Containing Aspergillus flavus. J. Pediatr. Hematol. 2020, 42, 350–358. [Google Scholar] [CrossRef]
- Tebbi, C.K. Mycoviruses in Fungi: Carcinogenesis of Fungal Agents May Not Always Be Mycotoxin Related. J. Fungi 2023, 9, 368. [Google Scholar] [CrossRef]
- Kotta-Loizou, I.; Coutts, R.H.A. Mycoviruses in Aspergilli: A Comprehensive Review. Front. Microbiol. 2017, 8, 1699. [Google Scholar] [CrossRef]
- Schmidt, F.R. The RNA interference-virus interplay: Tools of nature for gene modulation, morphogenesis, evolution, and a possible mean for aflatoxin control. Appl. Microbiol. Biotechnol. 2009, 83, 611–615. [Google Scholar] [CrossRef]
- Schmidt, F.R.; Lemke, P.A.; Esser, K. Viral influences on aflatoxin formation by Aspergillus flavus. Appl. Microbiol. Biotechnol. 1986, 24, 248–252. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Badiga, A.; Sahakian, E.; Powers, J.J.; Achille, A.N.; Patel, S.; Migone, F. Exposure to a mycovirus containing Aspergillus Flavus reproduces acute lymphoblastic leukemia cell surface and genetic markers in cells from patients in remission and not controls. Cancer Treat. Res. Commun. 2021, 26, 100279. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Suzuki, N. Viruses of Plant Pathogenic Fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Clancey, S.A.; Ruchti, F.; LeibundGut-Landmann, S.; Heitman, J.; Ianiri, G. A Novel Mycovirus Evokes Transcriptional Rewir-ing in the Fungus Malassezia and Stimulates Beta Interferon Production in Macrophages. MBio 2020, 11, e01534-20. [Google Scholar]
- Sun, Q.; Choi, G.H.; Nuss, D.L. Hypovirus-responsive transcription factor gene pro1 of the chestnut blight fungus Cryphonectria parasitica is required for female fertility, asexual spore development, and stable maintenance of hypovirus in-fection. Eukaryot Cell 2009, 8, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Yu, J.; Kim, K.H. Five Questions about Mycoviruses. PLoS Pathog. 2015, 11, e1005172. [Google Scholar] [CrossRef] [PubMed]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal–plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef]
- Jiang, D.; Fu, Y.; Guoqing, L.; Ghabrial, S.A. Viruses of the plant pathogenic fungus Sclerotinia sclerotiorum. Adv. Virus Res. 2013, 86, 215–248. [Google Scholar]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef]
- Filtz, T.M.; Vogel, W.K.; Leid, M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 2014, 35, 76–85. [Google Scholar] [CrossRef]
- Familiades, J.; Bousquet, M.; Lafage-Pochitaloff, M.; Béné, M.-C.; Beldjord, K.; De Vos, J.; Dastugue, N.; Coyaud, E.; Struski, S.; Quelen, C.; et al. PAX5 mutations occur frequently in adult B-cell progenitor acute lymphoblastic leukemia and PAX5 haploinsufficiency is associated with BCR-ABL1 and TCF3-PBX1 fusion genes: A GRAALL study. Leukemia 2009, 23, 1989–1998. [Google Scholar] [CrossRef]
- Cobaleda, C.; Jochum, W.; Busslinger, M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted pro-genitors. Nature 2007, 449, 473–477. [Google Scholar] [CrossRef]
- Cobaleda, C.; Schebesta, A.; Delogu, A.; Busslinger, M. Pax5: The guardian of B cell identity and function. Nat. Immunol. 2007, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Cimmino, L.; Jude, J.G.; Hu, Y.; Witkowski, M.T.; McKenzie, M.D.; Kartal-Kaess, M.; Best, S.A.; Tuohey, L.; Liao, Y.; et al. Pax5 loss imposes a reversible differentiation block in B-progenitor acute lymphoblastic leukemia. Genes Dev. 2014, 28, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Nakitandwe, J.; Chen, S.-C.; Lenny, N.T.; Miller, C.B.; Su, X.; Mullighan, C.G.; Downing, J.R. Acute Lymphoblastic Leukemia-Associated PAX5 Mutations Induce Aberrant B Cell Development. Blood 2010, 116, 10. [Google Scholar] [CrossRef]
- Nowak, D.; Kawamata, N.; Niebuhr, B.; Nowak, V.; Mossner, M.; Nahar, R.R.; Thoennissen, N.H.; Iwanski, G.B.; Stocking, C.; Dugas, M.; et al. The Pax5 Fusion Product Pax5-C20orf112 Causes Downregulation of Pre-B Cell Receptor Genes and Induces Differential Proliferation Patterns in B-Lymphoblastic Cell Lines. Blood 2009, 114, 1284. [Google Scholar] [CrossRef]
- Jia, Z.; Gu, Z. PAX5 alterations in B-cell acute lymphoblastic leukemia. Front. Oncol. 2022, 12, 1023606. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Downing, J.R. Genome-wide profiling of genetic alterations in acute lymphoblastic leukemia: Recent insights and future directions. Leukemia 2009, 23, 1209–1218. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.H. BCR–ABL1 lymphoblastic leukaemia is charac-terized by the deletion of Ikaros. Nature 2008, 453, 110–114. [Google Scholar] [CrossRef]
- Yoshida, T.; Georgopoulos, K. Ikaros fingers on lymphocyte differentiation. Int. J. Hematol. 2014, 100, 220–229. [Google Scholar] [CrossRef]
- John, L.B.; Ward, A.C. The Ikaros gene family: Transcriptional regulators of hematopoiesis and immunity. Mol. Immunol. 2011, 48, 1272–1278. [Google Scholar] [CrossRef]
- Marke, R.; van Leeuwen, F.N.; Scheijen, B. The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia. Haematologica 2018, 103, 565–574. [Google Scholar] [CrossRef]
- Kuiper, R.P.; Schoenmakers, E.F.P.M.; Van Reijmersdal, S.V.; Hehir-Kwa, J.Y.; van Kessel, A.G.; Van Leeuwen, F.N.; Hoogerbrugge, P.M. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting path-ways involved in lymphocyte differentiation and cell cycle progression. Leukemia 2007, 21, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Storlazzi, C.T.; Cilloni, D.; Lonetti, A.; Ottaviani, E.; Soverini, S.; Astolfi, A.; Chiaretti, S.; Vitale, A.; Messa, F.; et al. Identification and molecular characterization of recurrent genomic de-letions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: On behalf of Gruppo Italiano Malattie Ematologiche dell’Adulto Acute Leukemia Working Party (GIMEMA AL WP). Blood 2009, 114, 2159–2167. [Google Scholar] [PubMed]
- Kastner, P.; Dupuis, A.; Gaub, M.-P.; Herbrecht, R.; Lutz, P.; Chan, S. Function of Ikaros as a tumor suppressor in B cell acute lymphoblastic leukemia. Am. J. Blood Res. 2013, 3, 1–13. [Google Scholar] [PubMed]
- Vairy, S.; Tran, T.H. IKZF1 alterations in acute lymphoblastic leukemia: The good, the bad and the ugly. Blood Rev. 2020, 44, 100677. [Google Scholar] [CrossRef]
- Stanulla, M.; Cave, H.; Moorman, A.V. IKZF1 deletions in pediatric acute lymphoblastic leukemia: Still a poor prognostic marker? Blood 2020, 135, 252–260. [Google Scholar] [CrossRef]
- Mi, J.Q.; Wang, X.; Yao, Y.; Lu, H.J.; Jiang, X.X.; Zhou, J.F.; Wang, J.H.; Jiao, B.; Shen, S.H.; Tang, J.Y.; et al. Newly diagnosed acute lymphoblastic leukemia in China (II): Prognosis related to genetic abnormalities in a se-ries of 1091 cases. Leukemia 2012, 26, 1507–1516. [Google Scholar] [CrossRef]
- Dupuis, A.; Gaub, M.P.; Legrain, M.; Drenou, B.; Mauvieux, L.; Lutz, P.; Herbrecht, R.; Chan, S.; Kastner, P. Biclonal and biallelic deletions occur in 20% of B-ALL cases with IKZF1 mutations. Leukemia 2012, 27, 503–507. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; Zhang, B.; Payne, J.L.; Song, C.; Ge, Z.; Gowda, C.; Iyer, S.; Dhanyamraju, P.K.; Dorsam, G.; Reeves, M.E.; et al. Ikaros tumor suppressor function includes induction of active enhancers and super-enhancers along with pio-neering activity. Leukemia 2019, 33, 2720–2731. [Google Scholar] [CrossRef]
- Davis, K.L. Ikaros: Master of hematopoiesis, agent of leukemia. Ther. Adv. Hematol. 2011, 2, 359–368. [Google Scholar] [CrossRef]
- Georgopoulos, K.; Moore, D.D.; Derfler, B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science 1992, 258, 808–812. [Google Scholar] [CrossRef]
- Kim, J.; Sif, S.; Jones, B.; Jackson, A.; Koipally, J.; Heller, E.; Winandy, S.; Viel, A.; Sawyer, A.; Ikeda, T.; et al. Ikaros DNA-Binding Proteins Direct Formation of Chromatin Remodeling Complexes in Lymphocytes. Immunity 1999, 10, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Koipally, J.; Renold, A.; Kim, J.; Georgopoulos, K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999, 18, 3090–3100. [Google Scholar] [CrossRef] [PubMed]
- Boutboul, D.; Kuehn, H.S.; Van de Wyngaert, Z.; Niemela, J.E.; Callebaut, I.; Stoddard, J.; Lenoir, C.; Barlogis, V.; Farnarier, C.; Vely, F.; et al. Dominant-negative IKZF1 mutations cause a T, B, and myeloid cell combined immunodeficiency. J. Clin. Investig. 2018, 128, 3071–3087. [Google Scholar] [CrossRef] [PubMed]
- Churchman, M.L.; Qian, M.; Kronnie, G.T.; Zhang, R.; Yang, W.; Zhang, H.; Lana, T.; Tedrick, P.; Baskin, R.; Verbist, K.; et al. Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer Cell 2018, 33, 937–948.e8. [Google Scholar] [CrossRef]
- Espinosa, L.; Cathelin, S.; D’Altri, T.; Trimarchi, T.; Statnikov, A.; Guiu, J.; Rodilla, V.; Inglés-Esteve, J.; Nomdedeu, J.; Bellosillo, B.; et al. The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer Cell 2010, 18, 268–281. [Google Scholar] [CrossRef]
- Bellavia, D.; Campese, A.F.; Alesse, E.; Vacca, A.; Felli, M.P.; Balestri, A.; Stoppacciaro, A.; Tiveron, C.; Tatangelo, L.; Giovarelli, M.; et al. Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 2000, 19, 3337–3348. [Google Scholar] [CrossRef]
- Beg, A.A.; Baltimore, D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Nuclear factor-κB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef]
- Jeremias, I.; Kupatt, C.; Baumann, B.; Herr, I.; Wirth, T.; Debatin, K.M. Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood 1998, 91, 4624–4631. [Google Scholar] [CrossRef]
- Greten, F.R.; Karin, M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004, 206, 193–199. [Google Scholar] [CrossRef]
- Turco, M.C.; Romano, M.F.; Petrella, A.; Bisogni, R.; Tassone, P.; Venuta, S. NF-kappaB/Rel-mediated regulation of apoptosis in hematologic malignancies and normal hematopoietic progenitors. Leukemia 2004, 18, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Carvalho, G.; Fabre, C.; Grosjean, J.; Fenaux, P.; Kroemer, G. Targeting NF-kappaB in hematologic malignancies. Cell Death Differ. 2006, 13, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Munzert, G.; Kirchner, D.; Ottmann, O.; Bergmann, L.; Schmid, R.M. Constitutive NF-kappab/Rel activation in philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL). Leuk. Lymphoma 2004, 45, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Luria, S. Viruses, Cancer Cells, and the Genetic Concept of Virus Infection. Cancer Res. 1960, 20, 677–688. [Google Scholar] [PubMed]
- Melana, S.M.; Nepomnaschy, I.; Sakalian, M.; Abbott, A.; Hasa, J.; Holland, J.F.; Pogo, B.G. Characterization of Viral Particles Isolated from Primary Cultures of Human Breast Cancer Cells. Cancer Res. 2007, 67, 8960–8965. [Google Scholar] [CrossRef]
- Pogo, B.G.; Holland, J.F. Possibilities of a viral etiology for human breast cancer. Biol. Trace Element Res. 1997, 56, 131–142. [Google Scholar] [CrossRef]
- Li, H.P.; Leu, Y.W.; Chang, Y.S. Epigenetic changes in virus-associated human cancers. Cell Res. 2005, 15, 262–271. [Google Scholar] [CrossRef]
- Burnett-Hartman, A.N.; Newcomb, P.A.; Potter, J.D. Infectious agents, and colorectal cancer: A review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2970–2979. [Google Scholar] [CrossRef]
- Parsa, N. Environmental Factors Inducing Human Cancers. Iran. J. Public Health 2012, 41, 1–9. [Google Scholar]
- Parsa, N.Z.; Mukherjee, A.B.; Chaganti, R.S.K.; Gaidano, G.; Hauptschein, R.S.; Dalla-Favera, R.; Lenoir, G. Cytogenetic and molecular analysis of 6q deletions in Burkitt’s lymphoma cell lines. Genes Chromosomes Cancer 1994, 9, 13–18. [Google Scholar] [CrossRef]
- Mager, D.L. Bacteria and cancer: Cause, coincidence, or cure? A review. J. Transl. Med. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Egi, Y.; Ito, M.; Tanaka, S.; Imagawa, S.; Takata, S.; Yoshihara, M.; Haruma, K.; Chayama, K. Role of Helicobacter pylori Infection and Chronic Inflammation in Gastric Cancer in the Cardia. Ultrasound Med. Biol. 2007, 37, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C. Carcinogenesis and Leukemogenesis of Microorganisms. J. 21st Century Pathol. 2022, 2, 109. [Google Scholar]
- Lyles, D.S. Cytopathogenesis and Inhibition of Host Gene Expression by RNA Viruses. Microbiol. Mol. Biol. Rev. 2000, 64, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Desfarges, S.; Ciuffi, A. Viral integration and consequences on host gene expression. In Viruses: Essential Agents of Life; Springer: Dordrecht, The Netherlands, 2012; pp. 147–175. [Google Scholar] [CrossRef]
- Her, L.-S.; Lund, E.; Dahlberg, J.E. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science 1997, 276, 1845–1848. [Google Scholar] [CrossRef]
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer. Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef]
- Park, M.; Cho, Y.-J.; Kim, D.; Yang, C.-S.; Lee, S.M.; Dawson, T.L.; Nakamizo, S.; Kabashima, K.; Lee, Y.W.; Jung, W.H. A Novel Virus Alters Gene Expression and Vacuolar Morphology in Malassezia Cells and Induces a TLR3-Mediated Inflammatory Immune Response. MBio 2020, 11, e01521-20. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting transcription factors in cancer—From undruggable to reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef]
- Lebraud, H.; Wright, D.J.; Johnson CNHeightman, T.D. Protein Degradation by In-Cell Self-Assembly of Proteolysis Tar-geting Chimeras. ACS Cent. Sci. 2016, 2, 927–934. [Google Scholar] [CrossRef]
- Zeng, S.; Huang, W.; Zheng, X.; Cheng, L.; Zhang, Z.; Wang, J.; Shen, Z. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges. Eur. J. Med. Chem. 2021, 210, 112981. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020, 181, 102–114. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebbi, C.K.; Yan, J.; Sahakian, E.; Mediavilla-Varela, M.; Pinilla-Ibarz, J.; Patel, S.; Rottinghaus, G.E.; Liu, R.Y.; Dennison, C. Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2024, 25, 10361. https://doi.org/10.3390/ijms251910361
Tebbi CK, Yan J, Sahakian E, Mediavilla-Varela M, Pinilla-Ibarz J, Patel S, Rottinghaus GE, Liu RY, Dennison C. Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells. International Journal of Molecular Sciences. 2024; 25(19):10361. https://doi.org/10.3390/ijms251910361
Chicago/Turabian StyleTebbi, Cameron K., Jiyu Yan, Eva Sahakian, Melanie Mediavilla-Varela, Javier Pinilla-Ibarz, Saumil Patel, George E. Rottinghaus, Rachel Y. Liu, and Clare Dennison. 2024. "Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells" International Journal of Molecular Sciences 25, no. 19: 10361. https://doi.org/10.3390/ijms251910361
APA StyleTebbi, C. K., Yan, J., Sahakian, E., Mediavilla-Varela, M., Pinilla-Ibarz, J., Patel, S., Rottinghaus, G. E., Liu, R. Y., & Dennison, C. (2024). Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells. International Journal of Molecular Sciences, 25(19), 10361. https://doi.org/10.3390/ijms251910361




