A Novel Prognostic Model of Hepatocellular Carcinoma per Two NAD+ Metabolic Synthesis-Associated Genes
Abstract
:1. Introduction
2. Results
2.1. NAD+-Associated Differentially Expressed Genes (DEGs)
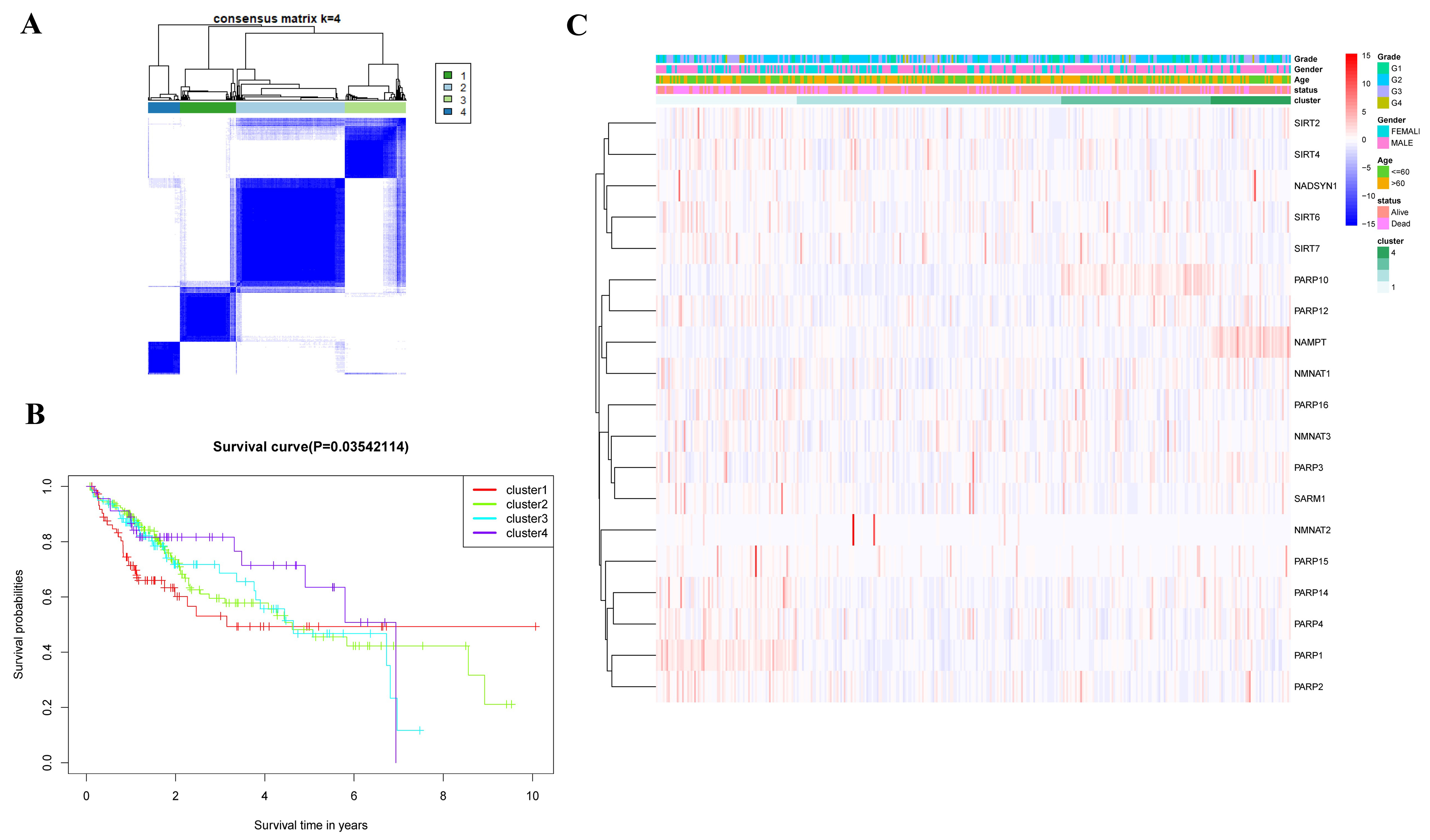
2.2. Consensus Clustering Analysis
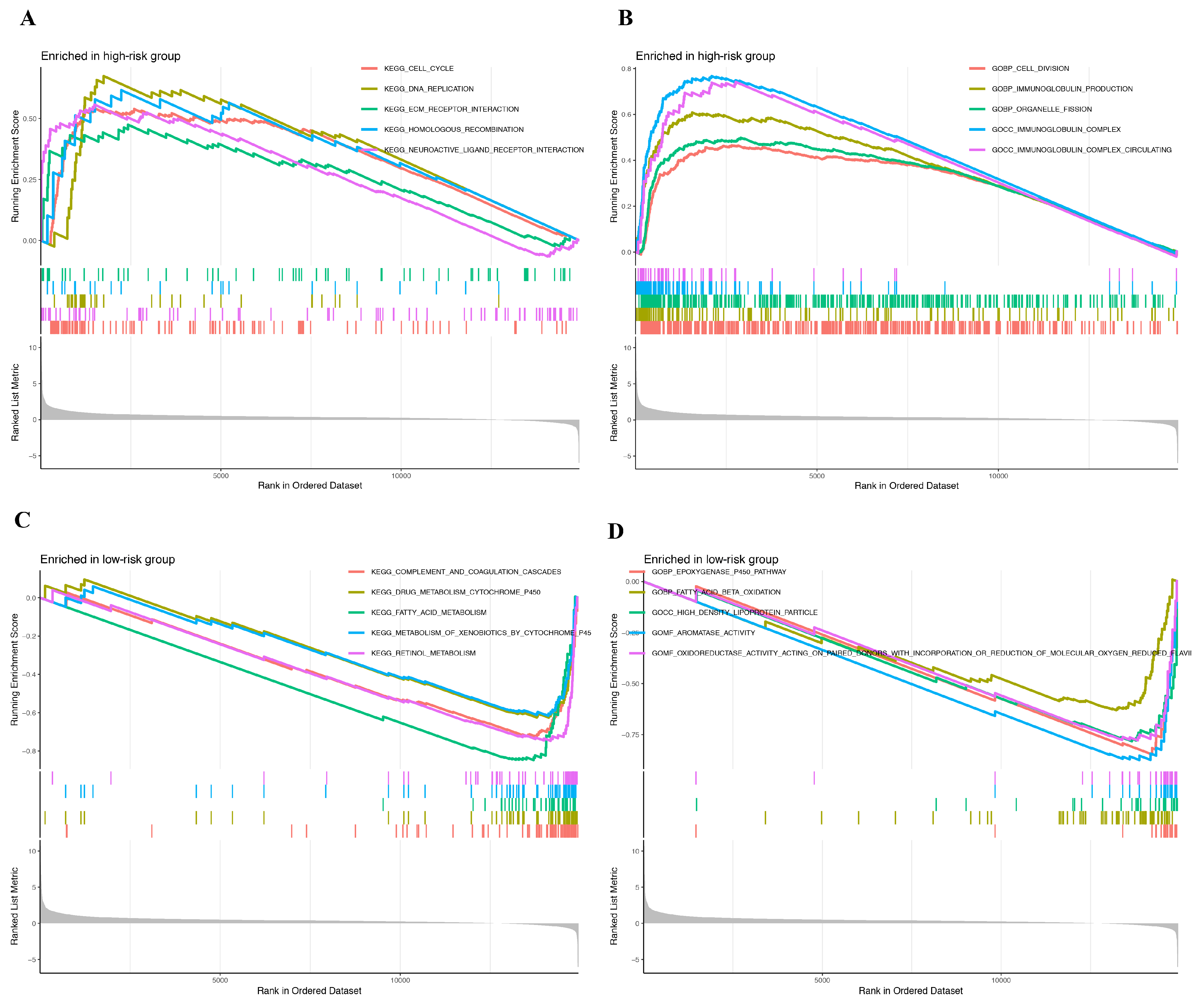
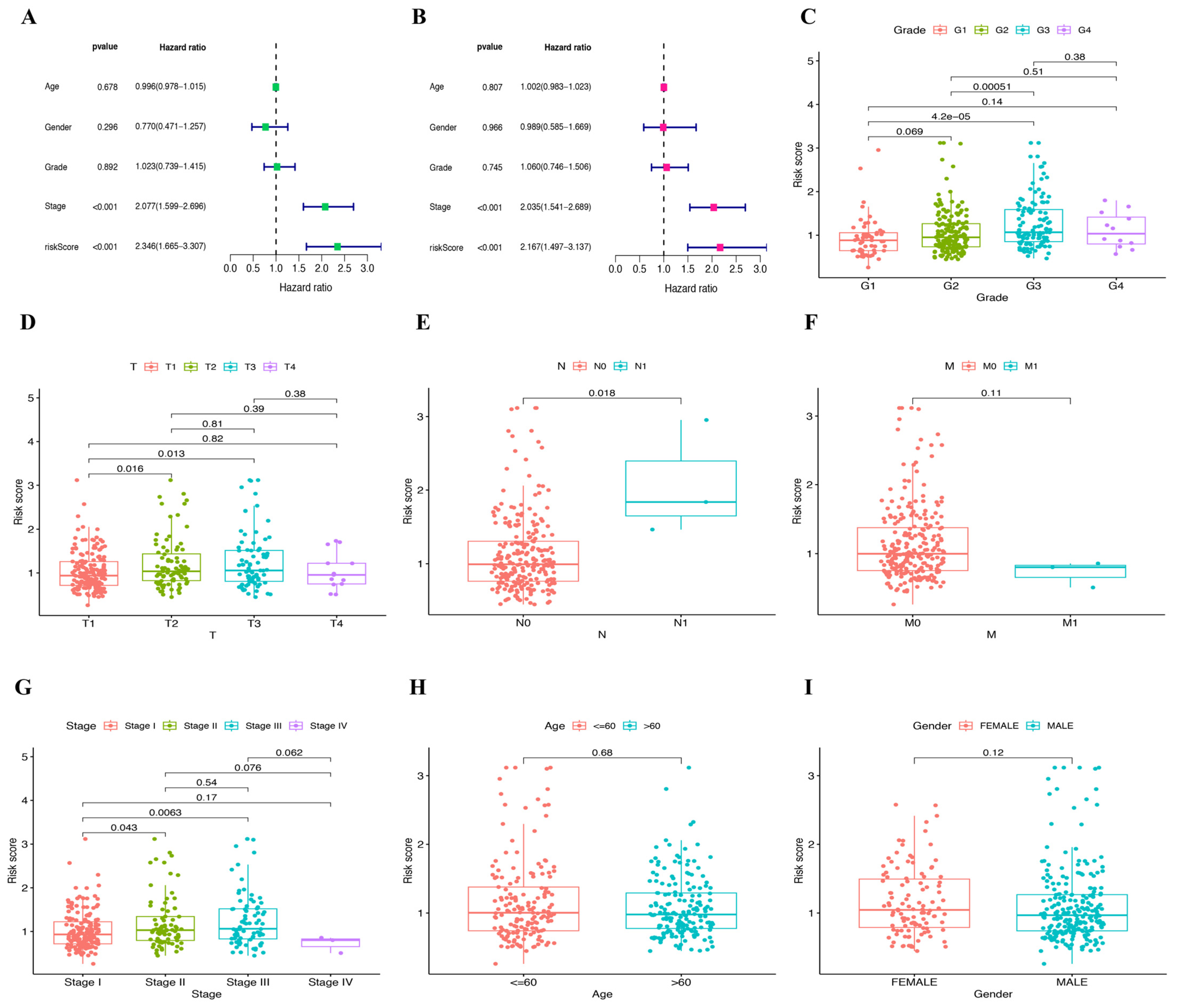
2.3. Establishment and Validation of Prognostic Model
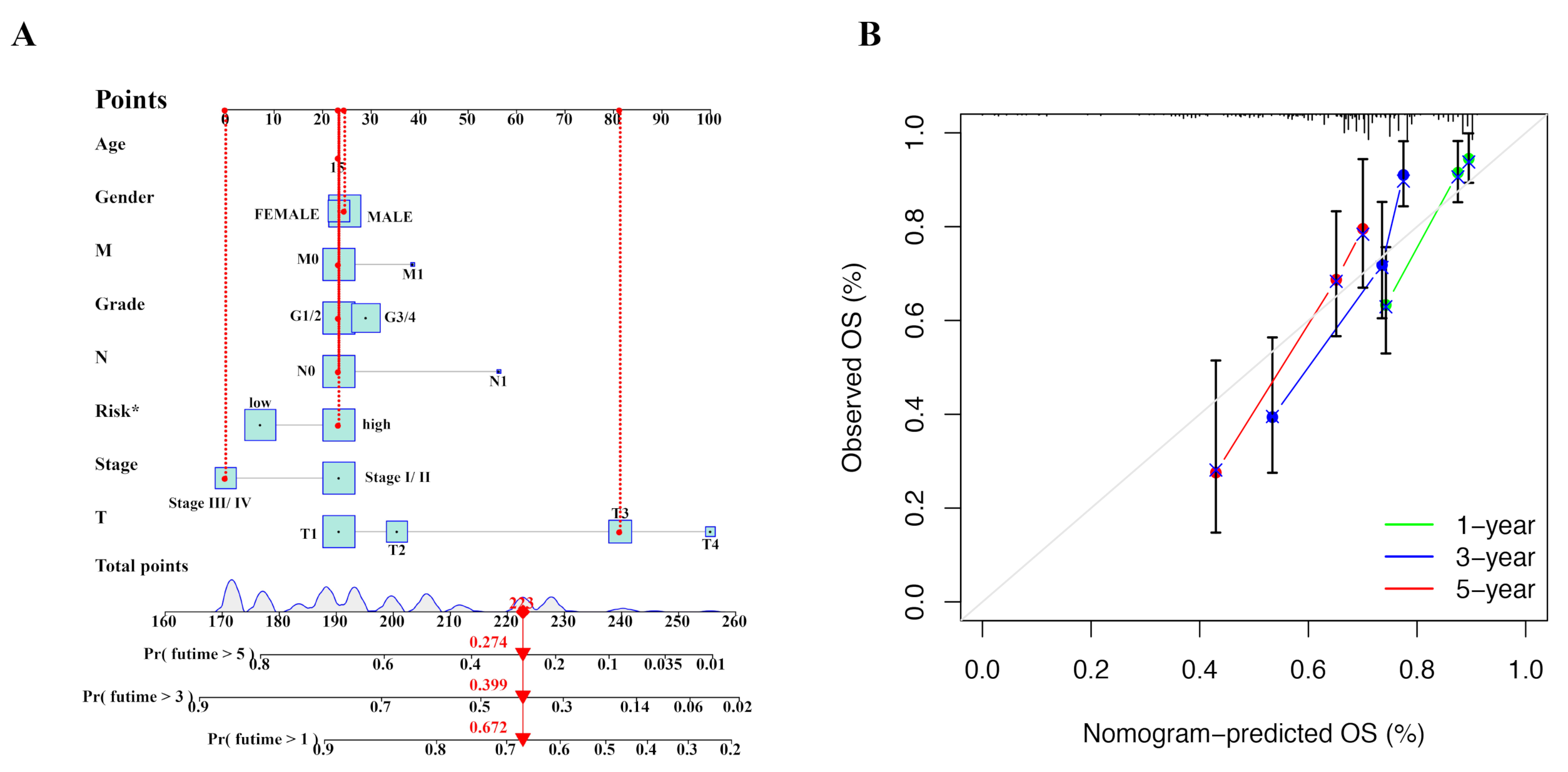
2.4. Nomogram Was Constructed to Predict Prognosis of HCC Patients
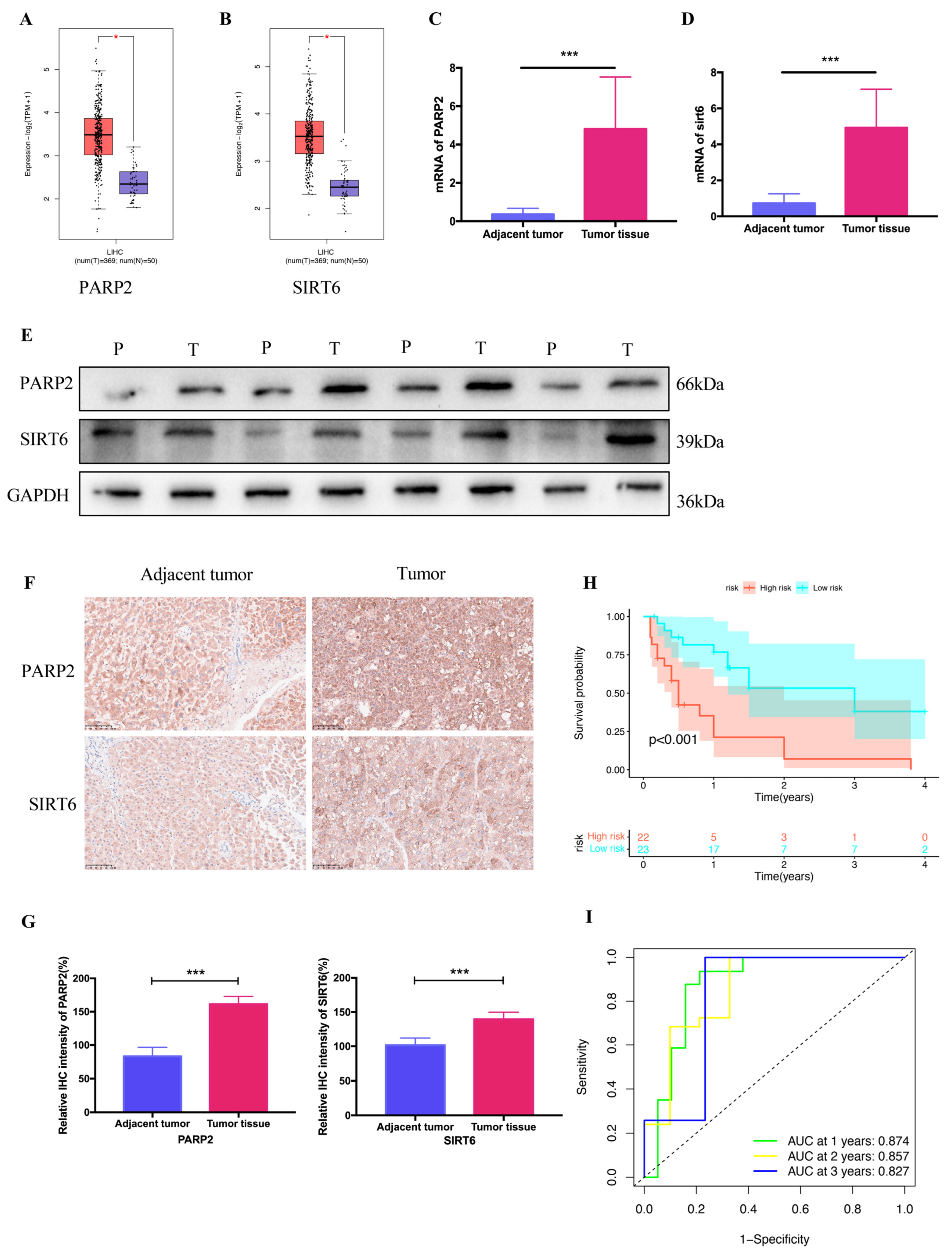
2.5. Verification of Clinical Tissue Samples
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Data Sources
4.3. Screening of DEGs Associated with NAD+
4.4. Establishment and Validation of Prognostic Model
4.5. Immunohistochemistry (IHC)
4.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Western Blotting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEGs | Differentially expressed genes |
| EMT | Epithelial–mesenchymal transition |
| GEPIA2.0 | Gene expression profiling interactive analysis 2.0 |
| GO | Gene ontology |
| HCC | Hepatocellular carcinoma |
| IHC | Immunohistochemistry |
| KEGG | Kyoto encyclopedia of genes and genomes |
| KM | Kaplan–Meier |
| MCU | Mitochondrial Ca2+ uniporter |
| NAD | Nicotinamide adenine dinucleotide |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| PARP2 | Poly (ADP-Ribose) polymerase 2 |
| PCA | Principal component analysis |
| PPI | Protein–protein interaction |
| ROS | Reactive oxygen species |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| SIRT6 | Sirtuin 6 |
| SIRT6/7 | Sirtuin 6/7 |
| SLC25A51 | Solute carrier family 25 member 20 |
| TCGA | The cancer genome atlas |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular Carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.; Sedej, S.; Kroemer, G. NAD(+) Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021, 144, 1795–1817. [Google Scholar] [CrossRef] [PubMed]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Chmielewski, J.P.; Bowlby, S.C.; Wheeler, F.B.; Shi, L.; Sui, G.; Davis, A.L.; Howard, T.D.; D’Agostino, R.B., Jr.; Miller, L.D.; Sirintrapun, S.J.; et al. CD38 Inhibits Prostate Cancer Metabolism and Proliferation by Reducing Cellular NAD(+) Pools. Mol. Cancer Res. 2018, 16, 1687–1700. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Q.; Ma, Z.; Yuan, M.; Zhao, S. Identification of immune-related genes that predict prognosis and risk of bladder cancer-bioinformatics analysis of TCGA database. Aging 2021, 13, 19352–19374. [Google Scholar] [CrossRef]
- Quan, Q.; Xiong, X.; Wu, S.; Yu, M. Identification of Autophagy-Related Prognostic Signature and Analysis of Immune Cell Infiltration in Low-Grade Gliomas. Biomed. Res. Int. 2021, 2021, 7918693. [Google Scholar] [CrossRef]
- Lucena-Cacace, A.; Otero-Albiol, D.; Jimenez-Garcia, M.P.; Peinado-Serrano, J.; Carnero, A. NAMPT overexpression induces cancer stemness and defines a novel tumor signature for glioma prognosis. Oncotarget 2017, 8, 99514–99530. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, D.; Zhang, X.; Liang, G.; Liu, W.; Qiao, S. FK866 inhibits the epithelial-mesenchymal transition of hepatocarcinoma MHCC97-H cells. Oncol. Lett. 2018, 16, 7231–7238. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Nakagawa, T. NAD metabolism: Implications in aging and longevity. Ageing Res. Rev. 2018, 47, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Y.; Wang, X.; Wang, Q.; Deng, H. ROS-Mediated 15-Hydroxyprostaglandin Dehydrogenase Degradation via Cysteine Oxidation Promotes NAD+-Mediated Epithelial-Mesenchymal Transition. Cell Chem. Biol. 2018, 25, 255–261.e254. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD(+) in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yang, Z.-X.; Ma, P.-F.; Liu, J.-S.; Wang, D.-S.; Yu, H.-C. Overexpression of SLC25A51 promotes hepatocellular carcinoma progression by driving aerobic glycolysis through activation of SIRT5. Free Radic. Biol. Med. 2022, 182, 11–22. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, H.; Wang, J.; Zhu, J.; Jin, M.; Wu, Y.; Guo, X.; Ji, L.; Huang, Q.; Zhang, H.; et al. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene 2017, 36, 5897–5909. [Google Scholar] [CrossRef]
- Chen, Q.; Kassab, M.A.; Dantzer, F.; Yu, X. PARP2 mediates branched poly ADP-ribosylation in response to DNA damage. Nat. Commun. 2018, 9, 3233. [Google Scholar] [CrossRef]
- Zuo, H.; Yang, D.; Yang, Q.; Tang, H.; Fu, Y.X.; Wan, Y. Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nat. Commun. 2020, 11, 1578. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, W.; Wang, Z.; Liu, S. Long non-coding RNA PTTG3P functions as an oncogene by sponging miR-383 and up-regulating CCND1 and PARP2 in hepatocellular carcinoma. BMC Cancer 2019, 19, 731. [Google Scholar] [CrossRef]
- Gerossier, L.; Dubois, A.; Paturel, A.; Fares, N.; Cohen, D.; Merle, P.; Lachuer, J.; Wierinckx, A.; Saintigny, P.; Bancel, B.; et al. PARP inhibitors and radiation potentiate liver cell death in vitro. Do hepatocellular carcinomas have an achilles’ heel? Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101553. [Google Scholar] [CrossRef]
- Khongkow, M.; Olmos, Y.; Gong, C.; Gomes, A.R.; Monteiro, L.J.; Yagüe, E.; Cavaco, T.B.; Khongkow, P.; Man, E.P.S.; Laohasinnarong, S.; et al. SIRT6 modulates paclitaxel and epirubicin resistance and survival in breast cancer. Carcinogenesis 2013, 34, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Andreani, C.; Bartolacci, C.; Persico, G.; Casciaro, F.; Amatori, S.; Fanelli, M.; Giorgio, M.; Galie, M.; Tomassoni, D.; Wang, J.; et al. SIRT6 promotes metastasis and relapse in HER2-positive breast cancer. Sci. Rep. 2023, 13, 22000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Q.R.; Wang, B.; Shao, J.; Zhang, T.; Liu, T.; Huang, G.; Xia, W. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell 2013, 4, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xie, Q.R.; Li, F.; Cheng, Y.; Wu, T.; Zhang, Y.; Lu, X.; Wong, A.S.T.; Sha, J.; Xia, W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 2021, 11, 6526–6541. [Google Scholar] [CrossRef]
- Han, L.L.; Jia, L.; Wu, F.; Huang, C. Sirtuin6 (SIRT6) Promotes the EMT of Hepatocellular Carcinoma by Stimulating Autophagic Degradation of E-Cadherin. Mol. Cancer Res. 2019, 17, 2267–2280. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhang, Y.; Fang, X.; Chen, N.; Zhou, X.; Wang, X. Sirt6 promotes tumorigenesis and drug resistance of diffuse large B-cell lymphoma by mediating PI3K/Akt signaling. J. Exp. Clin. Cancer Res. 2020, 39, 142. [Google Scholar] [CrossRef]
- Sebastian, C.; Zwaans, B.M.; Silberman, D.M.; Gymrek, M.; Goren, A.; Zhong, L.; Ram, O.; Truelove, J.; Guimaraes, A.R.; Toiber, D.; et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 2012, 151, 1185–1199. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, A.; Yu, X.; Zhu, J.; Dai, H. SIRT6 inhibits growth of gastric cancer by inhibiting JAK2/STAT3 pathway. Oncol. Rep. 2017, 38, 1059–1066. [Google Scholar] [CrossRef]
- Li, N.; Mao, D.; Cao, Y.; Li, H.; Ren, F.; Li, K. Downregulation of SIRT6 by miR-34c-5p is associated with poor prognosis and promotes colon cancer proliferation through inhibiting apoptosis via the JAK2/STAT3 signaling pathway. Int. J. Oncol. 2018, 52, 1515–1527. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, N.; Sun, H.; Su, L.; Zhang, C.; Xu, H.; Feng, J.; Wang, M.; Chen, J.; Liu, L.; et al. Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells. Gastroenterology 2020, 158, 664–678.e24. [Google Scholar] [CrossRef]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Lu, S.; Mao, L.; Zhong, M.; Feng, G.; He, S.; Yuan, G. A Novel Prognostic Model of Hepatocellular Carcinoma per Two NAD+ Metabolic Synthesis-Associated Genes. Int. J. Mol. Sci. 2024, 25, 10362. https://doi.org/10.3390/ijms251910362
Dai L, Lu S, Mao L, Zhong M, Feng G, He S, Yuan G. A Novel Prognostic Model of Hepatocellular Carcinoma per Two NAD+ Metabolic Synthesis-Associated Genes. International Journal of Molecular Sciences. 2024; 25(19):10362. https://doi.org/10.3390/ijms251910362
Chicago/Turabian StyleDai, Luo, Shiliu Lu, Linfeng Mao, Mingbei Zhong, Gangping Feng, Songqing He, and Guandou Yuan. 2024. "A Novel Prognostic Model of Hepatocellular Carcinoma per Two NAD+ Metabolic Synthesis-Associated Genes" International Journal of Molecular Sciences 25, no. 19: 10362. https://doi.org/10.3390/ijms251910362





