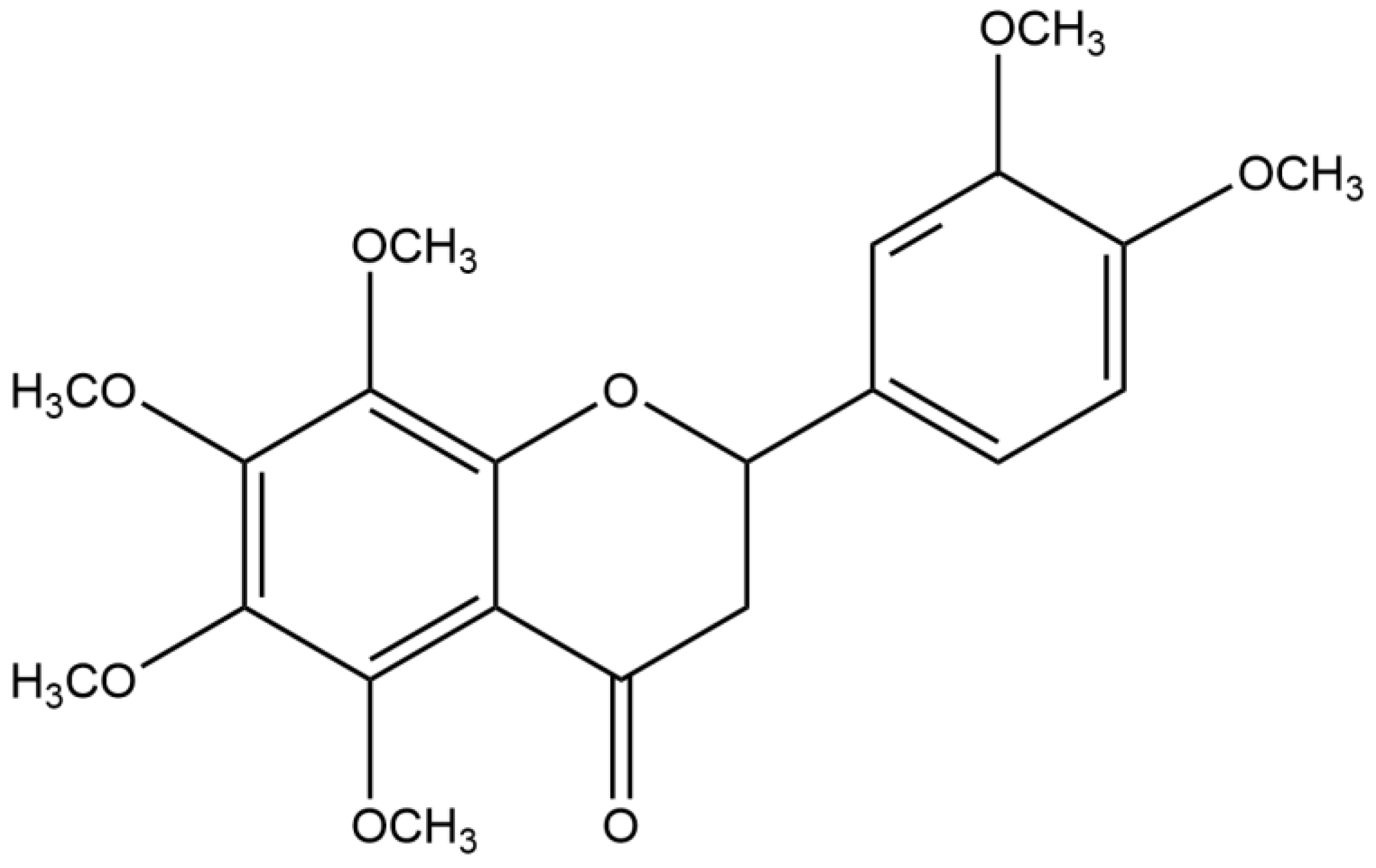
Nobiletin, as a Novel PDE4B Inhibitor, Alleviates Asthma Symptoms by Activating the cAMP-PKA-CREB Signaling Pathway
Abstract
:1. Introduction
2. Results
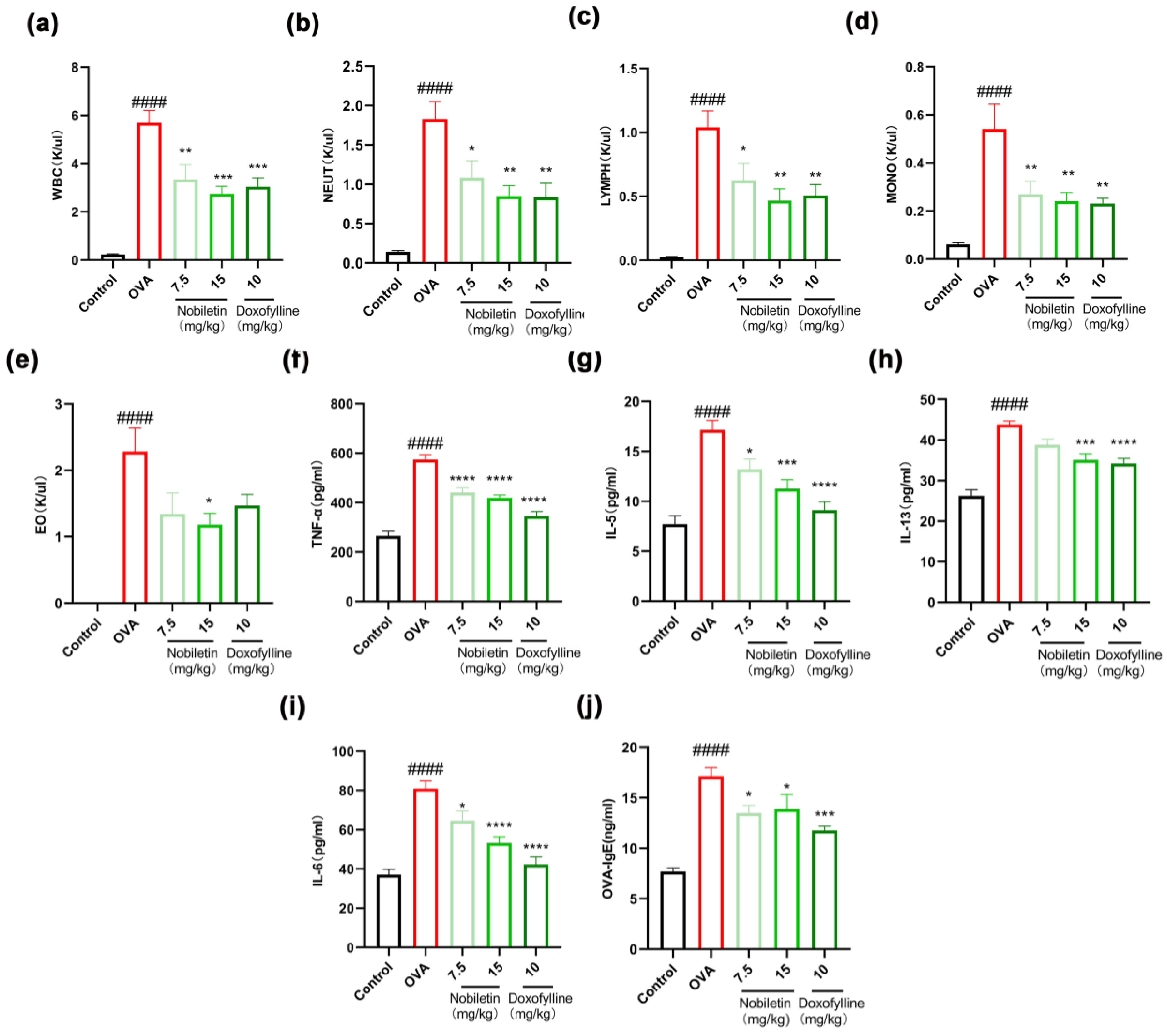
2.1. Nobiletin Decreased the Levels of Inflammation in Bronchoalveolar Lavage Fluid (BALF) and the Content of Specific IgE in Serum
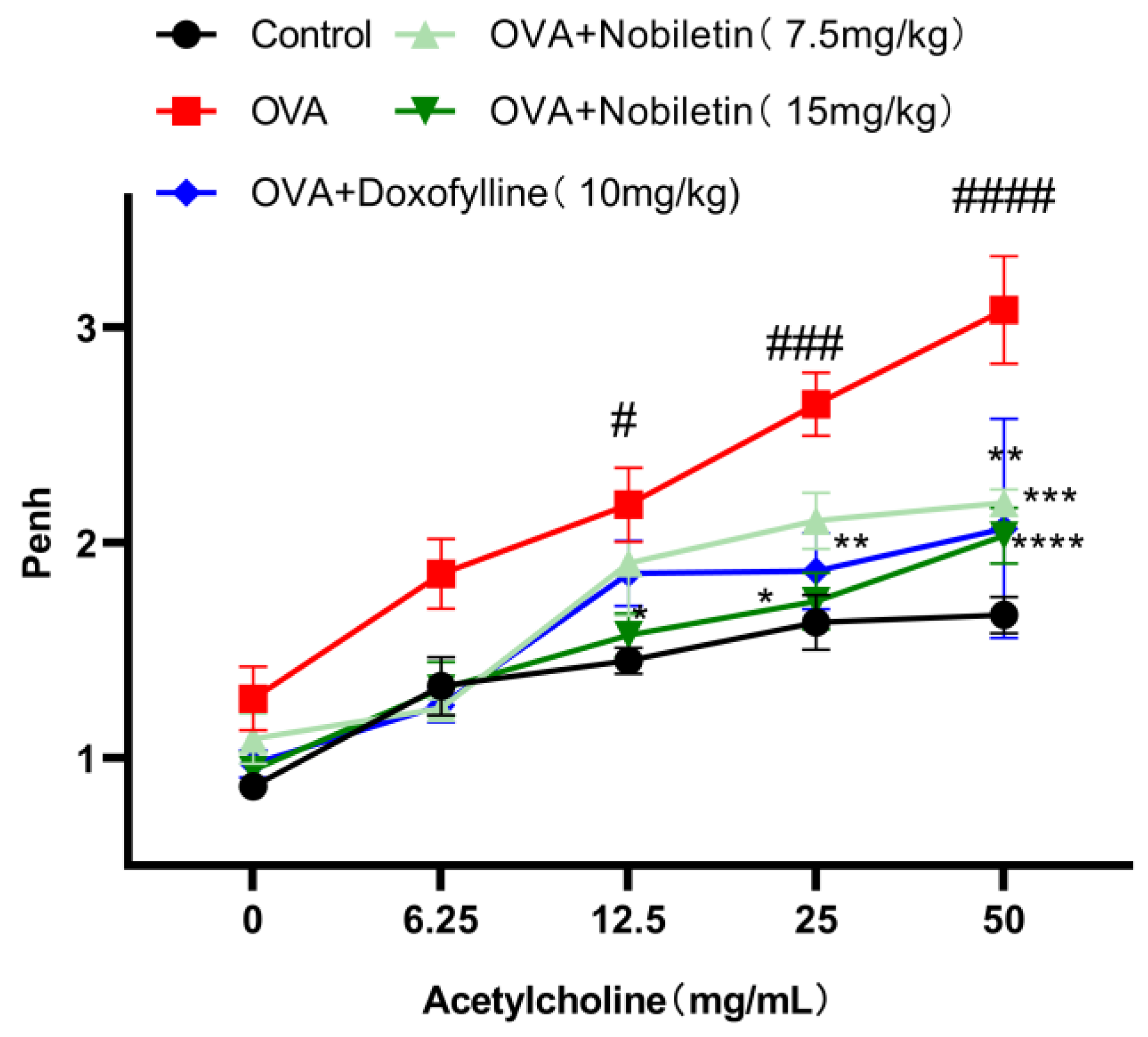
2.2. Nobiletin Attenuated Enhanced Pause (Penh) in Asthmatic Mice
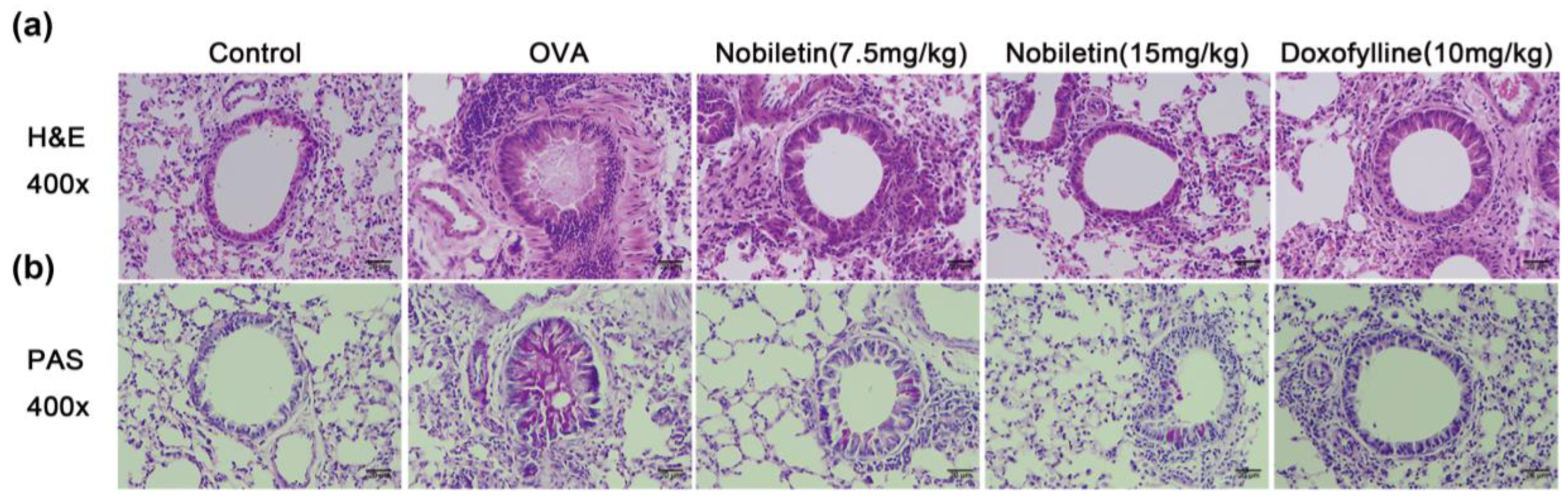
2.3. Effect of Nobiletin on Pathological Changes in Lung Tissue in Asthmatic Mice
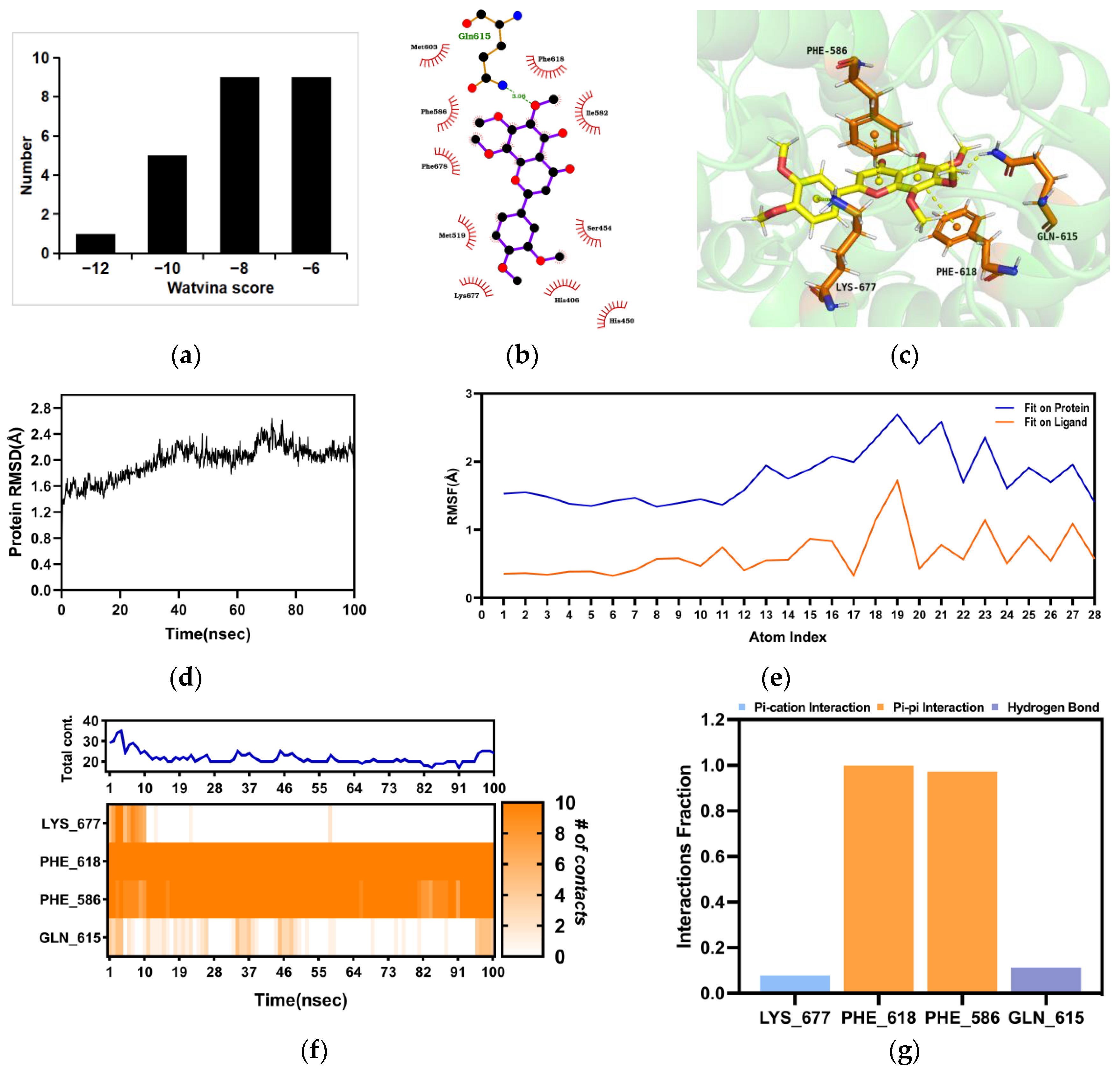
2.4. Molecular Docking and Molecular Dynamics (MD) between Nobiletin and PDE4B
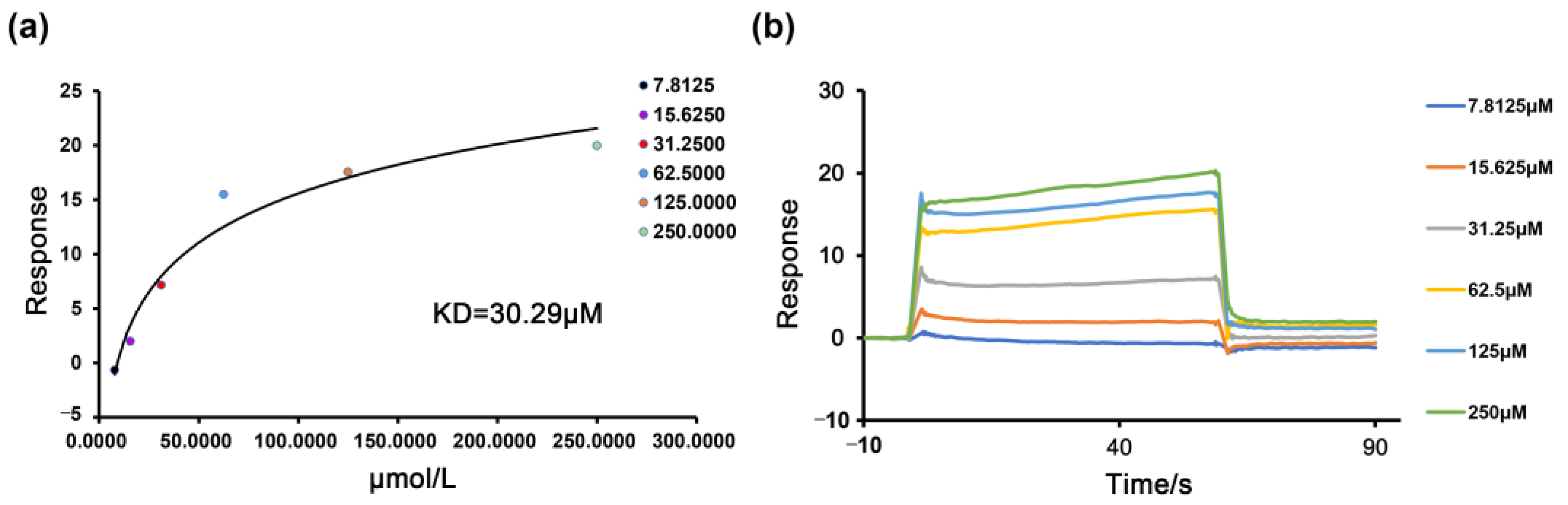
2.5. The Binding of the PDE4B Protein with Nobiletin
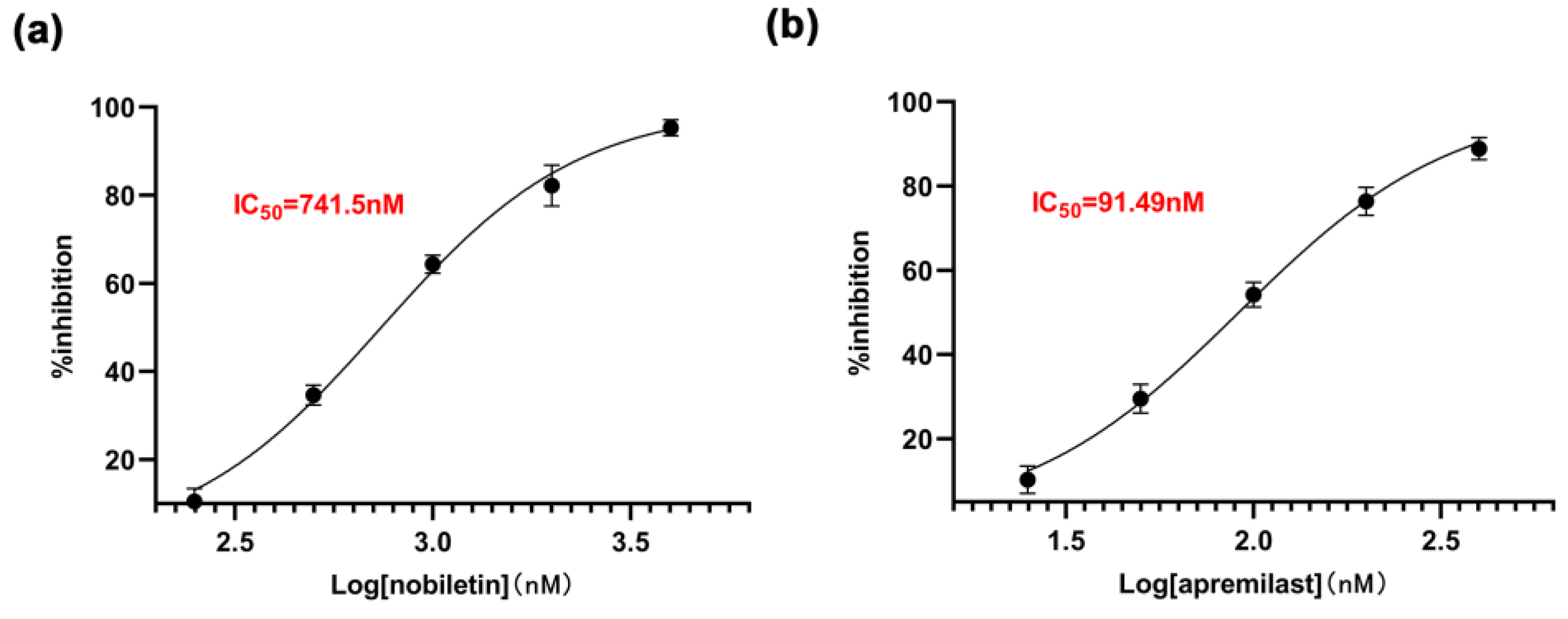
2.6. The Inhibitory Effect of Nobiletin on PDE4B
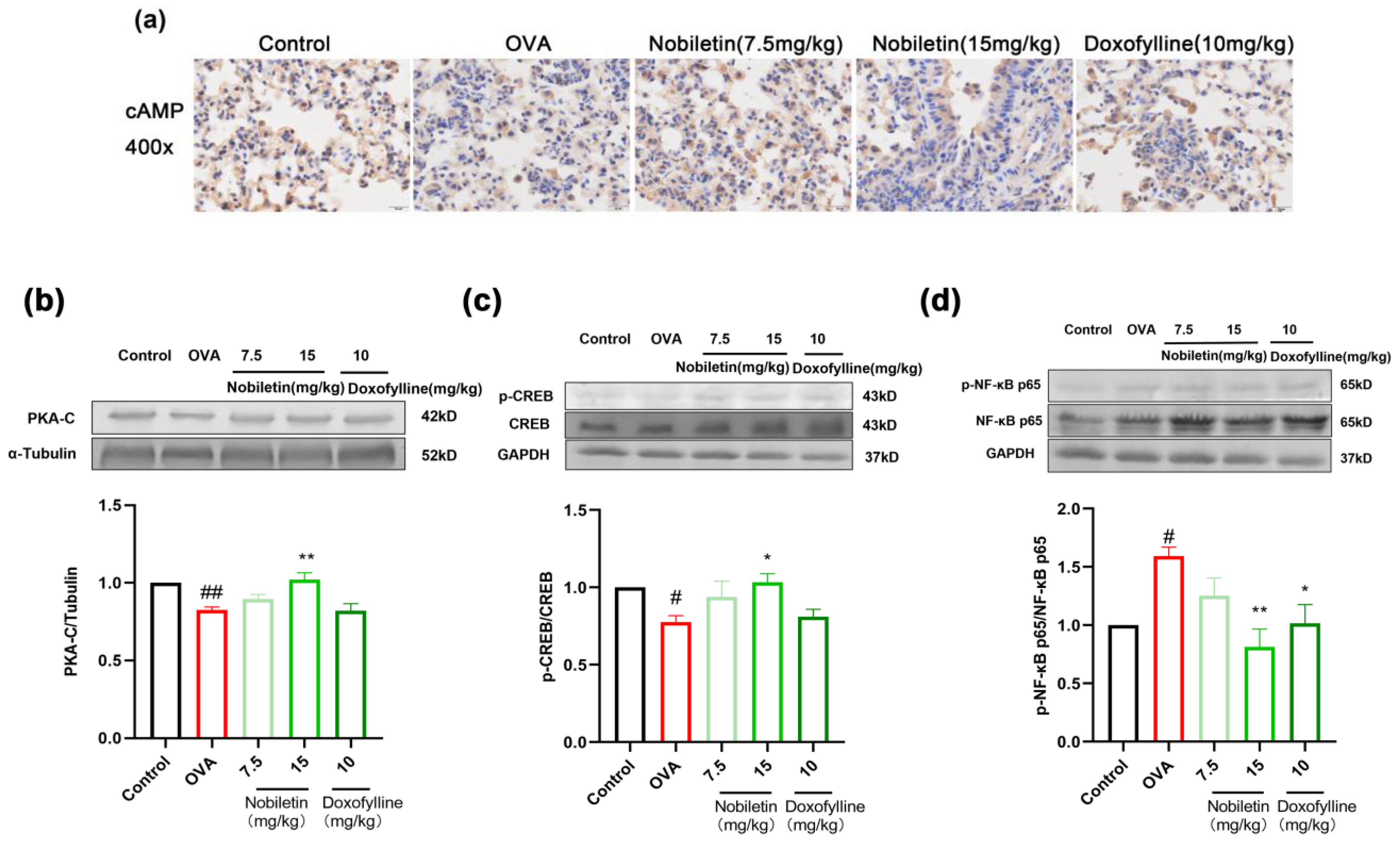
2.7. Effect of Nobiletin on the cAMP-PKA-CREB and NF-κB Signaling Pathways
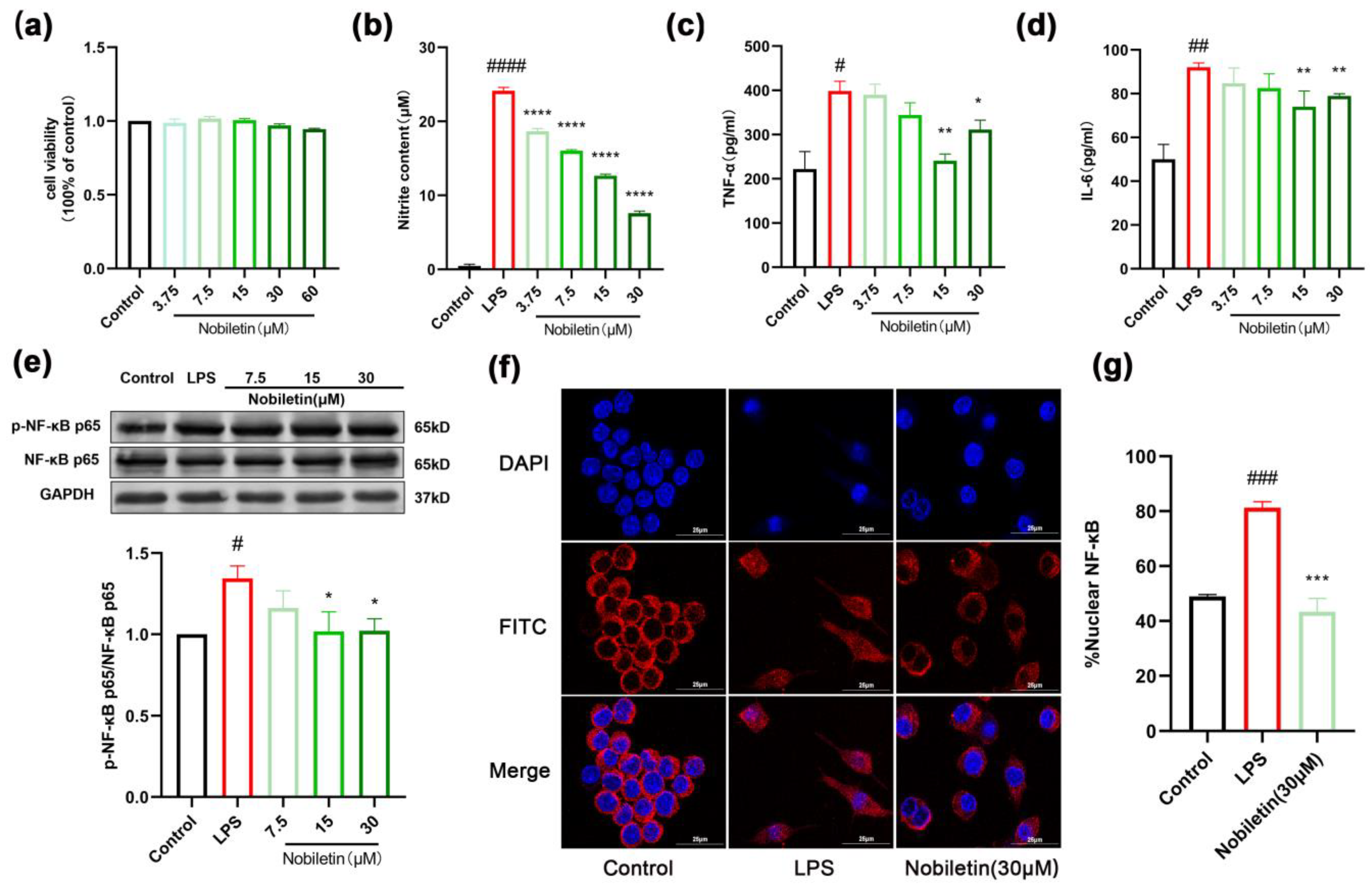
2.8. Nobiletin Decreased the Level of Inflammation in RAW264.7 Cells
2.9. Effects of Nobiletin on the cAMP-PKA-CREB Signaling Pathway in RAW264.7 Cells
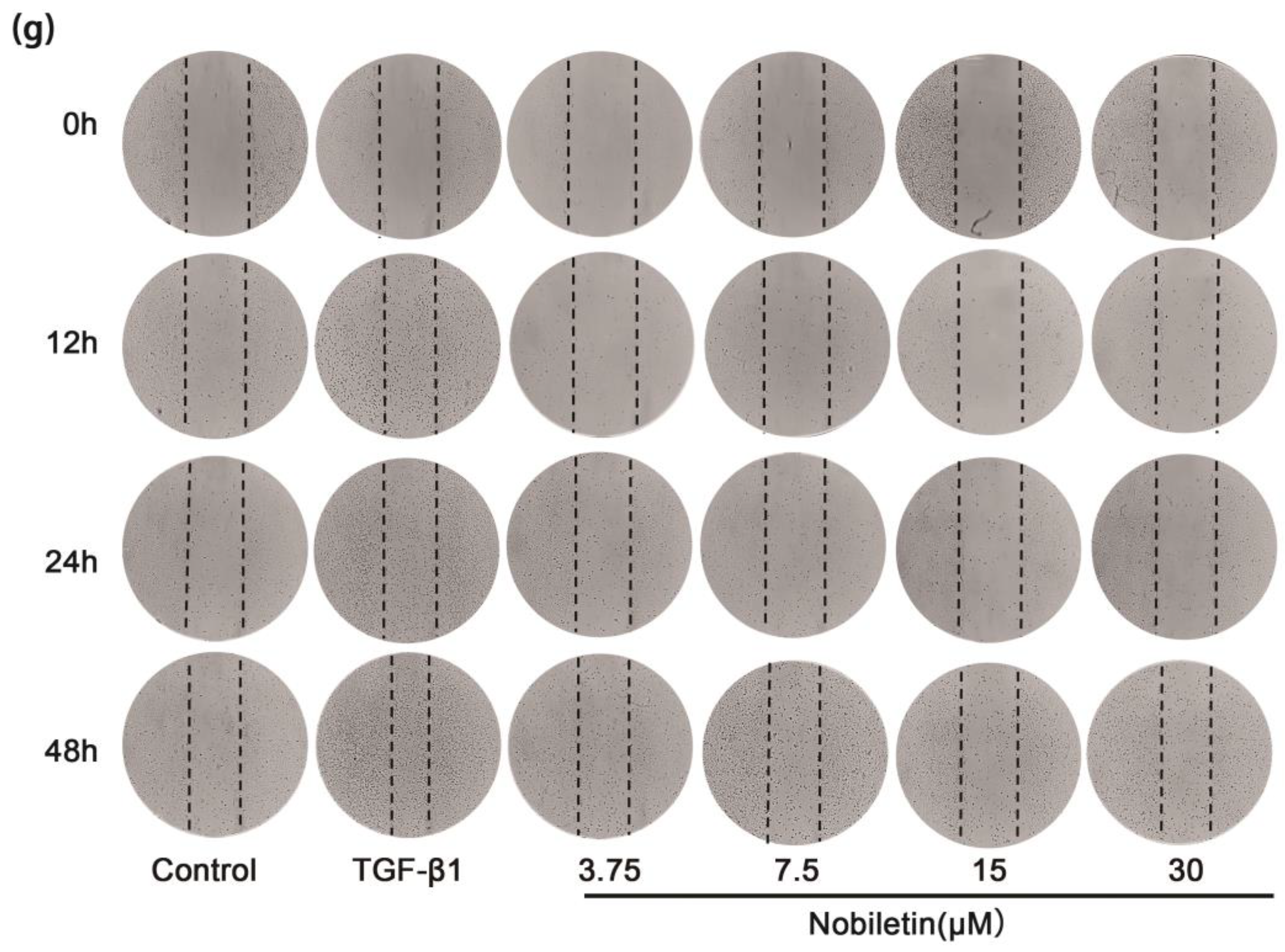
2.10. Nobiletin Decreased the Proliferation of ASM Cells Induced by TGF-β1
2.11. Effects of Nobiletin on the cAMP-PKA-CREB Signaling Pathway in ASM Cells Induced by TGF-β1
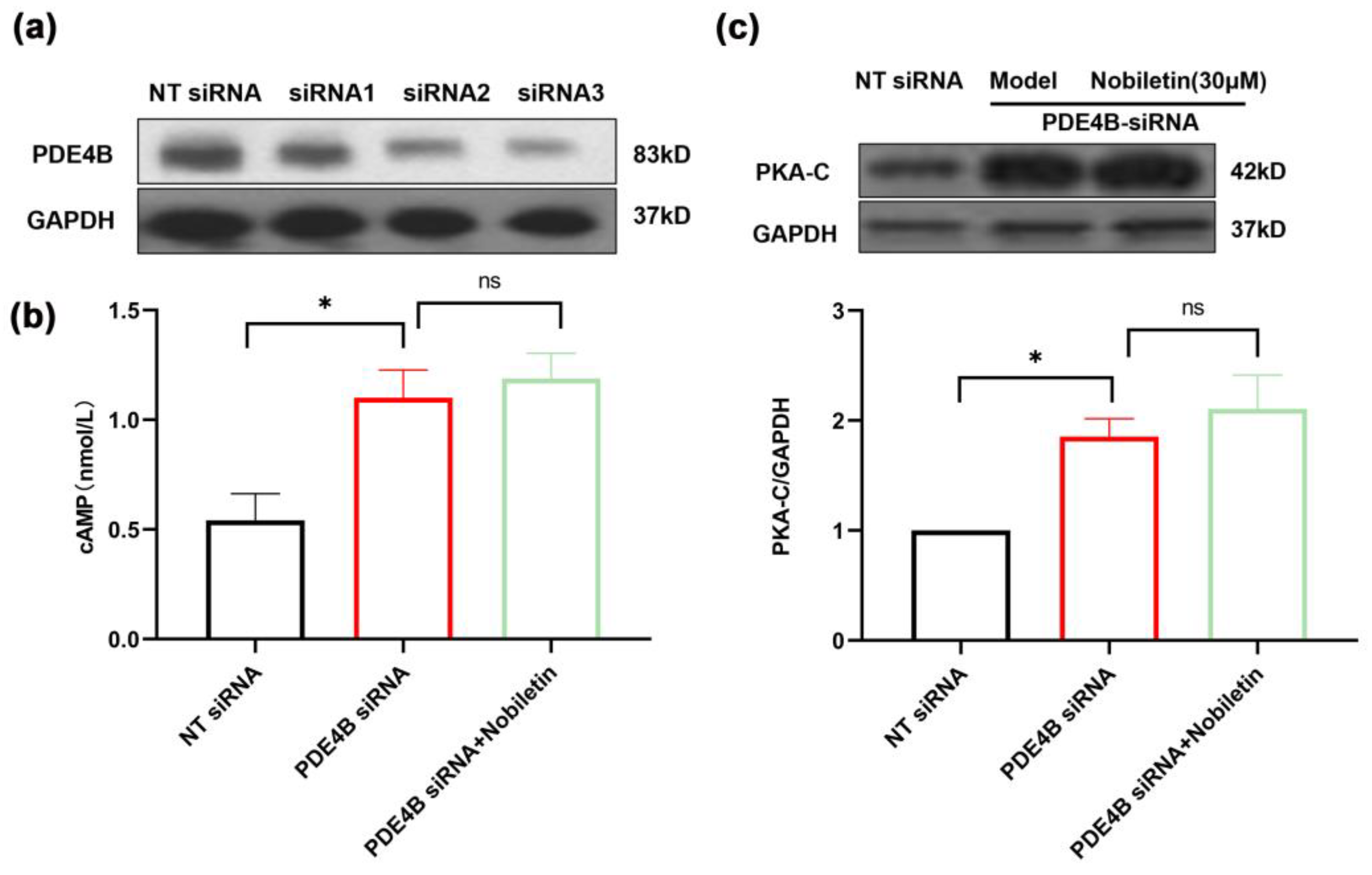
2.12. Nobiletin Played a Role in Increasing cAMP Levels by Depending on PDE4B
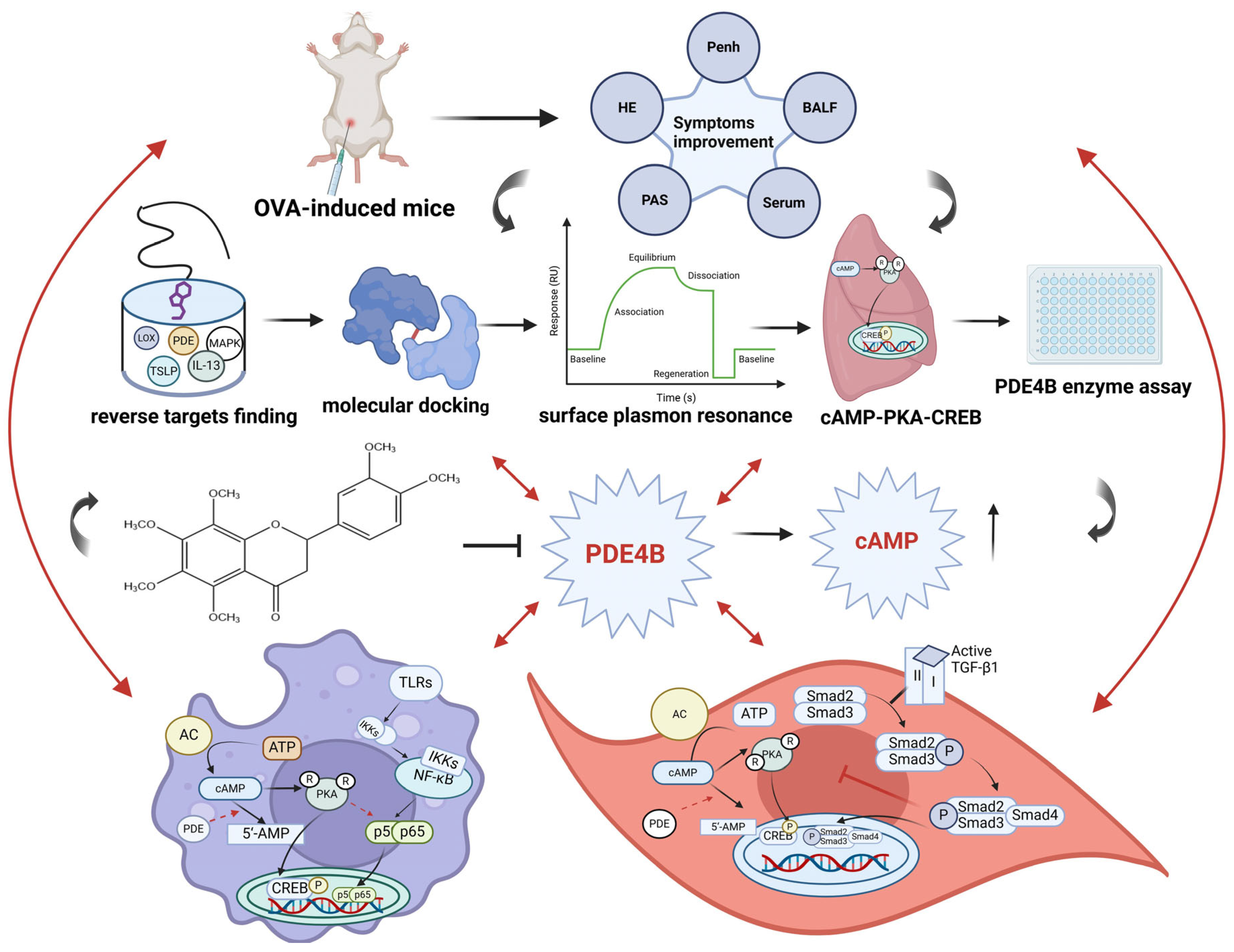
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animals
4.3. Animal Experimental Protocol
4.4. BALF Collection and Leukocyte Counts
4.5. Determination of IL-5, IL-13, IL-6, and OVA-IgE Levels
4.6. Detection of Airway Hyperresponsiveness
4.7. Histopathologic Evaluation of the Lung Tissue
4.8. Ligand Profiling and Molecular Docking
4.9. MD Simulation
4.10. SPR
4.11. PDE4B Inhibition Assay
4.12. Immunohistochemical Staining
4.13. Cell Culture and Treatments
4.14. MTT Assay
4.15. Measurement of NO, TNF-α, and IL-6
4.16. Immunofluorescence Analysis
4.17. Cell Proliferation Assay
4.18. Cell Migration Assay
4.19. Measurement of cAMP Levels
4.20. siRNA Transfection
4.21. Western Blotting Analysis
4.22. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTT | 3-(4:5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| FBS | fetal bovine serum |
| BALF | bronchoalveolar lavage fluid |
| OVA | ovalbumin |
| LPS | lipopolysaccharide |
| TGF-β1 | transforming growth factor β1 |
| SPR | surface plasmonic resonance |
| ASM | airway smooth muscle |
| PDE4B | phosphodiesterase 4B |
| MD | molecular dynamics. |
| Penh | enhanced pause |
| cAMP | 3′,5′-cyclic adenosine monophosphate |
| PKA | protein kinase A |
| CREB | cyclic-AMP response binding protein |
References
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Meghdadpour, S.; Lugogo, N.L. Medication Regimens for Managing Stable Asthma. Respir. Care 2018, 63, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Liu, Q.; Yu, C.; Chen, X.; Zhang, W.; Chen, Y.; Wang, T.; Luo, Y.; Jiang, G.; Zhu, M.; et al. The Protective Effects of Maresin 1 in the OVA-Induced Asthma Mouse Model. Mediat. Inflamm. 2021, 2021, 4131420. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, M. Th2 cells as targets for therapeutic intervention in allergic bronchial asthma. Expert. Rev. Mol. Diagn. 2009, 9, 85–100. [Google Scholar] [CrossRef] [PubMed]
- León, B.; Ballesteros-Tato, A. Modulating Th2 Cell Immunity for the Treatment of Asthma. Front. Immunol. 2021, 12, 637948. [Google Scholar] [CrossRef]
- Liang, H.E.; Reinhardt, R.L.; Bando, J.K.; Sullivan, B.M.; Ho, I.C.; Locksley, R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2011, 13, 58–66. [Google Scholar] [CrossRef]
- Wang, C.; Choi, Y.H.; Xian, Z.; Zheng, M.; Piao, H.; Yan, G. Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int. Immunopharmacol. 2018, 65, 571–579. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- Vilela, D.A.D.; Silva, B.A.O.; Brito, M.C.; Menezes, P.M.N.; Bomfim, H.F.; Duarte-Filho, L.; Silva, T.; Ribeiro, L.A.A.; Lucchese, A.M.; Silva, F.S. Lippia alnifolia essential oil induces relaxation on Guinea-pig trachea by multiple pathways. J. Ethnopharmacol. 2020, 246, 112162. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Feilhauer, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Citrus peel polymethoxyflavones nobiletin and tangeretin suppress LPS- and IgE-mediated activation of human intestinal mast cells. Eur. J. Nutr. 2017, 56, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.M. Anti-IL-4/-13 based therapy in asthma. Expert. Opin. Emerg. Drugs 2015, 20, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ma, X.; Xun, Y.; Pan, L. Protective effect of forsythiaside A on OVA-induced asthma in mice. Eur. J. Pharmacol. 2017, 812, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A. The TGF-beta1 paradox in asthma. Trends Immunol. 2001, 22, 299–300. [Google Scholar] [CrossRef]
- Ojiaku, C.A.; Chung, E.; Parikh, V.; Williams, J.K.; Schwab, A.; Fuentes, A.L.; Corpuz, M.L.; Lui, V.; Paek, S.; Bexiga, N.M.; et al. Transforming Growth Factor-β1 Decreases β(2)-Agonist-induced Relaxation in Human Airway Smooth Muscle. Am. J. Respir. Cell Mol. Biol. 2019, 61, 209–218. [Google Scholar] [CrossRef]
- Solomon, Y.; Malkamu, B.; Berhan, A.; Eyayu, T.; Almaw, A.; Legese, B.; Woldu, B. Peripheral blood eosinophilia in adult asthmatic patients and its association with the severity of asthma. BMC Pulm. Med. 2023, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, R.S.; Schatz, M.; Dalal, A.A.; Chen, W.; Sadikova, E.; Suruki, R.Y.; Kawatkar, A.A.; Qian, L. Blood Eosinophil Count and Outcomes in Severe Uncontrolled Asthma: A Prospective Study. J. Allergy Clin. Immunol. Pr. 2017, 5, 144–153.e8. [Google Scholar] [CrossRef]
- Al-Sajee, D.; Yin, X.; Gauvreau, G.M. An evaluation of roflumilast and PDE4 inhibitors with a focus on the treatment of asthma. Expert. Opin. Pharmacother. 2019, 20, 609–620. [Google Scholar] [CrossRef]
- Kung, T.T.; Crawley, Y.; Luo, B.; Young, S.; Kreutner, W.; Chapman, R.W. Inhibition of pulmonary eosinophilia and airway hyperresponsiveness in allergic mice by rolipram: Involvement of endogenously released corticosterone and catecholamines. Br. J. Pharmacol. 2000, 130, 457–463. [Google Scholar] [CrossRef]
- Han, Y.; Hou, R.; Zhang, X.; Liu, H.; Gao, Y.; Li, X.; Qi, R.; Cai, R.; Qi, Y. Amlexanox exerts anti-inflammatory actions by targeting phosphodiesterase 4B in lipopolysaccharide-activated macrophages. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118766. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Pszczoła, K.; Chłoń-Rzepa, G.; Jankowska, A.; Ellen, E.; Świerczek, A.; Pociecha, K.; Koczurkiewicz, P.; Piska, K.; Gawędzka, A.; Wyska, E.; et al. Novel phosphodiesterases inhibitors from the group of purine-2,6-dione derivatives as potent modulators of airway smooth muscle cell remodelling. Eur. J. Pharmacol. 2019, 865, 172779. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Huang, G.; Wang, Y.M.; Cheng, M.; Zhu, F.F.; Zhong, J.N.; Gao, Y.D. Exchange protein directly activated by cAMP (Epac) protects against airway inflammation and airway remodeling in asthmatic mice. Respir. Res. 2019, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. cAMP regulation of airway smooth muscle function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Al-Nema, M.; Gaurav, A.; Lee, V.S. Docking based screening and molecular dynamics simulations to identify potential selective PDE4B inhibitor. Heliyon 2020, 6, e04856. [Google Scholar] [CrossRef]
- Yuasa, K.; Kanoh, Y.; Okumura, K.; Omori, K. Genomic organization of the human phosphodiesterase PDE11A gene. Evolutionary relatedness with other PDEs containing GAF domains. Eur. J. Biochem. 2001, 268, 168–178. [Google Scholar] [CrossRef]
- Gaurav, A.; Gautam, V. Pharmacophore Based Virtual Screening Approach to Identify Selective PDE4B Inhibitors. Iran. J. Pharm. Res. 2017, 16, 910–923. [Google Scholar]
- Padda, I.S.; Bhatt, R.; Parmar, M. Apremilast. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Xu, T.; Ge, X.; Lu, C.; Dai, W.; Chen, H.; Xiao, Z.; Wu, L.; Liang, G.; Ying, S.; Zhang, Y.; et al. Baicalein attenuates OVA-induced allergic airway inflammation through the inhibition of the NF-κB signaling pathway. Aging 2019, 11, 9310–9327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.G.; Diao, B.Z.; Zhou, W.; Feng, J.L. Oroxylin A Inhibits Allergic Airway Inflammation in Ovalbumin (OVA)-Induced Asthma Murine Model. Inflammation 2016, 39, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Inam, A.; Shahzad, M.; Shabbir, A.; Shahid, H.; Shahid, K.; Javeed, A. Carica papaya ameliorates allergic asthma via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-ĸB, and iNOS levels. Phytomedicine 2017, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Li, W.; Wu, B.; Chen, W.M.; Chen, L.H.; Mo, G.W.; Zhang, Q.F.; Gong, L.; Li, J.; Zhang, H.C.; et al. Pheretima aspergillum decoction suppresses inflammation and relieves asthma in a mouse model of bronchial asthma by NF-κB inhibition. J. Ethnopharmacol. 2016, 189, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; He, X.; Hu, J.; Cai, W. Emodin inhibits NF-κB signaling pathway to protect obese asthmatic rats from pathological damage via Visfatin. Tissue Cell 2022, 74, 101713. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Falcão, M.A.P.; Pinto, F.J.; Bernardo, J.T.G.; Lopes-Ferreira, M. The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment. Cells 2023, 12, 924. [Google Scholar] [CrossRef]
- Chan, C.K.; Kuo, M.L.; Shen, J.J.; See, L.C.; Chang, H.H.; Huang, J.L. Ding Chuan Tang, a Chinese herb decoction, could improve airway hyper-responsiveness in stabilized asthmatic children: A randomized, double-blind clinical trial. Pediatr. Allergy Immunol. 2006, 17, 316–322. [Google Scholar] [CrossRef]
- Li, X.M. Treatment of asthma and food allergy with herbal interventions from traditional chinese medicine. Mt. Sinai J. Med. 2011, 78, 697–716. [Google Scholar] [CrossRef]
- Huang, T.P.; Liu, P.H.; Lien, A.S.; Yang, S.L.; Chang, H.H.; Yen, H.R. Characteristics of traditional Chinese medicine use in children with asthma: A nationwide population-based study. Allergy 2013, 68, 1610–1613. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhang, Y.; Yuan, H.Y.; Liang, J. The treatment of asthma using the Chinese Materia Medica. J. Ethnopharmacol. 2021, 269, 113558. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liang, J.J.; Wang, D.L.; Chen, J.B.; Cao, J.P.; Wang, Y.; Sun, C.D. Nobiletin as a chemopreventive natural product against cancer, a comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 6309–6329. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Alternat. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yang, J.; Li, H.; Li, J. Recent advances in the therapeutic potential of nobiletin against respiratory diseases. Phytomedicine 2024, 128, 155506. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yuan, Q.; Zhang, S.; He, C.; Wei, X.; Liu, M.; Jiang, N.; Huang, L.; Zhuang, L.; Wang, P. Biomimetic in vitro respiratory system using smooth muscle cells on ECIS chips for anti-asthma TCMs screening. Anal. Chim. Acta 2021, 1162, 338452. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Zhou, C.H.; Tao, J.; Li, S.N. Antagonistic effects of nobiletin, a polymethoxyflavonoid, on eosinophilic airway inflammation of asthmatic rats and relevant mechanisms. Life Sci. 2006, 78, 2689–2696. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Ouchi, K.; Okuyama, S.; Furukawa, Y.; Yoshida, T. Characterization of constituents in the peel of Citrus kawachiensis (Kawachibankan). Biosci. Biotechnol. Biochem. 2013, 77, 1977–1980. [Google Scholar] [CrossRef]
- Bai, D.; Sun, T.; Lu, F.; Shen, Y.; Zhang, Y.; Zhang, B.; Yu, G.; Li, H.; Hao, J. Eupatilin Suppresses OVA-Induced Asthma by Inhibiting NF-κB and MAPK and Activating Nrf2 Signaling Pathways in Mice. Int. J. Mol. Sci. 2022, 23, 1582. [Google Scholar] [CrossRef]
- Ho, S.C.; Kuo, C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef]
- Guo, S.; Wu, X.; Zheng, J.; Song, M.; Dong, P.; Xiao, H. Anti-Inflammatory Property of 5-Demethylnobiletin (5-Hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone) and Its Metabolites in Lipopolysaccharide (LPS)-Induced RAW 264.7 Cells. Biology 2022, 11, 1820. [Google Scholar] [CrossRef]
- Chu, X.; Ci, X.; He, J.; Wei, M.; Yang, X.; Cao, Q.; Li, H.; Guan, S.; Deng, Y.; Pang, D.; et al. A novel anti-inflammatory role for ginkgolide B in asthma via inhibition of the ERK/MAPK signaling pathway. Molecules 2011, 16, 7634–7648. [Google Scholar] [CrossRef] [PubMed]
- Farahi, N.; Paige, E.; Balla, J.; Prudence, E.; Ferreira, R.C.; Southwood, M.; Appleby, S.L.; Bakke, P.; Gulsvik, A.; Litonjua, A.A.; et al. Neutrophil-mediated IL-6 receptor trans-signaling and the risk of chronic obstructive pulmonary disease and asthma. Hum. Mol. Genet. 2017, 26, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Namakanova, O.A.; Gorshkova, E.A.; Zvartsev, R.V.; Nedospasov, S.A.; Drutskaya, M.S.; Gubernatorova, E.O. Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma. Int. J. Mol. Sci. 2022, 23, 3521. [Google Scholar] [CrossRef]
- Murphy, D.M.; O’Byrne, P.M. Recent advances in the pathophysiology of asthma. Chest 2010, 137, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qin, X.; Fu, T.; Li, C.; Wang, D.; Hu, Y.; Li, Y.; Chen, Y.; Cui, Y.; Wang, J.; et al. Attenuated airways inflammation and remodeling in IL-37a and IL-37b transgenic mice with an ovalbumin-induced chronic asthma. Cell Immunol. 2023, 391–392, 104759. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Cho, A.E. Using reverse docking to identify potential targets for ginsenosides. J. Ginseng Res. 2017, 41, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Cattani-Cavalieri, I.; Musheshe, N.; Nikolaev, V.O.; Schmidt, M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol. Ther. 2019, 197, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qiu, P.; Xu, G.; Wu, X.; Dong, P.; Yang, G.; Zheng, J.; McClements, D.J.; Xiao, H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J. Agric. Food Chem. 2012, 60, 2157–2164. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Z.; Wang, Y.; Jing, H.; Meng, Y.; Geng, Y.; Li, X.M.; Miao, M. Reduction of Th2 inflammation and fibrosis in eosinophilic esophagitis in a murine model by citri reticulatae pericarpium. J. Ethnopharmacol. 2023, 317, 116767. [Google Scholar] [CrossRef]
- Li, Y.; Ren, R.; Wang, L.; Peng, K. Eupatilin alleviates airway remodeling via regulating phenotype plasticity of airway smooth muscle cells. Biosci. Rep. 2020, 40, BSR20191445. [Google Scholar] [CrossRef]
- Morgan, S.J.; Deshpande, D.A.; Tiegs, B.C.; Misior, A.M.; Yan, H.; Hershfeld, A.V.; Rich, T.C.; Panettieri, R.A.; An, S.S.; Penn, R.B. β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J. Biol. Chem. 2014, 289, 23065–23074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Su, X.; Cao, A.; Chen, F.; Chen, P.; Yan, F.; Hu, H. SREBP2 promotes the viability, proliferation, and migration and inhibits apoptosis in TGF-β1-induced airway smooth muscle cells by regulating TLR2/NF-κB/NFATc1/ABCA1 regulatory network. Bioengineered 2022, 13, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Pszczoła, K.; Chłoń-Rzepa, G.; Jankowska, A.; Ślusarczyk, M.; Ferdek, P.E.; Kusiak, A.A.; Świerczek, A.; Pociecha, K.; Koczurkiewicz-Adamczyk, P.; Wyska, E.; et al. A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-β Signaling and Activation of cAMP/PKA Signaling. Int. J. Mol. Sci. 2020, 21, 4008. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Kolahian, S.; Shahbazfar, A.A.; Ansarin, K.; Pour Moghaddam, M.; Sakhinia, M.; Sakhinia, E.; Vafa, M. Effects of the flavanone combination hesperetin-naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother. Res. 2015, 29, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Ojiaku, C.A.; Cao, G.; Zhu, W.; Yoo, E.J.; Shumyatcher, M.; Himes, B.E.; An, S.S.; Panettieri, R.A., Jr. TGF-β1 Evokes Human Airway Smooth Muscle Cell Shortening and Hyperresponsiveness via Smad3. Am. J. Respir. Cell Mol. Biol. 2018, 58, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, M.; Huang, D.; Li, B.; Xu, R.; Gao, F.; Chen, Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials 2021, 271, 120761. [Google Scholar] [CrossRef]
- Meslamani, J.; Li, J.; Sutter, J.; Stevens, A.; Bertrand, H.O.; Rognan, D. Protein-ligand-based pharmacophores: Generation and utility assessment in computational ligand profiling. J. Chem. Inf. Model. 2012, 52, 943–955. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Schuster, D.; Danzl, B.; Schwaiger, S.; Markt, P.; Schmidtke, M.; Gertsch, J.; Raduner, S.; Wolber, G.; Langer, T.; et al. In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens. Planta Med. 2009, 75, 195–204. [Google Scholar] [CrossRef]
- Geladopoulos, T.P.; Sotiroudis, T.G.; Evangelopoulos, A.E. A malachite green colorimetric assay for protein phosphatase activity. Anal. Biochem. 1991, 192, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wu, W.H.; Gao, L.; Zheng, Z.Q.; Liu, D.; Mei, H.Y.; Zhang, Z.L.; Jing, Z.C. Oestradiol ameliorates monocrotaline pulmonary hypertension via NO, prostacyclin and endothelin-1 pathways. Eur. Respir. J. 2013, 41, 1116–1125. [Google Scholar] [CrossRef]
- Sun, T.; Li, H.; Zhang, Y.; Xiong, G.; Liang, Y.; Lu, F.; Zheng, R.; Zou, Q.; Hao, J. Inhibitory Effects of 3-Cyclopropylmethoxy-4-(difluoromethoxy) Benzoic Acid on TGF-β1-Induced Epithelial-Mesenchymal Transformation of In Vitro and Bleomycin-Induced Pulmonary Fibrosis In Vivo. Int. J. Mol. Sci. 2023, 24, 6172. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, Y.; Liang, H.; Liang, Y.; Xiong, G.; Lu, F.; Yang, K.; Zou, Q.; Zhang, X.; Du, G.; et al. Nobiletin, as a Novel PDE4B Inhibitor, Alleviates Asthma Symptoms by Activating the cAMP-PKA-CREB Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 10406. https://doi.org/10.3390/ijms251910406
Zhang Y, Yang Y, Liang H, Liang Y, Xiong G, Lu F, Yang K, Zou Q, Zhang X, Du G, et al. Nobiletin, as a Novel PDE4B Inhibitor, Alleviates Asthma Symptoms by Activating the cAMP-PKA-CREB Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(19):10406. https://doi.org/10.3390/ijms251910406
Chicago/Turabian StyleZhang, Yan, Yaping Yang, Huicong Liang, Yuerun Liang, Guixin Xiong, Fang Lu, Kan Yang, Qi Zou, Xiaomin Zhang, Guanhua Du, and et al. 2024. "Nobiletin, as a Novel PDE4B Inhibitor, Alleviates Asthma Symptoms by Activating the cAMP-PKA-CREB Signaling Pathway" International Journal of Molecular Sciences 25, no. 19: 10406. https://doi.org/10.3390/ijms251910406







