Influence of Primary Light Exposure on the Morphophysiological Characteristics and Phenolic Compounds Accumulation of a Tea Callus Culture (Camellia sinensis L.)
Abstract
:1. Introduction
2. Results
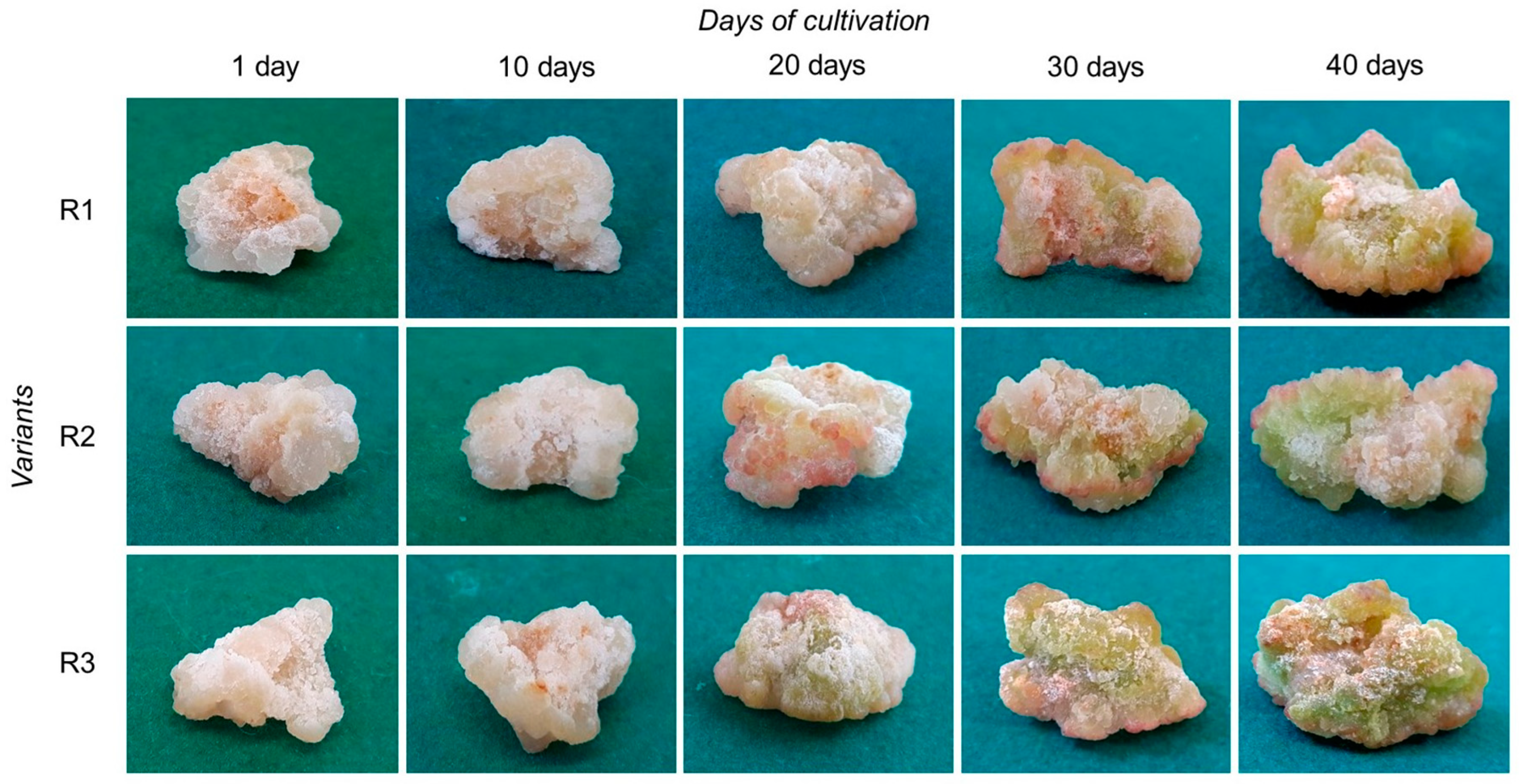
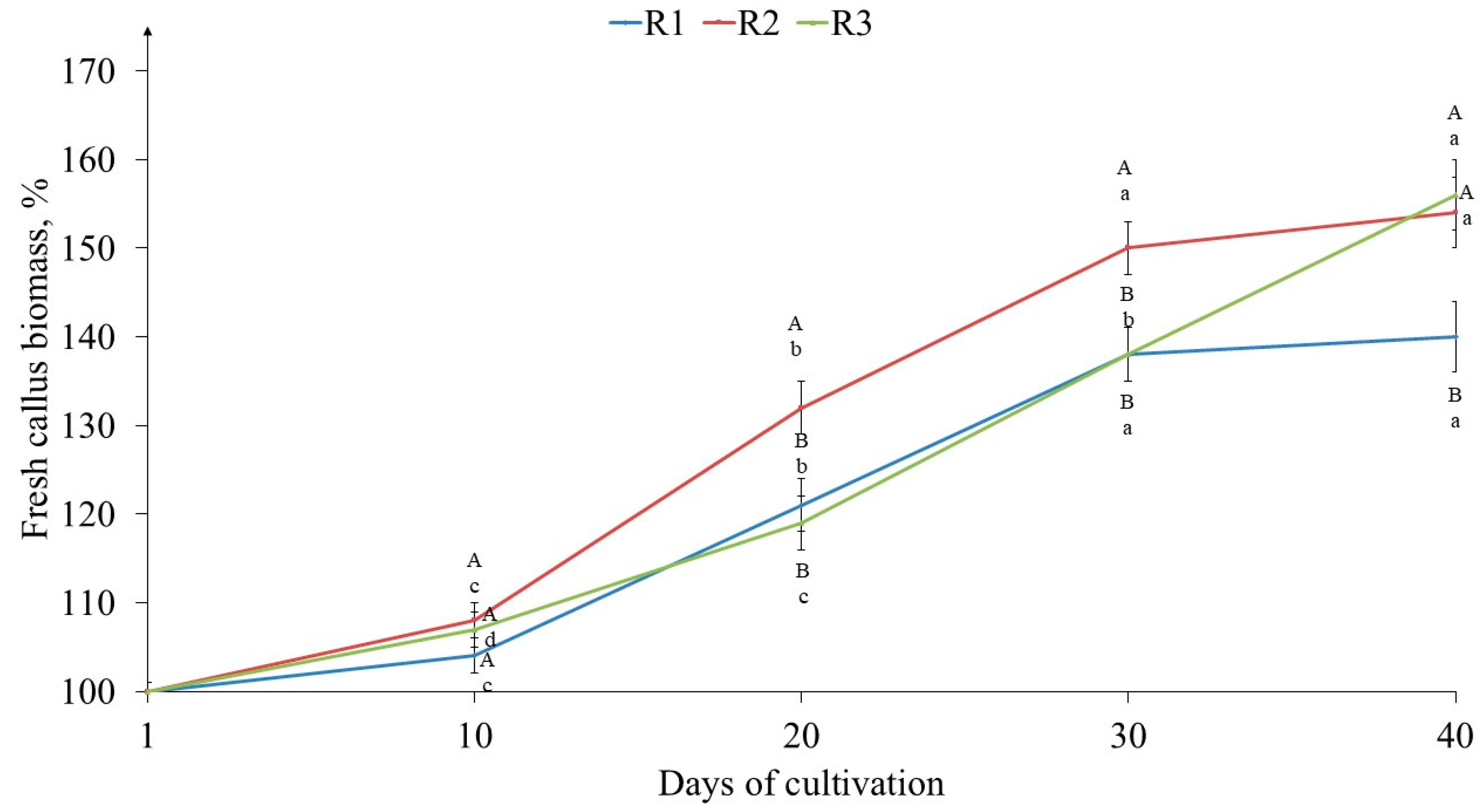
2.1. The Effects of Light on Tea Callus Culture Morphophysiological Parameters
2.2. The Effects of Light on Tea Callus Culture Lipid Peroxidation Level
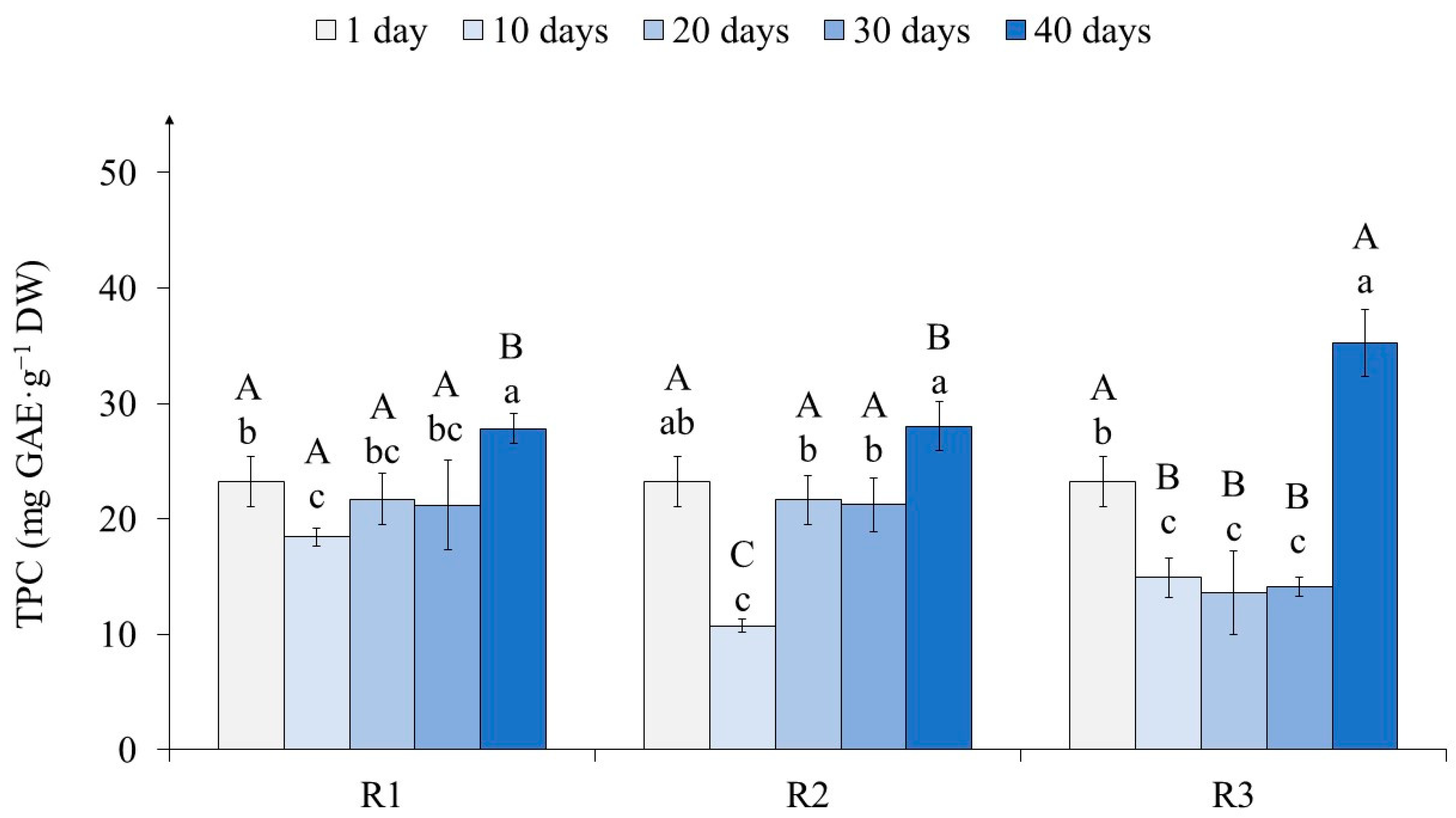
2.3. The Effects of Light on Tea Callus Culture Phenolic Content
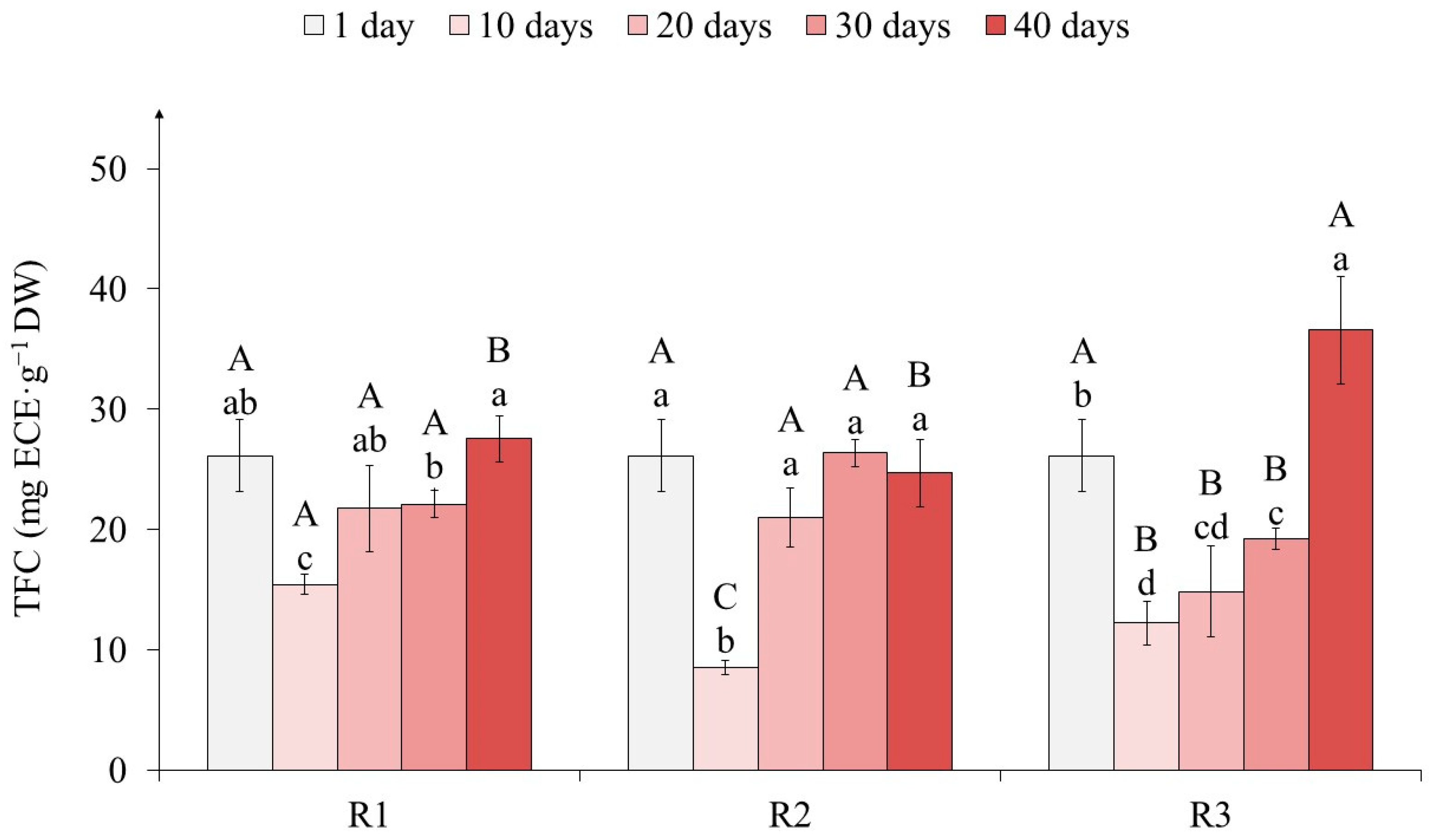
2.4. The Effects of Light on Tea Callus Culture Catechin Complex
3. Discussion
3.1. The Effects of Light on Tea Callus Culture Morphophysiological Parameters
3.2. The Effects of Light on Tea Callus Culture Peroxidation Level
3.3. The Effects of Light on Tea Callus Culture Phenolic Content
3.4. The Effects of Light on the Tea Callus Culture Catechin Complex
4. Materials and Methods
4.1. Plant Material and General Growth Conditions
4.2. Determination of Morphological Parameters of Tea Callus Cultures
4.3. Determination of Tea Callus Culture Growth
4.4. Determination of the Water Content in Tea Callus Cultures
4.5. Determination of the Chlorophyll a and b Fluorescence Intensity in Tea Callus Cultures
4.6. Determination of the Lipid Peroxidation Level in Tea Callus Cultures
4.7. Extraction of Phenolic Compounds from Tea Callus Cultures
4.8. Determination of the Total Phenolic Content in Tea Callus Cultures
4.9. Determination of the Phenylpropanoid Contents in Tea Callus Cultures
4.10. Determination of the Flavanol Contents in Tea Callus Cultures
4.11. Determination of Individual Phenolic Compounds Using the HPLC Method in Tea Callus Cultures
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, R.T. Distinct Responses to Light in Plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Kusnetsov, V.V.; Doroshenko, A.S.; Kudryakova, N.V.; Danilova, M.N. Role of phytohormones and light in de-etiolation. Rus. J. Plant. Physiol. 2020, 67, 971–984. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, T.; Liu, H. How plants coordinate their development in response to light and temperature signals. Plant Cell 2022, 34, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Siddiqui, H.; Upadhyaya, C.P.; Pichtel, J.; Hayat, S. Critical factors responsible for potato tuberization. Bot. Rev. 2023, 89, 421–437. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light perception: A matter of time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Allakhverdiev, S.I.; Kuznetsov, V.V. Signaling role of reactive oxygen species in plants under stress. Russ. J. Plant Physiol. 2012, 59, 141–154. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-H.; Lv, Y.-Q.; Liu, S.-R.; Jin, J.; Wang, Y.-F.; Wei, C.-L.; Zhao, S.-Q. Effects of light intensity and spectral composition on the transcriptome profiles of leaves in shade grown tea plants (Camellia sinensis L.) and regulatory network of flavonoid biosynthesis. Molecules 2021, 26, 5836. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.U.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation, and practical applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Feng, K.; Xie, M.; Barros, J.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.C. Phylogenetic occurrence of the phenylpropanoid pathway and lignin biosynthesis in plants. Front. Plant Sci. 2021, 12, 704697. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis regulation of folates and phenols in plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhang, M.H.; Su, H.Y.; Li, M.L.; Wang, Y.Y.; Jin, L.; Li, M.F. Integrated metabolomic and transcriptomic analysis reveals differential mechanism of flavonoid biosynthesis in two cultivars of Angelica sinensis. Molecules 2022, 27, 306. [Google Scholar] [CrossRef]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol. Biol. Rep. 2020, 47, 1459–1470. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodriguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and cancer: Potential for natural polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.; Fekri, A.; Kesar, L.A.; Othman, A.I. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother. Res. 2021, 35, 2879–2889. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant resources, chemical constituents, and bioactivities of tea plants from the genus Camellia Section Thea. J. Agric. Food Chem. 2018, 67, 5318–5349. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Wu, T.; Kerbler, S.M.; Fernie, A.R.; Zhang, Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Comm. 2021, 2, 100235. [Google Scholar] [CrossRef]
- Partap, M.; Chhimwal, J.; Kumar, P.; Kumar, D.; Padwad, Y.; Warghat, A.R. Growth dynamics and differential accumulation of picrosides and its precursor metabolites in callus cell lines of Picrorhiza kurroa with distinct anti-steatotic potential. Process Biochem. 2022, 120, 85–100. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern trends in the in vitro production and use of callus, suspension cells and root cultures of medicinal plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef]
- Samanta, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Lian, S.; Wu, N.; Zhou, Y.; Zhao, S.; Zhang, L.; Cheng, L.; Yuan, H. Integrated transcriptomics and metabolomics analysis of catechins, caffeine and theanine biosynthesis in tea plant (Camellia sinensis) over the course of seasons. BMC Plant Biol. 2020, 20, 294. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, T.; Li, W.; Jiang, Z.; Zhou, T.; Zhang, L.; Yu, Y. Widely targeted metabolomics using UPLCQTRAP-MS/MS reveals chemical changes during the processing of black tea from the cultivar Camellia sinensis (L.) O. Kuntze cv. Huangjinya. Food Res. Int. 2022, 162, 112169. [Google Scholar] [CrossRef]
- Wu, W.; Shi, J.; Jin, J.; Liu, Z.; Yuan, Y.; Chen, Z.; Zhang, S.; Dai, W.; Lin, Z. Comprehensive metabolic analyses provide new insights into primary and secondary metabolites in different tissues of Jianghua Kucha tea (Camellia sinensis var. assamica cv. Jianghua). Front. Nutr. 2023, 10, 1181135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.-T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis Teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Klepacka, J.; Tońska, E.; Rafałowski, R.; Czarnowska-Kujawska, M.; Opara, B. Tea as a source of biologically active compounds in the human diet. Molecules 2021, 26, 1487. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.Y.; Kim, Y.J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Y.; Zhang, S.; Wu, T. Induction of callus and establishment of cell suspension and effects on tea polyphenols in the cell suspension system of tea plants by PGRs. Sci. Hortic. 2023, 310, 111770. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Tian, B.; Huang, J.; Zhang, R.; Lin, Y.; Xiao, Z. Proliferation, accumulation of polyphenols, and antioxidant activities of callus from the ‘Anji Baicha’ cultivar of tea [Camellia sinensis (L.) O. Ktze.]. Hortic. Sci. Technol. 2017, 35, 252–264. [Google Scholar] [CrossRef]
- Movahedi, A.; Ahmadi Golsepidi, M.; Ghorbanli, M.; Alizadeh, M.; Ghasemi, K. The impact of Thiourea on Tea (Camellia sinensis) callus proliferation and secondary metabolites content. J. Plant Mol. Breed. 2019, 7, 37–44. [Google Scholar] [CrossRef]
- Aksenova, M.A.; Nechaeva, T.L.; Zubova, M.Y.; Goncharuk, E.A.; Kazantseva, V.V.; Katanskaya, V.M.; Lapshin, P.V.; Zagoskina, N.V. Influence of different precursors on content of polyphenols in Camellia sinensis in vitro callus culture. Plants 2023, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Usik, T.U.; Zaprometov, M.N. Effect of lighting duration on phenolic metabolism of photomixotrophic callus cultures of the tea plant. Russ. J. Plant Physiol. 1990, 37, 1089–1095. [Google Scholar]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In Vitro technology in plant conservation: Relevance to biocultural diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Sallahuddin, N.; Ahmed Shamsudin, N.H.; Mohd Zain, N.A.; Ibrahim, M.H.; Yaacob, J.S. Abiotic stress induces morphological, physiological, and genetic changes in Orthosiphon stamineus Benth. in vitro cultures. Horticulturae 2022, 8, 153. [Google Scholar] [CrossRef]
- Kruglova, N.; Zinatullina, A.; Yegorova, N. Histological approach to the study of morphogenesis in callus cultures in vitro: A Review. Int. J. Plant Biol. 2023, 14, 533–545. [Google Scholar] [CrossRef]
- Ossipov, V.; Zubova, M.; Nechaeva, T.; Zagoskina, N.; Salminen, J.P. The regulating effect of light on the content of flavan-3-ols and derivatives of hydroxybenzoic acids in the callus culture of the tea plant, Camellia sinensis L. Biochem. Syst. Ecol. 2022, 101, 104383. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Z.; Lee, J.; Li, X.; Sun, W. Proteomic analysis of tea plants (Camellia sinensis) with purple young shoots during leaf development. PLoS ONE 2017, 12, e0177816. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Kusnetsov, V.V. Endosymbiotic origin of chloroplasts in plant cells evolution. Rus. J. Plant Physiol. 2021, 68, 1–16. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sensors Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Mishra, V.K.; Bajpai, R.; Chaturvedi, R. Androgenic Haploid Plant Development via Embryogenesis with Simultaneous Determination of Bioactive Metabolites in Cambod Tea (Camellia assamica Ssp. Lasiocalyx). Plant Cell Tissue Organ Cult. 2022, 148, 515–531. [Google Scholar] [CrossRef]
- Biswal, B.; Jena, B.; Giri, A.K.; Acharya, L. Monochromatic light elicited biomass accumulation, antioxidant activity, and secondary metabolite production in callus culture of Operculina turpethum (L.). Plant Cell Tissue Organ Cult. 2022, 149, 123–134. [Google Scholar] [CrossRef]
- Siegień, I.; Adamczuk, A.; Wróblewska, K. Light affects in vitro organogenesis of Linum usitatissimum L. and its cyanogenic potential. Acta Physiol. Plant. 2012, 35, 781–789. [Google Scholar] [CrossRef]
- Phillips, G.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Suhartanto, B.; Astutik, M.; Umami, N.; Suseno, N.; Haq, M.S. The effect of explants and light conditions on callus induction of srikandi putih maize (Zea mays L.). IOP Conf. Ser. Earth Environ. Sci. 2022, 1001, 012006. [Google Scholar] [CrossRef]
- Batista, D.; Felipe, S.H.S.; Silva, T.D.; De Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.; Ríos, A.M.R.; Faria, D.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? Vitr. Cell. Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Usman, H.; Ullah, M.A.; Jan, H.; Siddiquah, A.; Drouet, S.; Anjum, S.; Giglioli-Guviarc’h, N.; Hano, C.; Abbasi, B.H. Interactive effects of wide-spectrum monochromatic lights on phytochemical production, antioxidant and biological activities of Solanum xanthocarpum callus cultures. Molecules 2020, 25, 2201. [Google Scholar] [CrossRef] [PubMed]
- Sawwan, J.S.; Al-Abdallat, A.M.; Al-Qudah, T.S.; Kushad, M.; Zucoloto, M. Hawthorn (Crataegus aronia L.) callus growth dynamics and polyphenol production under different light intensities. J. Food Agric. Environ. 2016, 14, 40–45. [Google Scholar]
- Yue, C.; Wang, Z.; Yang, P. Review: The effect of light on the key pigment compounds of photosensitive etiolated tea plant. Bot. Stud. 2021, 62, 21. [Google Scholar] [CrossRef]
- Lysenko, V.; Kirichenko, E.; Logvinov, A.; Azarov, A.; Rajput, V.D.; Chokheli, V.; Karaseva, T. Ultrastructure, CO2 Assimilation and Chlorophyll Fluorescence Kinetics in Photosynthesizing Glycine max Callus and Leaf Mesophyll Tissues. Horticulturae 2023, 9, 1211. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Ivanov, Y.; Ivanova, A.; Kreslavski, V.D.; Vereshchagin, M.; Tatarkina, P.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of light of different spectral compositions on growth parameters, photosynthetic pigment contents and gene expression in Scots pine plantlets. Int. J. Mol. Sci. 2023, 24, 2063. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light Acts as a Stressor and Influences Abiotic and Biotic Stress Responses in Plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A.; Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay. In Plant-Microbe Interactions; Springer: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Jeong, B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B Biol. 2019, 196, 111509. [Google Scholar] [CrossRef]
- Zaprometov, M.N. Metabolism of phenolic compounds in plants. Biochemistry 1977, 42, 3–20. [Google Scholar]
- Nazir, M.; Ullah, M.A.; Younas, M.; Siddiquah, A.; Shah, M.; Giglioli-Guivarc’H, N.; Hano, C.; Abbasi, B.H. Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Plant Cell Tissue Organ Cult. 2020, 142, 107–120. [Google Scholar] [CrossRef]
- Rahim, M.A.; Zhang, X.; Busatto, N. Editorial: Phenylpropanoid biosynthesis in plants. Front. Plant Sci. 2023, 14, 1230664. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Ineiguez-Franco, F.; Soto-Valdez, H.; Peralta, E.; Ayala-Zavala, J.F.; Auras, R.; Gamez-Meza, N. Antioxidant activity and diffusion of catechin and epicatechin from antioxidant active films made of poly(L-lactic acid). J. Agric. Food Chem. 2012, 60, 6515–6523. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.B.; Hahn, B.S.; Kim, K.H.; Ha, S.H.; Kim, J.B.; Kim, Y.H. EST analysis of genes involved in secondary metabolism in Camellia sinensis (tea), using suppression subtractive hybridization. Plant Sci. 2004, 166, 953–961. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Liu, L.; Yang, Q.; Lu, Z.; Nie, Z.; Wang, Y.; Xia, T. Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis). J. Biol. Chem. 2012, 287, 44406–44417. [Google Scholar] [CrossRef] [PubMed]
- Eeuwens, C.J. Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucifera) and cultured in vitro. Physiol. Plant. 1976, 36, 23–28. [Google Scholar] [CrossRef]
- Nikolaeva, T.N.; Zagoskina, N.V.; Zaprometov, M.N. Production of phenolic compounds in callus cultures of tea plant under the effect of 2, 4-D and NAA. Russ. J. Plant Physiol. 2009, 56, 45–49. [Google Scholar] [CrossRef]
- Schween, G.; Schwenkel, H.G. Effect of genotype on callus induction, shoot regeneration, and phenotypic stability of regenerated plants in the greenhouse of Primula ssp. Plant Cell Tissue Organ Cult. 2003, 72, 53–61. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Bucinski, A.; Luczkiewicz, M. Light and temperature conditions affect bioflavonoid accumulation in callus cultures of Cyclopia subternata Vogel (honeybush). Plant Cell Tissue Organ Cult. 2014, 118, 589–593. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Doroshenko, A.S.; Danilova, M.N.; Medvedeva, A.S.; Kusnetsov, V.V. Influence of blue-light signaling components on the regulation of cytokinin-dependent Arabidopsis thaliana seedlings’ greening. Russ. J. Plant Physiol. 2019, 66, 864–871. [Google Scholar] [CrossRef]
- Lukatkin, A.S. Contribution of oxidative stress to the development of cold-induced damage to leaves of chilling-sensitive plants: 1. Reactive oxygen species formation during plant chilling. Russ. J. Plant Physiol. 2002, 49, 622–627. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactivesubstances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Olenichenko, N.A.; Zagoskina, N.V.; Astakhova, N.V.; Trunova, T.I.; Kuznetsov, Y.V. Primary and secondary metabolism of winter wheat under cold hardening and treatment with antioxidants. Appl. Biochem. Microbiol. 2008, 44, 535. [Google Scholar] [CrossRef]
- Nikolaeva, T.N.; Lapshin, P.V.; Zagoskina, N.V. Method for determining the total content of phenolic compounds in plant extracts with Folin–Denis reagent and Folin–Ciocalteu reagent: Modification and comparison. Russ. J. Bioorg. Chem. 2022, 48, 1519–1525. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela Raverntos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Kurdyukov, Е.Е.; Vodop’yanova, O.A.; Mitishev, A.V.; Moiseev, Y.P.; Semenova, E.F. Method for quantifying the amount of phenylpropanoids in Stevia raw materials. Khim. Rastit. Syr’ya. 2020, 3, 115–121. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Howarth, R.E. Specificity of the vanillin test for flavanols. J. Agric. Food Chem. 1976, 24, 317–320. [Google Scholar] [CrossRef]
- Kazantseva, V.V.; Goncharuk, E.A.; Zaitsev, G.P.; Zagoskina, N.V.; Klykov, A.G. Plant ploidy level and the presence of cadmium in the growing environment changes the content of the main components of the phenolic complex in buckwheat sprouts. Dokl. Biochem. Biophys. 2022, 502, 10–14. [Google Scholar] [CrossRef]
- Shirokova, A.V.; Volovik, V.T.; Zagoskina, N.V.; Zaitsev, G.P.; Khudyakova, H.K.; Korovina, L.M.; Baranova, E.N. From dimness to glossiness—Characteristics of the spring rapeseed mutant form without glaucous Bloom (Brassica napus L.). Agronomy 2020, 10, 1563. [Google Scholar] [CrossRef]



| Days of Cultivation | Water Content, % | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| 1 | 91.74 ± 0.81 Aa | 91.74 ± 0.81 Aa | 91.74 ± 0.81 Aa |
| 10 | 89.77 ± 0.21 Ab | 89.08 ± 0.22 Bbc | 89.34 ± 0.21 Bb |
| 20 | 89.32 ± 0.18 Ac | 89.60 ± 0.37 Ab | 87.43 ± 0.19 Bc |
| 30 | 89.65 ± 0.30 Abc | 86.54 ± 2.45 Bc | 87.24 ± 1.65 Bc |
| 40 | 88.37 ± 0.18 Cd | 90.84 ± 0.74 Aa | 89.55 ± 0.10 Bb |
| Variants | Days of Cultivation | Chlorophyll FI, c.u. | ||
|---|---|---|---|---|
| a | b | a + b | ||
| R1 | 10 | 24.50 ± 4.71 Ad | 10.20 ± 1.57 Ad | 34.70 ± 6.28 Ad |
| 20 | 78.56 ± 5.81 Bc | 28.82 ± 2.44 Bc | 107.38 ± 8.25 Bc | |
| 30 | 333.86 ± 57.97 ABb | 107.22 ± 39.36 ABb | 441.08 ± 97.33 ABb | |
| 40 | 542.14 ± 22.16 Ba | 227.71 ± 9.87 Ba | 769.85 ± 32.04 Ba | |
| R2 | 10 | 11.97 ± 1.71 Bd | 5.86 ± 0.56 Bd | 17.83 ± 2.26 Bd |
| 20 | 47.07 ± 5.86 Cc | 18.98 ± 2.28 Bc | 66.05 ± 8.14 Cc | |
| 30 | 272.93 ± 15.42 Bb | 94.24 ± 5.29 Bb | 367.17 ± 20.71 Bb | |
| 40 | 572.45 ± 41.37 Ba | 210.63 ± 10.81 Ba | 783.08 ± 52.18 Ba | |
| R3 | 10 | 6.38 ± 1.07 Cd | 4.09 ± 0.58 Cc | 10.47 ± 1.66 Cd |
| 20 | 279.36 ± 19.71 Ac | 93.75 ± 7.27 Ab | 373.11 ± 26.98 Ac | |
| 30 | 339.48 ± 14.23 Ab | 111.77 ± 10.85 Ab | 451.25 ± 25.08 Ab | |
| 40 | 788.57 ± 77.90 Aa | 271.65 ± 27.87 Aa | 1060.22 ± 105.76 Aa | |
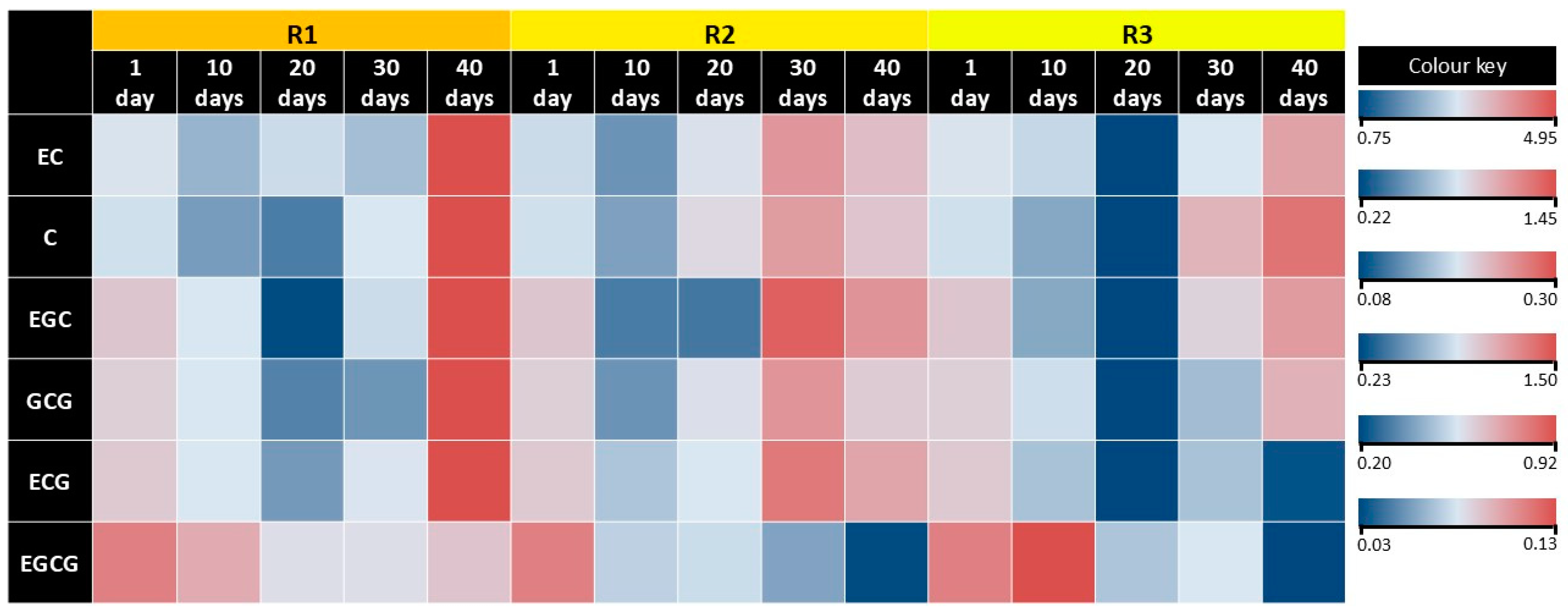
| Content of Catechins, mg·g−1 DW | |||||||
|---|---|---|---|---|---|---|---|
| Variants | Days of Cultivation | EGC | C | EC | EGCG | GCG | ECG |
| R1 | 1 | 0.17 ± 0.03 Ab | 0.59 ± 0.01 Ab | 1.93 ± 0.04 Ab | 0.09 ± 0.01 Aa | 0.82 ± 0.02 Ab | 0.51±0.03 Ab |
| 10 | 0.13 ± 0.01 Ab | 0.40 ± 0.01 Bd | 1.39 ± 0.01 Be | 0.09 ± 0.01 Ba | 0.66 ± 0.01 Ac | 0.35±0.01 Ac | |
| 20 | 0.08 ± 0.01 Ac | 0.33 ± 0.01 Be | 1.62 ± 0.01 Bc | 0.07 ± 0.01 Aa | 0.39 ± 0.06 Bd | 0.27±0.06 Ac | |
| 30 | 0.13 ± 0.01 Bb | 0.56 ± 0.01 Cc | 1.45 ± 0.01 Cd | 0.07 ± 0.01 Aa | 0.44 ± 0.05 Cd | 0.36±0.09 Bc | |
| 40 | 0.30 ± 0.01 Aa | 1.45 ± 0.01 Aa | 4.95 ± 0.01 Aa | 0.08 ± 0.01 Aa | 1.50 ± 0.01 Aa | 0.92±0.01 Aa | |
| R2 | 1 | 0.17 ± 0.03 Ac | 0.59 ± 0.01 Ad | 1.93 ± 0.04 Ac | 0.09 ± 0.01 Aa | 0.82 ± 0.02 Ab | 0.51±0.03 Ac |
| 10 | 0.10 ± 0.01 Bd | 0.41 ± 0.04 ABe | 1.20 ± 0.04 Cd | 0.06 ± 0.03 Bab | 0.43 ± 0.04 Bd | 0.31±0.01 Ae | |
| 20 | 0.10 ± 0.01 Ad | 0.66 ± 0.03 Ac | 1.85 ± 0.09 Ac | 0.06 ± 0.01 Ab | 0.71 ± 0.05 Ac | 0.34±0.01 Ad | |
| 30 | 0.28 ± 0.01 Aa | 1.01 ± 0.04 Aa | 3.45 ± 0.11 Aa | 0.05 ± 0.01 Ab | 1.13 ± 0.04 Aa | 0.77±0.02 Aa | |
| 40 | 0.23 ± 0.01 Bb | 0.77 ± 0.03 Cb | 2.61 ± 0.04 Cb | 0.03 ± 0.02 Ba | 0.82 ± 0.05 Cb | 0.60±0.01 Bb | |
| R3 | 1 | 0.17 ± 0.03 Ac | 0.59 ± 0.01 Ac | 1.93 ± 0.04 Ab | 0.09 ± 0.01 Ab | 0.82 ± 0.02 Ab | 0.51±0.03 Aa |
| 10 | 0.11 ± 0.01 ABc | 0.43 ± 0.01 Ad | 1.60 ± 0.01 Ac | 0.13 ± 0.01 Aa | 0.64 ± 0.01 Ac | 0.31±0.01 Ab | |
| 20 | 0.08 ± 0.01 Ad | 0.22 ± 0.01 Ce | 0.75 ± 0.01 Cd | 0.06 ± 0.01 Ac | 0.23 ± 0.02 Ce | 0.20±0.04 Bc | |
| 30 | 0.15 ± 0.02 Bb | 0.87 ± 0.03 Bb | 1.69 ± 0.08 Bc | 0.06 ± 0.01 Ac | 0.55 ± 0.03 Bd | 0.31±0.01 Bb | |
| 40 | 0.22 ± 0.01 Ba | 1.25 ± 0.01 Ba | 3.23 ± 0.01 Ba | 0.03 ± 0.01 Bd | 0.96 ± 0.01 Ba | 0.21±0.01 Cc | |
| Retention time, min | 13.23 | 13.65 | 15.33 | 15.54 | 15.67 | 17.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubova, M.Y.; Goncharuk, E.A.; Nechaeva, T.L.; Aksenova, M.A.; Zaitsev, G.P.; Katanskaya, V.M.; Kazantseva, V.V.; Zagoskina, N.V. Influence of Primary Light Exposure on the Morphophysiological Characteristics and Phenolic Compounds Accumulation of a Tea Callus Culture (Camellia sinensis L.). Int. J. Mol. Sci. 2024, 25, 10420. https://doi.org/10.3390/ijms251910420
Zubova MY, Goncharuk EA, Nechaeva TL, Aksenova MA, Zaitsev GP, Katanskaya VM, Kazantseva VV, Zagoskina NV. Influence of Primary Light Exposure on the Morphophysiological Characteristics and Phenolic Compounds Accumulation of a Tea Callus Culture (Camellia sinensis L.). International Journal of Molecular Sciences. 2024; 25(19):10420. https://doi.org/10.3390/ijms251910420
Chicago/Turabian StyleZubova, Maria Y., Evgenia A. Goncharuk, Tatiana L. Nechaeva, Maria A. Aksenova, Georgiy P. Zaitsev, Vera M. Katanskaya, Varvara V. Kazantseva, and Natalia V. Zagoskina. 2024. "Influence of Primary Light Exposure on the Morphophysiological Characteristics and Phenolic Compounds Accumulation of a Tea Callus Culture (Camellia sinensis L.)" International Journal of Molecular Sciences 25, no. 19: 10420. https://doi.org/10.3390/ijms251910420
APA StyleZubova, M. Y., Goncharuk, E. A., Nechaeva, T. L., Aksenova, M. A., Zaitsev, G. P., Katanskaya, V. M., Kazantseva, V. V., & Zagoskina, N. V. (2024). Influence of Primary Light Exposure on the Morphophysiological Characteristics and Phenolic Compounds Accumulation of a Tea Callus Culture (Camellia sinensis L.). International Journal of Molecular Sciences, 25(19), 10420. https://doi.org/10.3390/ijms251910420






