Gram-Negative Bacilli Blood Stream Infection in Patients with Severe Burns: Microbiological and Clinical Evidence from a 9-Year Cohort
Abstract
1. Introduction
2. Results
2.1. Demographics and Clinical Findings
2.2. Microbial Findings and Antimicrobial Treatment
2.3. Clinical Associations for BSI by MDR Strains and Mortality
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Data Collection
4.3. Strains Identification and Antimicrobial Susceptibility Profiles
4.4. Carbapenemase Detection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Burn Association. National Burn Repository (NBR). Available online: https://ameriburn.org/wp-content/uploads/2017/05/2016abanbr_final_42816.pdf (accessed on 18 June 2024).
- Pham, T.N.; Cancio, L.C.; Gibran, N.S.; American Burn Association. American Burn Association practice guidelines burn shock resuscitation. J. Burn Care Res. 2008, 29, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.M.; Peterson, J.M.; Wolf, S.E. Detection of Infection and Sepsis in Burns. Surg. Infect. 2021, 22, 20–27. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Sepsis in the burn patient: A different problem than sepsis in the general population. Burns Trauma 2017, 8, 23. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Voutsas, V.; Konoglou, M.; Karali, V.; Koukiasa, P.; Loridas, N.; Papaioannou, M.; Vasileiadou, G.; Bitzani, M. Determinants of Outcome in Burn ICU Patients with Septic Shock. J. Burn Care Res. 2017, 38, e172–e179. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Moins-Teisserenc, H.; Cordeiro, D.J.; Audigier, V.; Ressaire, Q.; Benyamina, M.; Lambert, J.; Maki, G.; Homyrda, L.; Toubert, A.; Legrand, M. Severe Altered Immune Status after Burn Injury Is Associated with Bacterial Infection and Septic Shock. Front. Immunol. 2021, 12, 586195. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; McManus, A. Nursing Committee of the International Society for Burn Injuries. Infection control in burn patients. Burns 2004, 30, A16–A24. [Google Scholar] [CrossRef]
- Erol, S.; Altoparlak, U.; Akcay, M.N.; Celebi, F.; Parlak, M. Changes of microbial flora and wound colonization in burned patients. Burns 2004, 30, 357–361. [Google Scholar] [CrossRef]
- Sousa, D.; Ceniceros, A.; Galeiras, R.; Pértega-Díaz, S.; Gutiérrez-Urbón, J.M.; Rodríguez-Mayo, M.; López-Suso, E.; Mourelo-Fariña, M.; Llinares, P. Microbiology in burns patients with blood stream infections: Trends over time and during the course of hospitalization. Infect. Dis. 2018, 50, 289–296. [Google Scholar] [CrossRef]
- Tang, C.Q.; Li, J.Q.; Shou, B.M.; Pan, B.H.; Chen, T.S.; Xiao, Y.Q.; Zheng, X.P.; Xiao, S.C.; Tan, Q.; Xia, Z.F. Epidemiology and outcomes of bloodstream infections in 177 severe burn patients from an industrial disaster: A multicentre retrospective study. Clin. Microbiol. Infect. 2018, 24, 199.e1–199.e7. [Google Scholar] [CrossRef]
- Ruegsegger, L.; Xiao, J.; Naziripour, A.; Kanumuambidi, T.; Brown, D.; Williams, F.; Marshall, S.H.; Rudin, S.D.; Yen, K.; Chu, T.; et al. Multidrug-Resistant Gram-Negative Bacteria in Burn Patients. Antimicrob. Agents Chemother. 2022, 66, e0068822. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Q.; Gong, Y.L.; Zhang, C.; Liu, M.X.; Shi, Y.L.; Peng, Y.Z.; Li, N. Analysis of distribution and drug resistance of pathogens isolated from 159 patients with catheter-related bloodstream infection in burn intensive care unit. Zhonghua Shao Shang Za Zhi 2020, 20, 24–31. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://stacks.cdc.gov/view/cdc/20705#:~:text=CDC%20estimates%20that%20in%20the,and%20are%20likely%20minimum%20estimates (accessed on 27 May 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 12, 629–655. [Google Scholar] [CrossRef] [PubMed]
- CDC. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection). Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 16 June 2024).
- Patel, B.M.; Paratz, J.D.; Mallet, A.; Lipman, J.; Rudd, M.; Muller, M.J.; Paterson, D.L.; Roberts, J.A. Characteristics of bloodstream infections in burn patients: An 11-year retrospective study. Burns 2012, 38, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Wu, P.F.; Chen, C.S.; Chen, I.H.; Huang, W.T.; Wang, F.D. Trends in microbial profile of burn patients following an event of dust explosion at a tertiary medical center. BMC Infect. Dis. 2020, 4, 193. [Google Scholar] [CrossRef]
- Öncül, O.; Öksüz, S.; Acar, A.; Ülkür, E.; Turhan, V.; Uygur, F.; Ulçay, A.; Erdem, H.; Özyurt, M.; Görenek, L. Nosocomial infection characteristics in a burn intensive care unit: Analysis of an eleven-year active surveillance. Burns 2014, 40, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Santucci, S.; Gobara, S.; Santos, C.; Fontana, C.; Levin, A. Infections in a burn intensive care unit: Experience of seven years. J. Hosp. Infect. 2003, 53, 6–13. [Google Scholar] [CrossRef]
- Escandón-Vargas, K.; Tangua, A.R.; Medina, P.; Zorrilla-Vaca, A.; Briceño, E.; Clavijo-Martínez, T.; Tróchez, J.P. Healthcare-associated infections in burn patients: Timeline and risk factors. Burns 2020, 46, 1775–1786. [Google Scholar] [CrossRef]
- Lee, H.G.; Jang, J.; Choi, J.E.; Chung, D.C.; Han, J.W.; Woo, H.; Jeon, W.; Chun, B.C. Blood stream infections in patients in the burn intensive care unit. Infect. Chemother. 2013, 45, 194–201. [Google Scholar] [CrossRef]
- Brusselaers, N.; Monstrey, S.; Snoeij, T.; Vandijck, D.; Lizy, C.; Hoste, E.; Lauwaert, S.; Colpaert, K.; Vandekerckhove, L.; Vogelaers, D.; et al. Morbidity and Mortality of Bloodstream Infections in Patients with Severe Burn Injury. Am. J. Crit. Care 2010, 19, e81–e87. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Raz-Pasteur, A.; Hussein, K.; Finkelstein, R.; Ullmann, Y.; Egozi, D. Blood stream infections (BSI) in severe burn patients—Early and late BSI: A 9-year study. Burns 2013, 39, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Ponce de León, S. Plan Universitario de Control de la Resistencia Antimicrobiana Estado Actual de La Resistencia Antimicrobiana En México. Available online: http://www.puis.unam.mx/slider_docs/plan-ucradigital.pdf (accessed on 15 May 2024).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Lopez-Jacome, L.E.; Fernandez-Rodriguez, D.; Franco-Cendejas, R.; Camacho-Ortiz, A.; Morfin-Otero, M.d.R.; Rodriguez-Noriega, E.; Ponce-De-Leon, A.; Ortiz-Brizuela, E.; Rojas-Larios, F.; Velazquez-Acosta, M.d.C.; et al. Increment Antimicrobial Resistance during the COVID-19 Pandemic: Results from the Invifar Network. Microb. Drug Resist. 2022, 28, 338–345. [Google Scholar] [CrossRef]
- Ramirez-Blanco, C.E.; Ramirez-Rivero, C.E.; Diaz-Martinez, L.A.; Sosa-Avila, M.L. Infection in burn patients in a referral center in Colombia. Burns 2017, 43, 642–653. [Google Scholar] [CrossRef]
- Zhang, D.; Micek, S.T.; Kollef, M.H. Time to appropriate antibiotic therapy is an independent determinant of postinfection ICU and hospital lengths of stay in patients with sepsis. Crit. Care Med. 2015, 43, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Garza-González, E.; Bocanegra-Ibarias, P.; Bobadilla-Del-Valle, M.; Ponce-De-León-Garduño, L.A.; Esteban-Kenel, V.; Silva-Sánchez, J.; Garza-Ramos, U.; Barrios-Camacho, H.; López-Jácome, L.E.; Colin-Castro, C.A.; et al. Drug resistance phenotypes and genotypes in Mexico in representative gram-negative species: Results from the infivar network. PLoS ONE 2021, 16, e0248614. [Google Scholar] [CrossRef]
- Vickers, M.L.; Dulhunty, J.M.; Ballard, E.; Chapman, P.; Muller, M.; Roberts, J.A.; Cotta, M.O. Risk factors for multidrug-resistant Gram-negative infection in burn patients. ANZ J. Surg. 2018, 88, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Mahar, P.; Padiglione, A.A.; Cleland, H.; Paul, E.; Hinrichs, M.; Wasiak, J. Pseudomonas aeruginosa bacteraemia in burns patients: Risk factors and outcomes. Burns 2010, 36, 1228–1233. [Google Scholar] [CrossRef]
- Fochtmann-Frana, A.; Freystätter, C.; Vorstandlechner, V.; Barth, A.; Bolliger, M.; Presterl, E.; Ihra, G.; Muschitz, G.; Mittlboeck, M.; Makristathis, A.; et al. Incidence of risk factors for bloodstream infections in patients with major burns receiving intensive care: A retrospective single-center cohort study. Burns 2018, 44, 784–792. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Approved Standard, M07-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
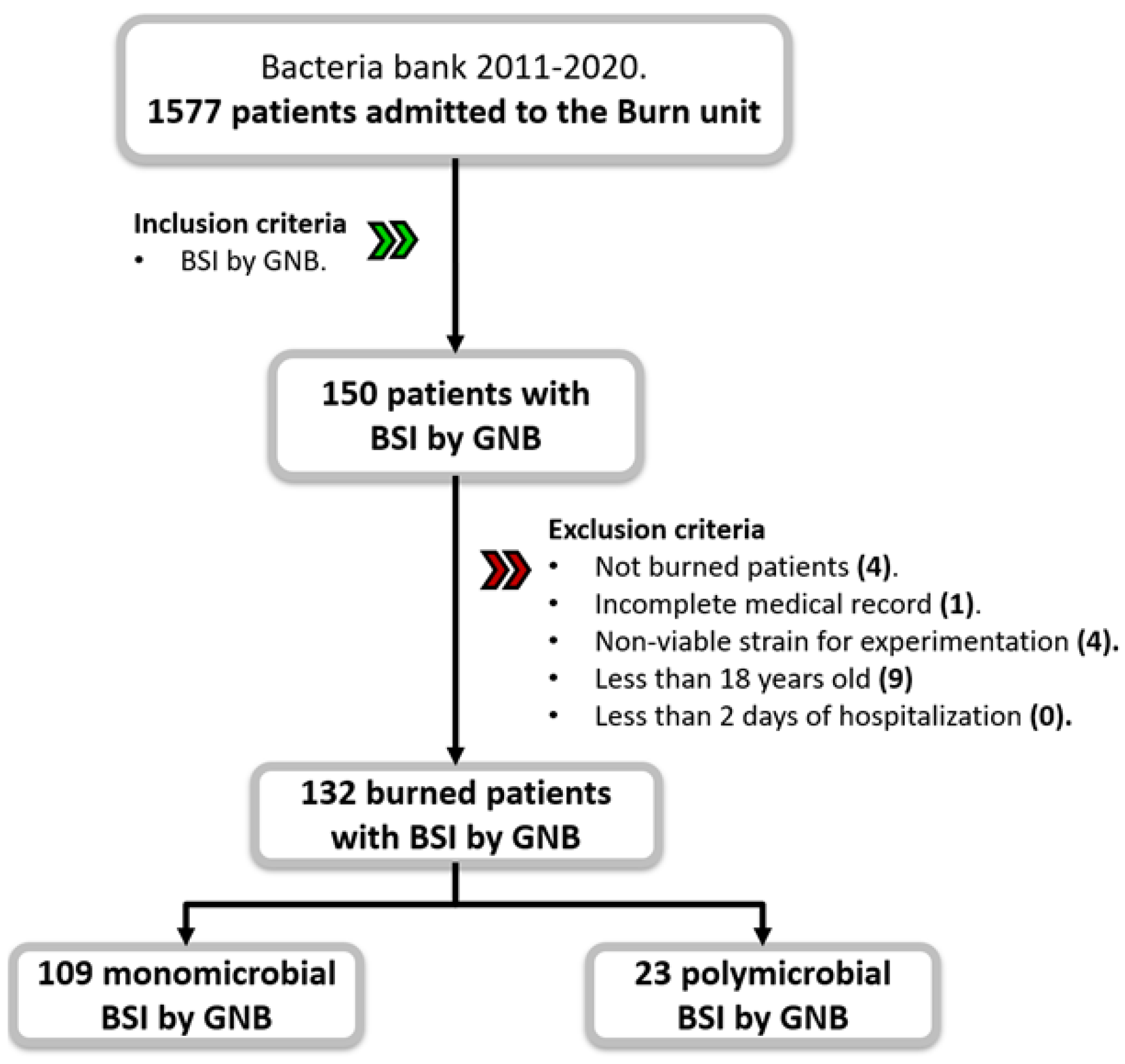
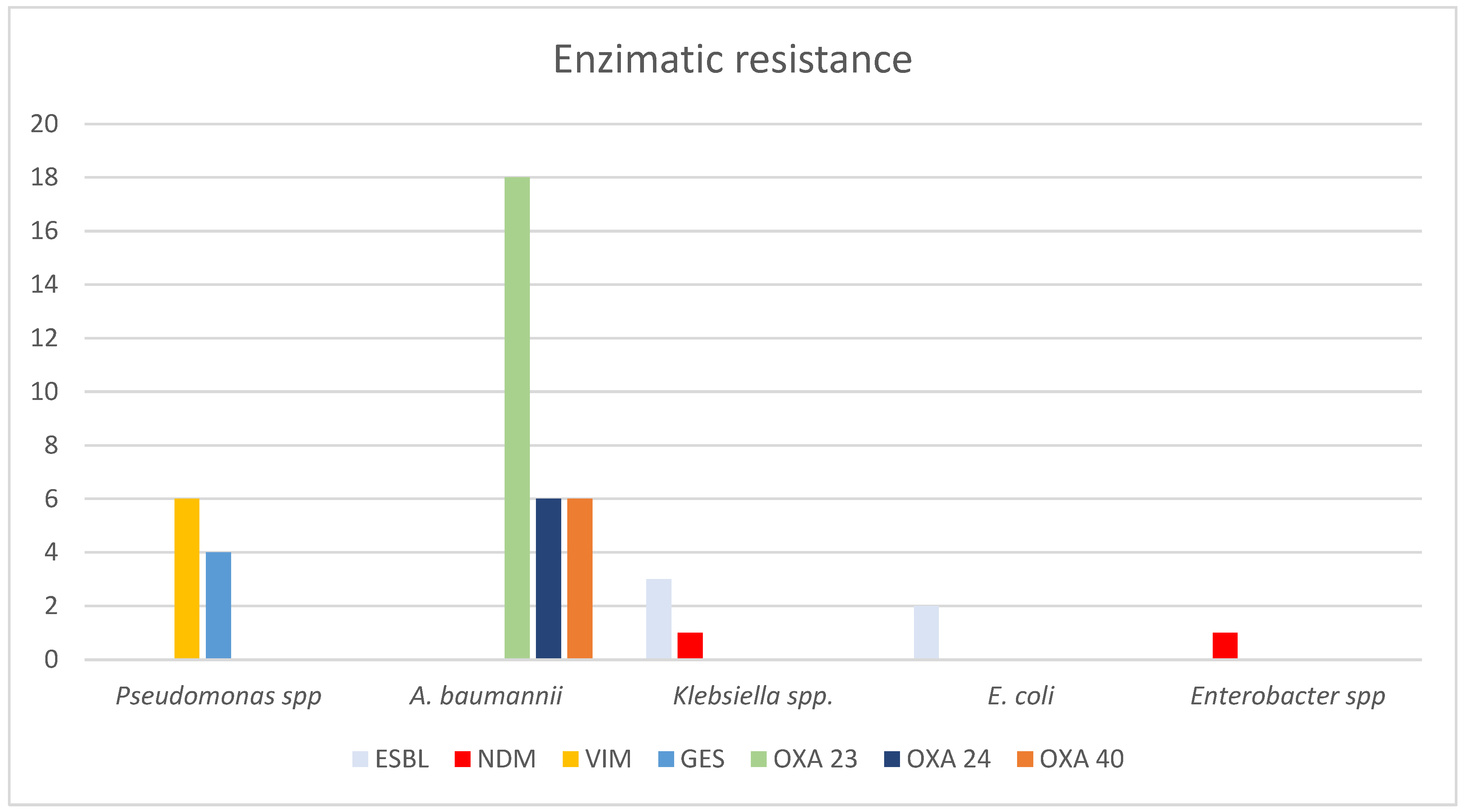
| Variable | Total |
|---|---|
| n = 132 (%) | |
| Sociodemographic data | |
| Sex, male | 93 (70) |
| Age, median (IQR) | 35.5 (25.5–49) |
| Referred patient | 123 (93) |
| Comorbidities | |
| Glucose > 200 mg/dL (on admission) | 11 (8.3) |
| Abnormal weight | 102 (77.3) |
| Low weight | 2 (1.5) |
| Overweight | 55 (41.7) |
| Obese | 45 (34.1) |
| Clinical presentation | |
| Type/mechanism of burn | |
| Fire | 99 (75.0) |
| Electricity | 25 (18.9) |
| Burned degree | |
| Second | 47 (35.6) |
| Third | 85 (64.4) |
| Inhalation injury | 37 (28.0) |
| Days to hospital admission, median (IQR) | 3 (1–8) |
| Days to isolation, median (IQR) | 10.5 (5–20.5) |
| LOS, median (IQR) | 41.5 (25.5–56.5) |
| TBSA, mean ± SD | 44.9 ± 20.7 |
| ABSI score < 8 points, ≥80% survival | 61 (46.2) |
| SOFA, mean ± SD | 5.5 ± 4.9 |
| PITT, mean ± SD | 4.4 ± 3.8 |
| Infections and outcome | |
| Monomicrobial BSI | 109 (82.6) |
| Primary BSI | 70 (53.0) |
| CLABSI | 56 (42.4) |
| CRBSI | 14 (10.6) |
| Secondary BSI | 62 (47.0) |
| Soft tissue | 55 (41.7) |
| Reinfection | 23 (17.4) |
| Relapse | 19 (14.4) |
| Death | 34 (25.8) |
| Candidemia | 12 (9.1) |
| C. difficile infection | 5 (3.8) |
| Invasive procedures | |
| Number of invasive procedures (types), median (IQR) | 3 (2–3) |
| Surgery | 129 (97.7) |
| Number of surgeries, median (IQR) | 5 (3–8) |
| CVC | 128 (97.0) |
| IMV | 92 (69.7) |
| RRT | 21 (15.9) |
| Antimicrobial | Pseudomonas spp. | A. baumannii | Klebsiella spp. | E. coli | Enterobacter spp. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Amikacin | 16 | 45.7 | 4 | 12 | 1 | 6.6 | 0 | 0 | 2 | 20 |
| Ceftazidime | 22 | 62.8 | 0 | 0 | 5 | 33.3 | 3 | 37.5 | 4 | 40 |
| Cefepime | 20 | 57.1 | 27 | 84.3 | 4 | 26.6 | 1 | 12.5 | 4 | 40 |
| Ciprofloxacin | 23 | 65.7 | 28 | 87.5 | 7 | 46.6 | 7 | 87.5 | 4 | 40 |
| Levofloxacin | 24 | 68.5 | 28 | 87.5 | 7 | 46.6 | 7 | 87.5 | 2 | 20 |
| Imipenem | 29 | 82.8 | 0 | 0 | 1 | 6.6 | 0 | 0 | 1 | 10 |
| Meropenem | 26 | 74.2 | 27 | 84.3 | 1 | 6.6 | 0 | 0 | 1 | 10 |
| Piperacillin/Tazobactam | 12 | 34.2 | 0 | 0 | 1 | 6.6 | 0 | 0 | 4 | 40 |
| Colistin | 1 | 2.8 | 0 | 0 | 2 | 13.3 | 0 | 0 | 0 | 0 |
| Tigecycline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total N (%) | Enterobacterales N (%) | Non-Fermenters N (%) | p | |
|---|---|---|---|---|
| Type of Antimicrobial Treatment | ||||
| In-care antimicrobial therapy | 102 (93.6) | 39 (97.5) | 63 (91.3) | >0.99 |
| Accurate empirical treatment (first 24 h) | 72 (66.1) | 25 (62.5) | 47/68.1) | 0.551 |
| Change/treatment upgrade | 32 (29.4) | 13 (32.5) | 19 (27.5) | 0.583 |
| Monotherapy | 20 (19.6) | 13 (33.3) | 7 (11.1) | 0.004 |
| Carbapenem | 16 (80) | 12 (92.3) | 4 (57.1) | 0.101 |
| Cephalosporin | 3 (15) | 1 (77) | 2 (28.6) | 0.27 |
| Quinolone | 1 (50) | 0 | 1 (14.3) | 0.35 |
| Combination | 87 (85.3) | 26 (66.7) | 61 (96.8) | 0.003 |
| Two antimicrobials | 61 (70.1) | 18 (69.2) | 43 (70.5) | 0.906 |
| Carbapenem and colistin | 49 (80.3) | 12 (66.7) | 37 (86) | 0.082 |
| Three antimicrobials | 15 (17.2) | 6 (23.1) | 9 (14.8) | 0.347 |
| Carbapenem, colistin, and rifampin | 5 (5.7) | 1 (3.8) | 4 (6.6) | 0.58 |
| Four antimicrobials | 9 (10.3) | 2 (7.7) | 7 (11.5) | 0.719 |
| Five antimicrobials | 2 (2.3) | 0 | 2 (3.3) | >0.99 |
| Variable | p | OR (95% CI) | p | aOR (95% CI) | p-Value |
|---|---|---|---|---|---|
| MDR isolation | |||||
| >3 days to burn unit admission | 0.01 | 2.87 (1.28–6.43) | 0.25 | 2.74 (1.13–6.63) | 0.046 |
| Secondary BSI | 0.001 | 3.9 (1.70–8.90) | 0.022 | 2.80 (1.15–6.77) | |
| CLABSI | 0.001 | 0.26 (0.11–0.56) | |||
| Soft tissue BSI | 0.004 | 3.44 (1.47–8.03) | |||
| Carbapenem and colistin | 0.012 | 4.88 (1.42–16.82) | |||
| Non-fermenter | <0.001 | 4.51 (2.03–9.98) | 0.001 | 4.13 (1.77–9.63) | |
| Mortality | |||||
| Male | 0.011 | 0.34 (0.15–0.78) | |||
| Fire (mechanism of burn) | 0.008 | 7.40 (1.66–32.86) | |||
| Third degree burn | 0.038 | 2.66 (1.05–6.70) | |||
| >10 days to bacterial isolation | 0.007 | 0.31 (0.13–0.72) | |||
| >42 days of hospital stay | <0.001 | 0.18 (0.07–0.46) | |||
| >1 BSI episode | 0.017 | 2.72 (1.19–6.23) | |||
| >3 invasive procedures (types) | 0.004 | 4.20 (1.59–11.12) | |||
| BSSA > 40% | 0.001 | 5.4 (2.04–12.93) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-González, M.F.; Fernández-Rodríguez, D.; Colín-Castro, C.A.; Hernández-Durán, M.; López-Jácome, L.E.; Franco-Cendejas, R. Gram-Negative Bacilli Blood Stream Infection in Patients with Severe Burns: Microbiological and Clinical Evidence from a 9-Year Cohort. Int. J. Mol. Sci. 2024, 25, 10458. https://doi.org/10.3390/ijms251910458
Fuentes-González MF, Fernández-Rodríguez D, Colín-Castro CA, Hernández-Durán M, López-Jácome LE, Franco-Cendejas R. Gram-Negative Bacilli Blood Stream Infection in Patients with Severe Burns: Microbiological and Clinical Evidence from a 9-Year Cohort. International Journal of Molecular Sciences. 2024; 25(19):10458. https://doi.org/10.3390/ijms251910458
Chicago/Turabian StyleFuentes-González, María Fernanda, Diana Fernández-Rodríguez, Claudia A. Colín-Castro, Melissa Hernández-Durán, Luis Esaú López-Jácome, and Rafael Franco-Cendejas. 2024. "Gram-Negative Bacilli Blood Stream Infection in Patients with Severe Burns: Microbiological and Clinical Evidence from a 9-Year Cohort" International Journal of Molecular Sciences 25, no. 19: 10458. https://doi.org/10.3390/ijms251910458
APA StyleFuentes-González, M. F., Fernández-Rodríguez, D., Colín-Castro, C. A., Hernández-Durán, M., López-Jácome, L. E., & Franco-Cendejas, R. (2024). Gram-Negative Bacilli Blood Stream Infection in Patients with Severe Burns: Microbiological and Clinical Evidence from a 9-Year Cohort. International Journal of Molecular Sciences, 25(19), 10458. https://doi.org/10.3390/ijms251910458






