S6K1 Controls DNA Damage Signaling Modulated by the MRN Complex to Induce Radioresistance in Lung Cancer
Abstract
1. Introduction
2. Results
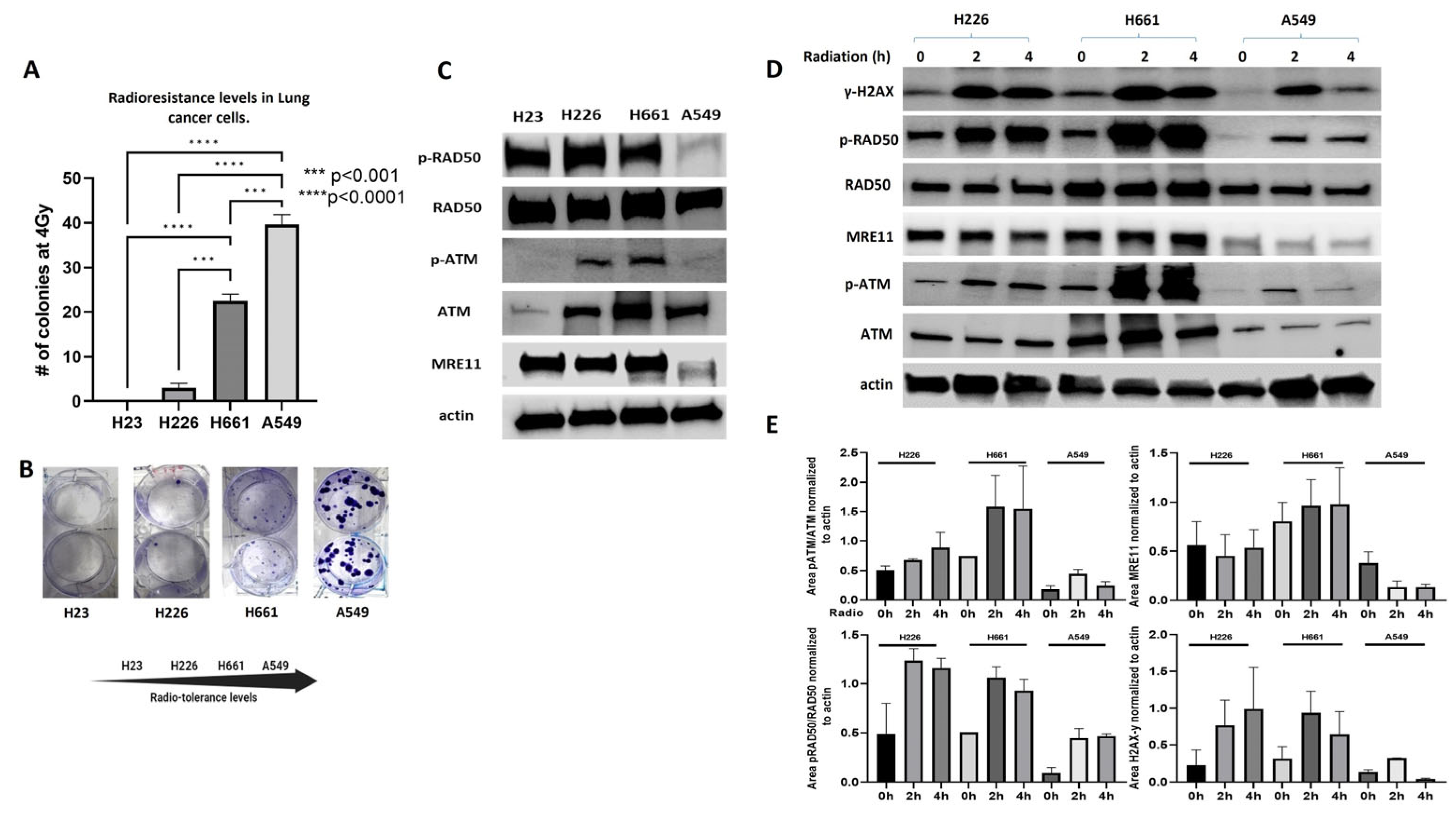
2.1. MRN Complex Signaling Is Diminished in Endogenously Radioresistant Cells
2.2. S6K1 Inhibition Sensitizes Lung Cancer Cells to Rradiation
2.3. S6K1 Modulates DNA Damage Signaling by Regulating Activation of MRN Complex Members
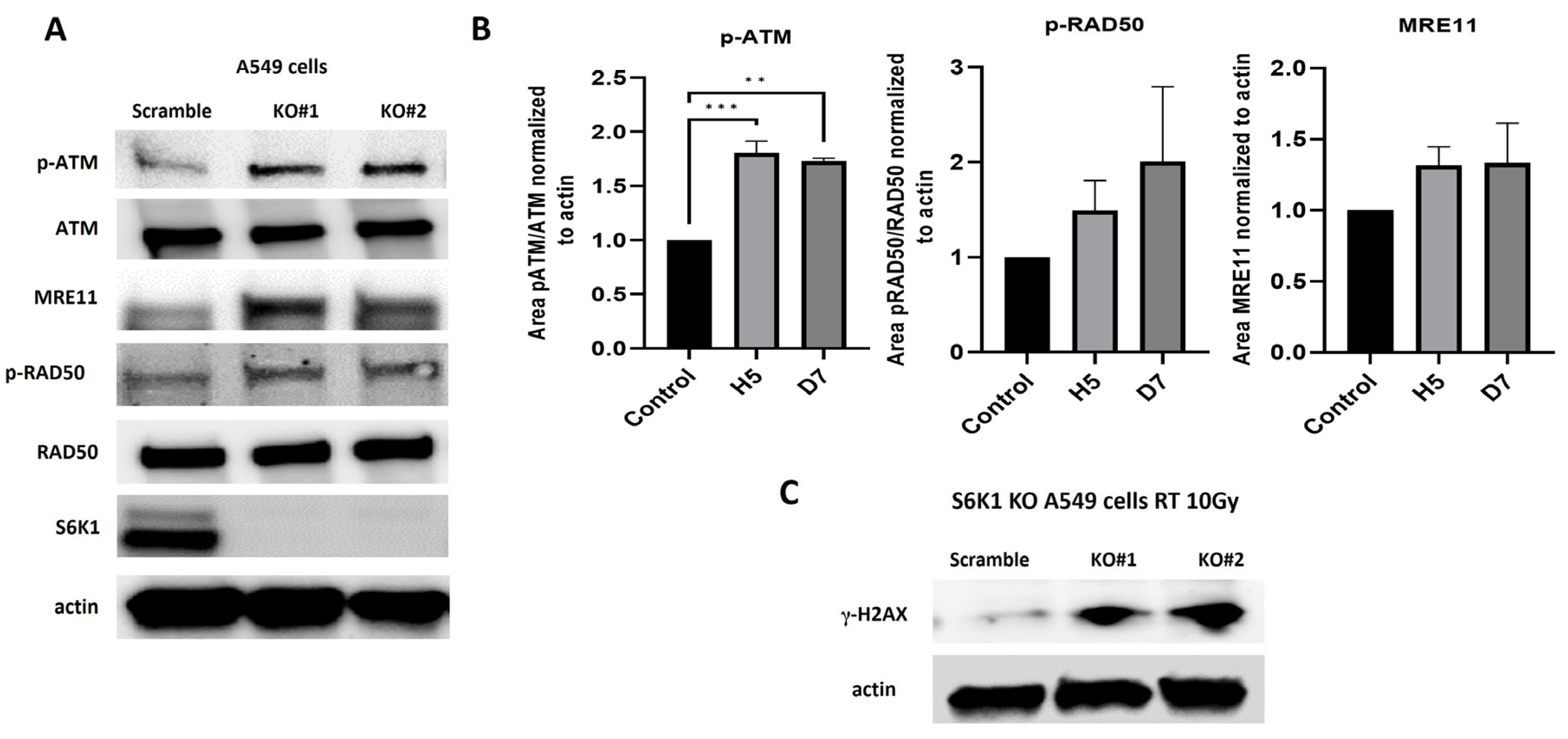
2.4. S6K1 Overexpression Increases Resistance to Radiation and Impairs Activation of MRN Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blot
4.3. Colony Formation Assay
4.4. Crispr-Cas9 Cells
4.5. Cell Transfection
4.6. Proliferation Assays
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Césaire, M.; Montanari, J.; Curcio, H.; Lerouge, D.; Gervais, R.; Demontrond, P.; Balosso, J.; Chevalier, F. Radioresistance of Non-Small Cell Lung Cancers and Therapeutic Perspectives. Cancers 2022, 14, 2829. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Eblan, M.J.; Deal, A.M.; Lipner, M.; Zagar, T.M.; Wang, Y.; Mavroidis, P.; Lee, C.B.; Jensen, B.C.; Rosenman, J.G.; et al. Cardiac Toxicity After Radiotherapy for Stage III Non–Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J. Clin. Oncol. 2017, 35, 1387–1394. [Google Scholar] [CrossRef]
- Nickoloff, J.A.; Taylor, L.; Sharma, N.; Kato, T.A. Exploiting DNA repair pathways for tumor sensitization, mitigation of resistance, and normal tissue protection in radiotherapy. Cancer Drug Resist. 2021, 4, 244–263. [Google Scholar] [CrossRef]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; Pavan, I.C.; Amaral, C.L.; Meneguello, L.; Luchessi, A.D.; Simabuco, F.M. The S6K protein family in health and disease. Life Sci. 2015, 131, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Andersen, J.S.; Mann, M.; Terada, N.; Korsmeyer, S.J. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 2001, 98, 9666–9670. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wong, A.S. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology 2006, 147, 2557–2566. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Zhou, X.-D.; Qian, G.; Shi, X.; Fang, J.; Jiang, B.-H. AKT1 Amplification Regulates Cisplatin Resistance in Human Lung Cancer Cells through the Mammalian Target of Rapamycin/p70S6K1 Pathway. Cancer Res. 2007, 67, 6325–6332. [Google Scholar] [CrossRef]
- Li, W.; Tan, D.; Zhang, Z.; Liang, J.J.; Brown, R.E. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol. Rep. 2008, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Flynn, D.C.; Zhang, Z.; Zhong, X.-S.; Walker, V.; Liu, K.J.; Shi, X.; Jiang, B.-H. G1cell cycle progression and the expression of G1cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am. J. Physiol. Physiol. 2004, 287, C281–C291. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Huang, S.-B.; Bedolla, R.G.; Rivas, P.; Basler, J.W.; Swanson, G.P.; Huang, T.H.-M.; Narayanasamy, G.; Papanikolaou, N.; Miyamoto, H.; et al. Suppression of ribosomal protein RPS6KB1 by Nexrutine increases sensitivity of prostate tumors to radiation. Cancer Lett. 2018, 433, 232–241. [Google Scholar] [CrossRef]
- Yu, C.-C.; Hung, S.-K.; Lin, H.-Y.; Chiou, W.-Y.; Lee, M.-S.; Liao, H.-F.; Huang, H.-B.; Ho, H.-C.; Su, Y.-C. Targeting the Pi3k/Akt/Mtor Signaling Pathway as an Effectively Radiosensitizing Strategy for Treating Human Oral Squamous Cell Carcinoma in Vitro and in Vivo. Oncotarget 2017, 8, 68641–68653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mei, H.; Shao, Q.; Wang, J.; Lin, Z. Association of ribosomal protein S6 kinase 1 with cellular radiosensitivity of non-small lung cancer. Int. J. Radiat. Biol. 2017, 93, 581–589. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, K.; Zhang, S.; Dong, X.; Yin, Z.; Meng, R.; Zhao, Y.; Dai, X.; Zhang, T.; Yang, K.; et al. S6K1 phosphorylation-dependent degradation of Mxi1 by beta-Trcp ubiquitin ligase promotes Myc activation and radioresistance in lung cancer. Theranostics 2018, 8, 1286–1300. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Meng, Y.; Zhang, M.; Li, D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: Implications for cancer treatment. Mol. Cancer 2019, 18, 169. [Google Scholar] [CrossRef]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Abubakar, A.; Lim, S.H.; Ng, W.; Lee, M.; de Souza, P.; Shin, J.-S.; et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer 2018, 18, 869. [Google Scholar] [CrossRef]
- Piscitello, D.; Varshney, D.; Lilla, S.; Vizioli, M.G.; Reid, C.; Gorbunova, V.; Seluanov, A.; A Gillespie, D.; Adams, P.D. AKT overactivation can suppress DNA repair via p70S6 kinase-dependent downregulation of MRE11. Oncogene 2017, 37, 427–438. [Google Scholar] [CrossRef]
- Lai, K.P.; Leong, W.F.; Chau, J.F.L.; Jia, D.; Zeng, L.; Liu, H.; He, L.; Hao, A.; Zhang, H.; Meek, D.; et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J. 2010, 29, 2994–3006. [Google Scholar] [CrossRef]
- Pearce, L.R.; Alton, G.R.; Richter, D.T.; Kath, J.C.; Lingardo, L.; Chapman, J.; Hwang, C.; Alessi, D.R. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1). Biochem. J. 2010, 431, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Gorgoulis, V.G.; Halazonetism, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Plo, I.; Laulier, C.; Gauthier, L.; Lebrun, F.; Calvo, F.; Lopez, B.S. AKT1 inhibits homologous recombination by inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res. 2008, 68, 9404–9412. [Google Scholar] [CrossRef] [PubMed]
- Stronach, E.A.; Chen, M.; Maginn, E.N.; Agarwal, R.; Mills, G.B.; Wasan, H.; Gabra, H. DNA-PK Mediates AKT Activation and Apoptosis Inhibition in Clinically Acquired Platinum Resistance. Neoplasia 2011, 13, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Shah, O.J.; Wang, Z.; Hunter, T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 2004, 14, 1650–1656. [Google Scholar] [CrossRef]
- Artemenko, M.; Zhong, S.S.; To, S.K.; Wong, A.S. p70 S6 kinase as a therapeutic target in cancers: More than just an mTOR effector. Cancer Lett. 2022, 535, 215593. [Google Scholar] [CrossRef]
- Zhang, P.; Catterson, J.H.; Grönke, S.; Partridge, L. Inhibition of S6K lowers age-related inflammation and increases lifespan through the endolysosomal system. Nat. Aging 2024, 4, 491–509. [Google Scholar] [CrossRef]
- Gallage, S.; Irvine, E.E.; Avila, J.E.B.; Reen, V.; Pedroni, S.M.A.; Duran, I.; Ranvir, V.; Khadayate, S.; Pombo, J.; Brookes, S.; et al. Ribosomal S6 kinase 1 regulates inflammaging via the senescence secretome. Nat. Aging 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Calderon-Aparicio, A.; Yamamoto, H.; De Vitto, H.; Zhang, T.; Wang, Q.; Bode, A.M.; Dong, Z. RCC2 Promotes Esophageal Cancer Growth by Regulating Activity and Expression of the Sox2 Transcription Factor. Mol. Cancer Res. 2020, 18, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Schalm, S.S.; Blenis, J. Identification of a Conserved Motif Required for mTOR Signaling. Curr. Biol. 2002, 12, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Schalm, S.S.; Tee, A.R.; Blenis, J. Characterization of a Conserved C-terminal Motif (RSPRR) in Ribosomal Protein S6 Kinase 1 Required for Its Mammalian Target of Rapamycin-dependent Regulation. J. Biol. Chem. 2005, 280, 11101–11106. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon-Aparicio, A.; He, J.; Simone, N.L. S6K1 Controls DNA Damage Signaling Modulated by the MRN Complex to Induce Radioresistance in Lung Cancer. Int. J. Mol. Sci. 2024, 25, 10461. https://doi.org/10.3390/ijms251910461
Calderon-Aparicio A, He J, Simone NL. S6K1 Controls DNA Damage Signaling Modulated by the MRN Complex to Induce Radioresistance in Lung Cancer. International Journal of Molecular Sciences. 2024; 25(19):10461. https://doi.org/10.3390/ijms251910461
Chicago/Turabian StyleCalderon-Aparicio, Ali, Jun He, and Nicole L. Simone. 2024. "S6K1 Controls DNA Damage Signaling Modulated by the MRN Complex to Induce Radioresistance in Lung Cancer" International Journal of Molecular Sciences 25, no. 19: 10461. https://doi.org/10.3390/ijms251910461
APA StyleCalderon-Aparicio, A., He, J., & Simone, N. L. (2024). S6K1 Controls DNA Damage Signaling Modulated by the MRN Complex to Induce Radioresistance in Lung Cancer. International Journal of Molecular Sciences, 25(19), 10461. https://doi.org/10.3390/ijms251910461





