Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice
Abstract
1. Introduction
2. Results
2.1. Gradual Inducement of Airway Allergy in Dams
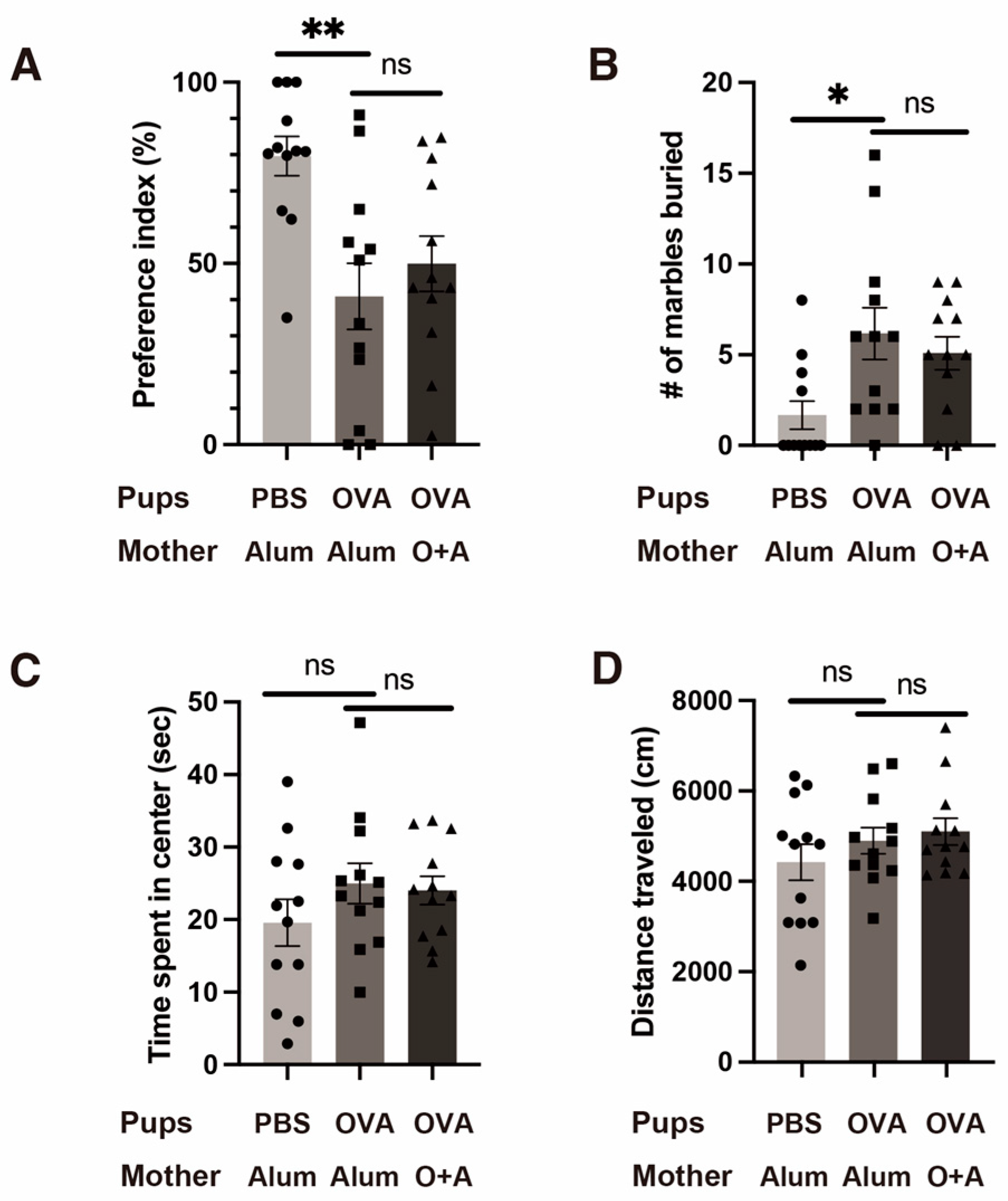
2.2. Mild Allergy-Induced ASD-like Behavioral Changes in the Offspring
2.3. Effects of Maternal Nursing Behavior
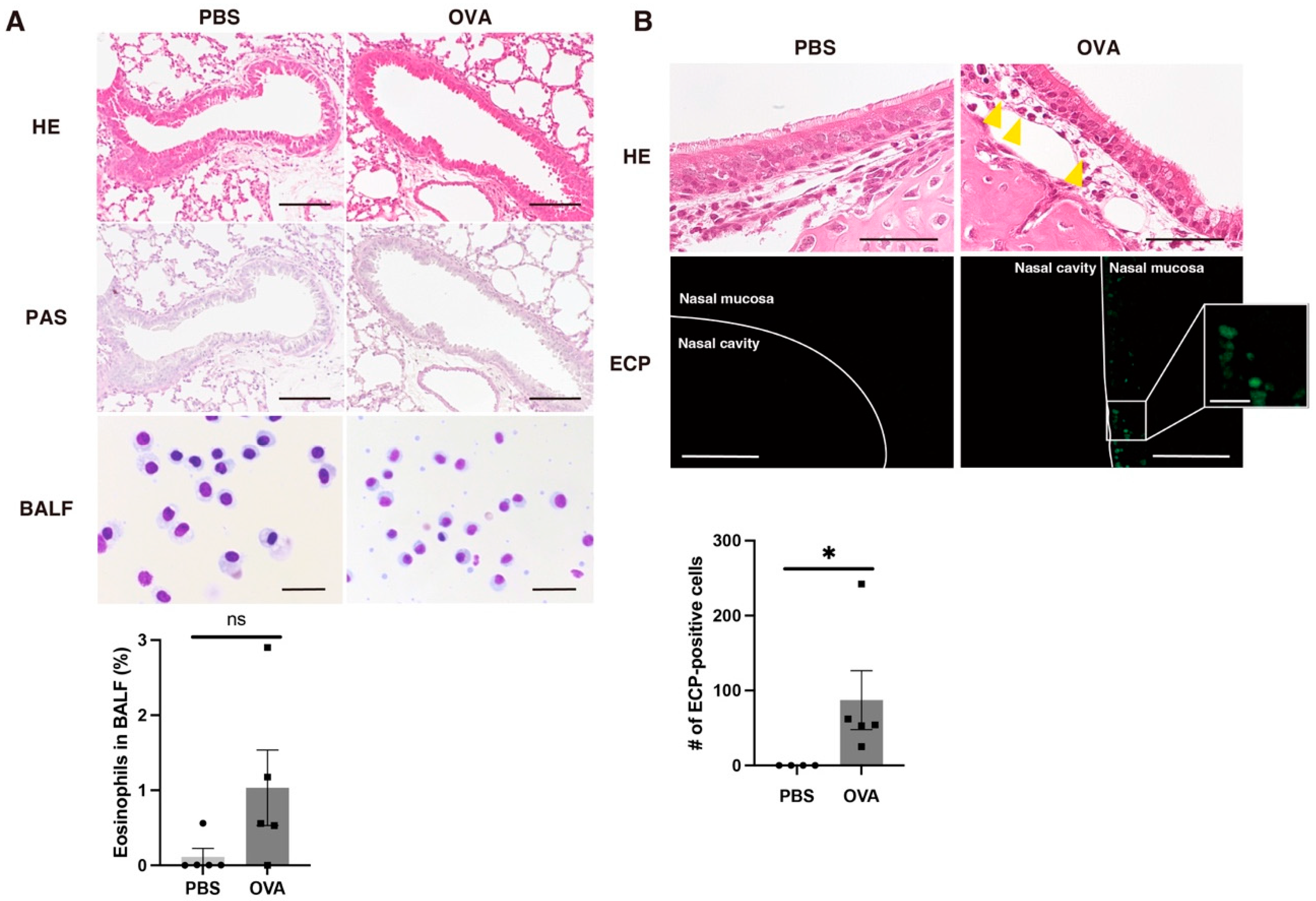
2.4. Allergic Rhinitis in OVA Group According to Histopathological Examination of Lung and Nasal Mucosa
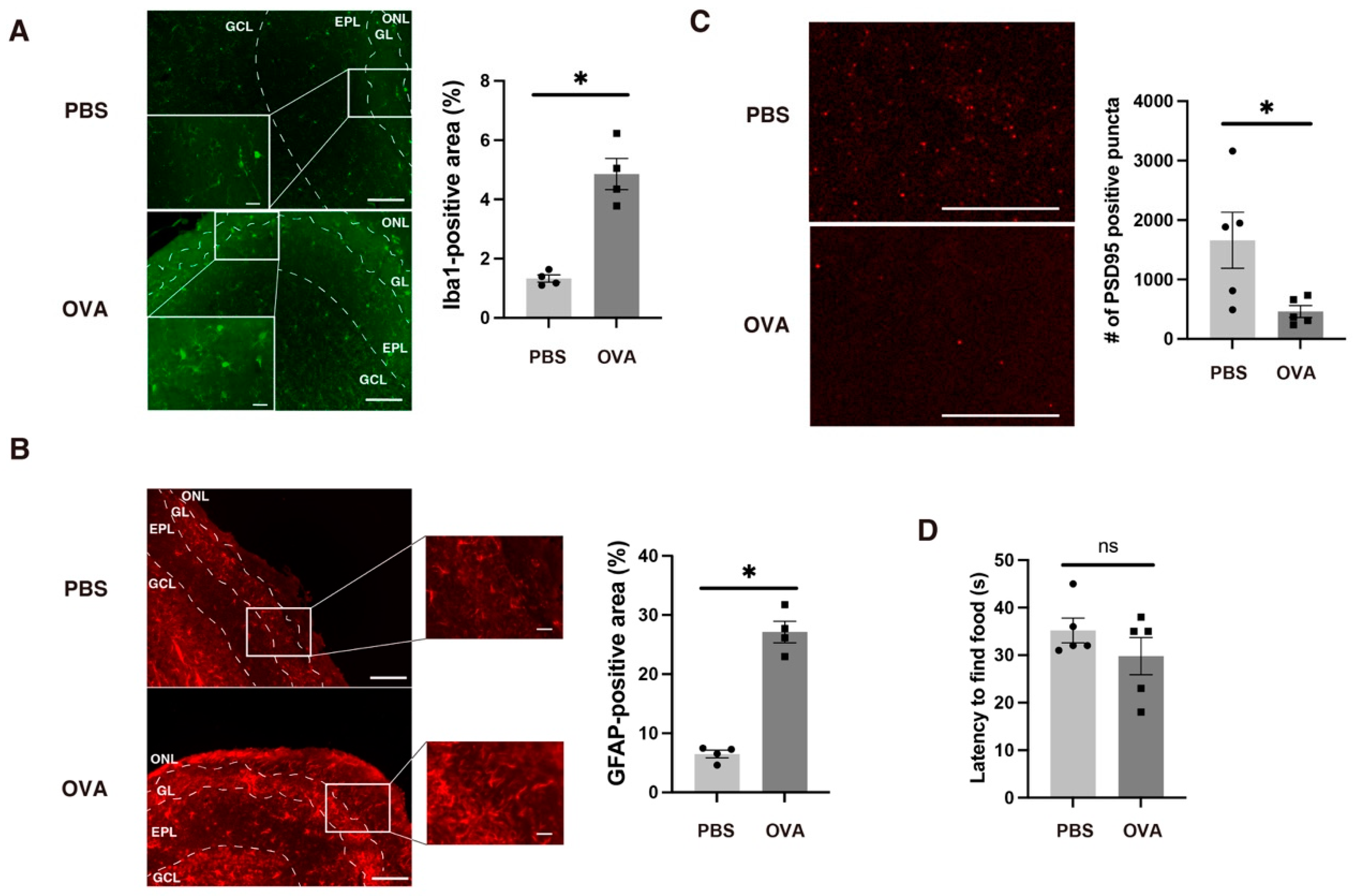
2.5. Allergic Rhinitis-Induced Glial Inflammation in the OB
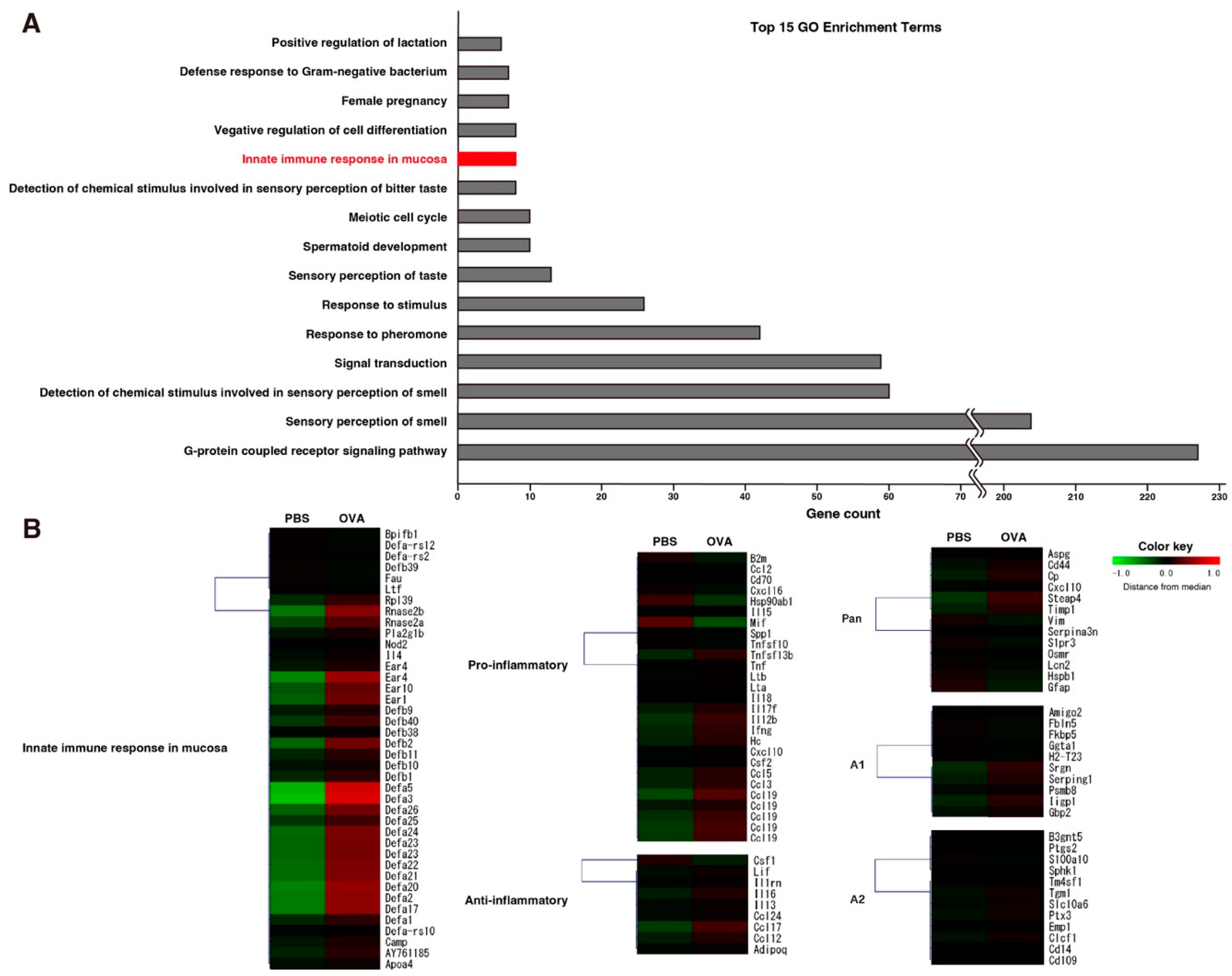
2.6. Eosinophilic Protein Upregulation in OVA Group in Microarray Analysis of OB
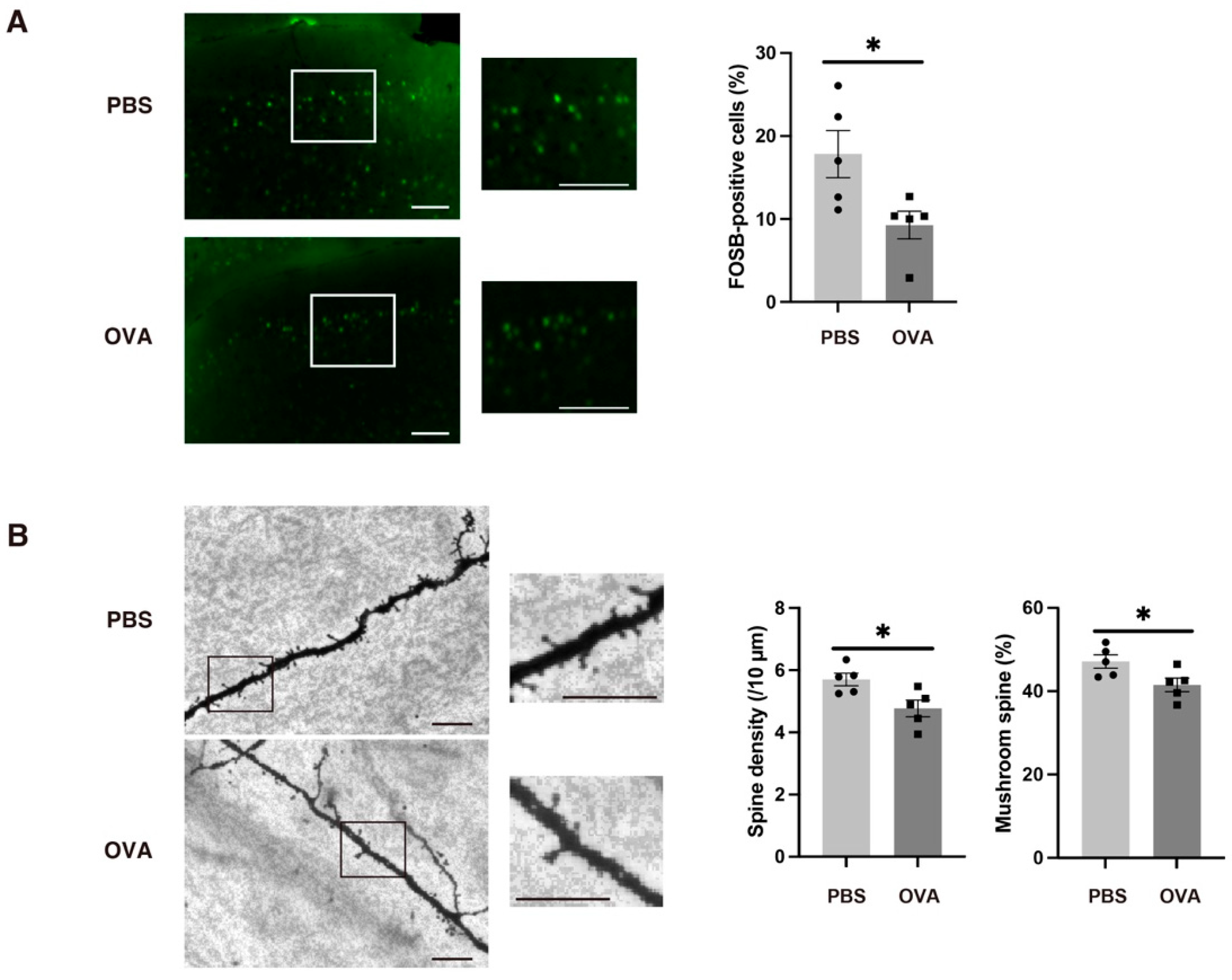
2.7. OB Glial Inflammation-Induced Synaptic Immaturity in the Medial Prefrontal Cortex
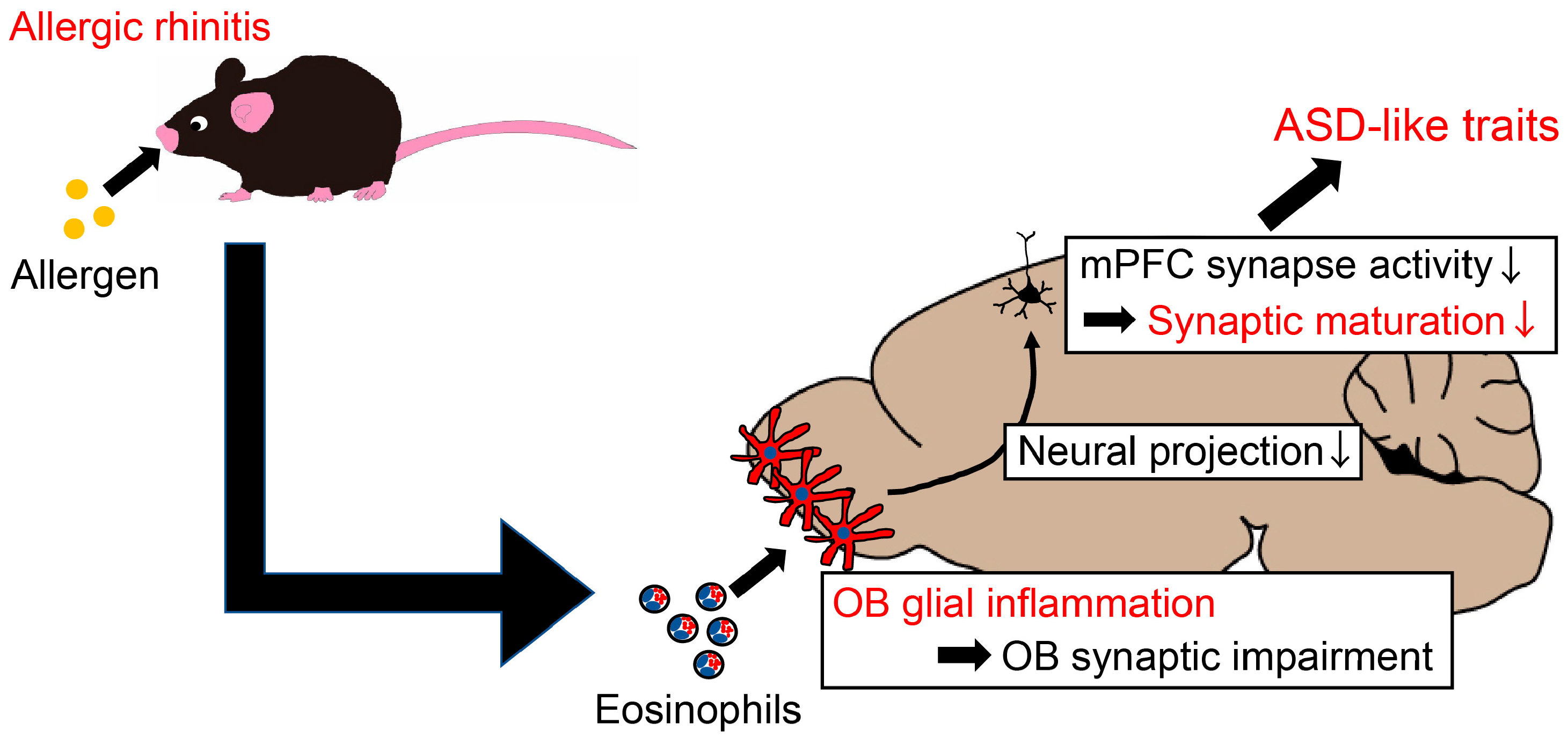
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Animals
4.3. Allergic Sensitization and Exposure
4.4. Three-Chamber Test
4.5. Marble Burying Test
4.6. Open Field Test
4.7. Buried Food Pellet Test
4.8. Maternal Behavior
4.9. Tissue Sample Preparation for Histological and Immunohistochemical Analyses
4.10. Immunofluorescence Analyses
4.11. Golgi–Cox Staining
4.12. Microarray
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhoury, M. Autistic Spectrum Disorders: A Review of Clinical Features, Theories and Diagnosis. Int. J. Dev. Neurosci. 2015, 43, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, Diagnosis and Therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic Diseases and Inflammation of the Brain in the Pathogenesis of Autism Spectrum Disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.X.; Tai, Y.H.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Increased Risk of Atopic Diseases in the Siblings of Patients with Autism Spectrum Disorder: A Nationwide Population-Based Cohort Study. J. Autism Dev. Disord. 2019, 49, 4626–4633. [Google Scholar] [CrossRef]
- Mazur, M.; Czarnobilska, M.; Dyga, W.; Czarnobilska, E. Trends in the Epidemiology of Allergic Diseases of the Airways in Children Growing Up in an Urban Agglomeration. J. Clin. Med. 2022, 11, 2188. [Google Scholar] [CrossRef]
- Blaiss, M.S.; Hammerby, E.; Robinson, S.; Kennedy-Martin, T.; Buchs, S. The Burden of Allergic Rhinitis and Allergic Rhinoconjunctivitis on Adolescents: A Literature Review. Ann. Allergy Asthma Immunol. 2018, 121, 43–52.e3. [Google Scholar] [CrossRef]
- Yang, L.; Sato, M.; Saito-Abe, M.; Miyaji, Y.; Shimada, M.; Sato, C.; Nishizato, M.; Kumasaka, N.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study. Nutrients 2022, 14, 4335. [Google Scholar] [CrossRef]
- Liao, T.C.; Lien, Y.T.; Wang, S.; Huang, S.L.; Chen, C.Y. Comorbidity of Atopic Disorders with Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder. J. Pediatr. 2016, 171, 248–255. [Google Scholar] [CrossRef]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of Food Allergy and Other Allergic Conditions with Autism Spectrum Disorder in Children. JAMA Netw. Open 2018, 1, e180279. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Su, T.P.; Chen, Y.S.; Hsu, J.W.; Huang, K.L.; Chang, W.H.; Chen, T.J.; Pan, T.L.; Bai, Y.M. Is Atopy in Early Childhood a Risk Factor for ADHD and ASD? A Longitudinal Study. J. Psychosom. Res. 2014, 77, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Chen, M.H.; Su, T.P.; Chen, Y.S.; Hsu, J.W.; Huang, K.L.; Chang, W.H.; Chen, T.J.; Bai, Y.M. Increased Risk of Autism Spectrum Disorder among Early Life Asthma Patients: An 8-Year Nationwide Population-Based Prospective Study. Res. Autism Spectr. Disord. 2014, 8, 381–386. [Google Scholar] [CrossRef]
- Ebrahim Soltani, Z.; Badripour, A.; Haddadi, N.S.; Elahi, M.; Kazemi, K.; Afshari, K.; Dehpour, A. Allergic Rhinitis in BALB/c Mice Is Associated with Behavioral and Hippocampus Changes and Neuroinflammation via the TLR4/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2022, 108, 108725. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.; Fujii, T.; Wang, B.; Masaki, K.; Kido, M.A.; Yoshida, M.; Matsushita, T.; Kira, J. Allergic Inflammation Leads to Neuropathic Pain via Glial Cell Activation. J. Neurosci. 2016, 36, 11929–11945. [Google Scholar] [CrossRef]
- Saitoh, B.; Tanaka, E.; Yamamoto, N.; van Kruining, D.; Iinuma, K.; Nakamuta, Y.; Yamaguchi, H.; Yamasaki, R.; Matsumoto, K.; Kira, J. ichi Early Postnatal Allergic Airway Inflammation Induces Dystrophic Microglia Leading to Excitatory Postsynaptic Surplus and Autism-like Behavior. Brain. Behav. Immun. 2021, 95, 362–380. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional Differences in Synaptogenesis in Human Cerebral Cortex. J. Comp. Neurol. 1997, 387, 167–178. [Google Scholar] [CrossRef]
- Plaschke, P.P.; Janson, C.; Norrman, E.; Bjornsson, E.; Ellbjar, S.; Jarvholm, B. Onset and Remission of Allergic Rhinitis and Asthma and the Relationship with Atopic Sensitization and Smoking. Am. J. Respir. Crit. Care Med. 2000, 162, 920–924. [Google Scholar] [CrossRef]
- Acevedo-Prado, A.; Seoane-Pillado, T.; López-Silvarrey-Varela, A.; Salgado, F.J.; Cruz, M.J.; Faraldo-Garcia, A.; Nieto-Fontarigo, J.J.; Pértega-Díaz, S.; Sanchez-Lastres, J.; San-José-González, M.A.; et al. Association of Rhinitis with Asthma Prevalence and Severity. Sci. Rep. 2022, 12, 6389. [Google Scholar] [CrossRef]
- Hasegawa-Ishii, S.; Shimada, A.; Imamura, F. Lipopolysaccharide-Initiated Persistent Rhinitis Causes Gliosis and Synaptic Loss in the Olfactory Bulb. Sci. Rep. 2017, 7, 11605. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, A.; Dobeš, J.; Oujezdská, J.; Ballek, O.; Benešová, M.; Šumník, Z.; Včeláková, J.; Koloušková, S.; Obermannová, B.; Kolář, M.; et al. Eosinophils from Patients with Type 1 Diabetes Mellitus Express High Level of Myeloid Alpha-Defensins and Myeloperoxidase. Cell. Immunol. 2012, 273, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; PassosdosSantos, R.; Gaestel, M.; David, S. TNF and Increased Intracellular Iron Alter Macrophage Polarization to a Detrimental M1 Phenotype in the Injured Spinal Cord. Neuron 2014, 83, 1098–1116. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Ghazvineh, S.; Zare, M.; Parsazadegan, T.; Dehdar, K.; Nazari, M.; Mirnajafi-Zadeh, J.; Jamaati, H.; Raoufy, M.R. Distraction of Olfactory Bulb-Medial Prefrontal Cortex Circuit May Induce Anxiety-like Behavior in Allergic Rhinitis. PLoS ONE 2019, 14, e0221978. [Google Scholar] [CrossRef]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism Spectrum Disorder: Neuropathology and Animal Models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Chua, R.X.Y.; Tay, M.J.Y.; Ooi, D.S.Q.; Siah, K.T.H.; Tham, E.H.; Shek, L.P.C.; Meaney, M.J.; Broekman, B.F.P.; Loo, E.X.L. Understanding the Link Between Allergy and Neurodevelopmental Disorders: A Current Review of Factors and Mechanisms. Front. Neurol. 2021, 11, 603571. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Croen, L.A.; Grether, J.K.; Yoshida, C.K.; Odouli, R.; De Water, J. Van Maternal Autoimmune Diseases, Asthma and Allergies, and Childhood Autism Spectrum Disorders: A Case-Control Study. Arch. Pediatr. Adolesc. Med. 2005, 159, 151–157. [Google Scholar] [CrossRef]
- Breach, M.R.; Dye, C.N.; Galan, A.; Lenz, K.M. Prenatal Allergic Inflammation in Rats Programs the Developmental Trajectory of Dendritic Spine Patterning in Brain Regions Associated with Cognitive and Social Behavior. Brain Behav. Immun. 2022, 102, 279–291. [Google Scholar] [CrossRef]
- Schwartzer, J.J.; Careaga, M.; Chang, C.; Onore, C.E.; Ashwood, P. Allergic Fetal Priming Leads to Developmental, Behavioral and Neurobiological Changes in Mice. Transl. Psychiatry 2015, 5, e543. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Bernardi, M.M. Prenatal Lipopolysaccharide Induces Hypothalamic Dopaminergic Hypoactivity and Autistic-like Behaviors: Repetitive Self-Grooming and Stereotypies. Behav. Brain Res. 2017, 331, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Gumusoglu, S.B.; Stevens, H.E. Maternal Inflammation and Neurodevelopmental Programming: A Review of Preclinical Outcomes and Implications for Translational Psychiatry. Biol. Psychiatry 2019, 85, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Song, M.K.; Lee, K. Comparison of Asthma Phenotypes in OVA-Induced Mice Challenged via Inhaled and Intranasal Routes. BMC Pulm. Med. 2019, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Coisne, C. Fluids and Barriers of the CNS Establish Immune Privilege by Confining Immune Surveillance to a Two-Walled Castle Moat Surrounding the CNS Castle. Fluids Barriers CNS 2011, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Shimada, A. Neuroinflammation and the Blood–Brain Interface: New Findings in Brain Pathology. Clin. Exp. Neuroimmunol. 2020, 11, 16–20. [Google Scholar] [CrossRef]
- Imamura, F.; Hasegawa-Ishii, S. Environmental Toxicants-Induced Immune Responses in the Olfactory Mucosa. Front. Immunol. 2016, 7, 475. [Google Scholar] [CrossRef]
- Yamasaki, R. Distinct Roles of Microglia and Monocytes in Central Nervous System Inflammation and Degeneration. Clin. Exp. Neuroimmunol. 2014, 5, 41–48. [Google Scholar] [CrossRef][Green Version]
- Morizawa, Y.M.; Hirayama, Y.; Ohno, N.; Shibata, S.; Shigetomi, E.; Sui, Y.; Nabekura, J.; Sato, K.; Okajima, F.; Takebayashi, H.; et al. Reactive Astrocytes Function as Phagocytes after Brain Ischemia via ABCA1-Mediated Pathway. Nat. Commun. 2017, 8, 28. [Google Scholar] [CrossRef]
- Koizumi, S.; Hirayama, Y.; Morizawa, Y.M. New Roles of Reactive Astrocytes in the Brain; an Organizer of Cerebral Ischemia. Neurochem. Int. 2018, 119, 107–114. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The Influence of Neuroinflammation in Autism Spectrum Disorder. Brain. Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.; Ahn, M.; Ekanayake, P.; Tanaka, A.; Matsuda, H.; Shin, T. Microglial and Astroglial Reaction in the Olfactory Bulb of Mice after Triton X-100 Application. Acta Histochem. 2019, 121, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.A.; Hummel, T. Olfaction in Allergic Rhinitis: A Systematic Review. J. Allergy Clin. Immunol. 2015, 136, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, Z.; Zou, Q.; Zhou, M.; Liu, H.; Fan, J. Construction of an Irreversible Allergic Rhinitis-Induced Olfactory Loss Mouse Model. Biochem. Biophys. Res. Commun. 2019, 513, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Koehler, L.; Fournel, A.; Albertowski, K.; Roessner, V.; Gerber, J.; Hummel, C.; Hummel, T.; Bensafi, M. Impaired Odor Perception in Autism Spectrum Disorder Is Associated with Decreased Activity in Olfactory Cortex. Chem. Senses 2018, 43, 627–634. [Google Scholar] [CrossRef]
- Cruz, F.C.; Koya, E.; Guez-Barber, D.H.; Bossert, J.M.; Lupica, C.R.; Shaham, Y.; Hope, B.T. New Technologies for Examining the Role of Neuronal Ensembles in Drug Addiction and Fear. Nat. Rev. Neurosci. 2013, 14, 743–754. [Google Scholar] [CrossRef]
- Fortier, A.V.; Meisner, O.C.; Nair, A.R.; Chang, S.W.C. Prefrontal Circuits Guiding Social Preference: Implications in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2022, 141, 104803. [Google Scholar] [CrossRef]
- Vitor-Vieira, F.; Vilela, F.C.; Giusti-Paiva, A. Hyperactivation of the Amygdala Correlates with Impaired Social Play Behavior of Prepubertal Male Rats in a Maternal Immune Activation Model. Behav. Brain Res. 2021, 414, 113503. [Google Scholar] [CrossRef]
- Williams, R.S.; Hauser, S.L.; Purpura, D.P.; DeLong, G.R.; Swisher, C.N. Autism and Mental Retardation: Neuropathologic Studies Performed in Four Retarded Persons with Autistic Behavior. Arch. Neurol. 1980, 37, 749–753. [Google Scholar] [CrossRef]
- Hutsler, J.J.; Zhang, H. Increased Dendritic Spine Densities on Cortical Projection Neurons in Autism Spectrum Disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef]
- Balamotis, M.A.; Tamberg, N.; Woo, Y.J.; Li, J.; Davy, B.; Kohwi-Shigematsu, T.; Kohwi, Y. Satb1 Ablation Alters Temporal Expression of Immediate Early Genes and Reduces Dendritic Spine Density during Postnatal Brain Development. Mol. Cell. Biol. 2012, 32, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Anthony Otero, P.; Fricklas, G.; Nigam, A.; Lizama, B.N.; Wills, Z.P.; Johnson, J.W.; Chu, C.T. Endogenous PTEN-Induced Kinase 1 Regulates Dendritic Architecture and Spinogenesis. J. Neurosci. 2022, 42, 7848–7860. [Google Scholar] [CrossRef]
- Fetit, R.; Hillary, R.F.; Price, D.J.; Lawrie, S.M. The Neuropathology of Autism: A Systematic Review of Post-Mortem Studies of Autism and Related Disorders. Neurosci. Biobehav. Rev. 2021, 129, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Bringas, M.E.; Carvajal-Flores, F.N.; López-Ramírez, T.A.; Atzori, M.; Flores, G. Rearrangement of the Dendritic Morphology in Limbic Regions and Altered Exploratory Behavior in a Rat Model of Autism Spectrum Disorder. Neuroscience 2013, 241, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.C.; Coulter, A.R. Adjuvants—A Classification and Review of Their Modes of Action. Vaccine 1997, 15, 248–256. [Google Scholar] [CrossRef]
- Hamilton, K.A.; Heinbockel, T.; Ennis, M.; Szabó, G.; Erdélyi, F.; Hayar, A. Properties of External Plexiform Layer Interneurons in Mouse Olfactory Bulb Slices. Neuroscience 2005, 133, 819–829. [Google Scholar] [CrossRef]
- Zhuang, T.; Pan, C.; Chen, J.; Han, H.; Zhu, X.; Xu, H.; Lu, Y. Chronic asthma-induced behavioral and hippocampal neuronal morphological changes are concurrent with BDNF, cofilin1 and Cdc42/RhoA alterations in immature mice. Brain Res. Bull. 2018, 143, 194–206. [Google Scholar] [CrossRef]
- Jain, V.V.; Kitagaki, K.; Businga, T.; Hussain, I.; George, C.; O’Shaughnessy, P.; Kline, J.N. CpG-Oligodeoxynucleotides Inhibit Airway Remodeling in a Murine Model of Chronic Asthma. J. Allergy Clin. Immunol. 2002, 110, 867–872. [Google Scholar] [CrossRef]
- Lo, S.C.; Scearce-Levie, K.; Sheng, M. Characterization of Social Behaviors in Caspase-3 Deficient Mice. Sci. Rep. 2016, 6, 18335. [Google Scholar] [CrossRef]
- Cunha, C.; Hort, Y.; Shine, J.; Doyle, K.L. Morphological and Behavioural Changes Occur Following the X-Ray Irradiation of the Adult Mouse Olfactory Neuroepithelium. BMC Neurosci. 2012, 13, 134. [Google Scholar] [CrossRef]
- Chourbaji, S.; Hoyer, C.; Richter, S.H.; Brandwein, C.; Pfeiffer, N.; Vogt, M.A.; Vollmayr, B.; Gass, P. Differences in Mouse Maternal Care Behavior—Is There a Genetic Impact of the Glucocorticoid Receptor? PLoS ONE 2011, 6, e19218. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.D.; Mueller, A.; Sisti, H.M.; Ogawa, S.; McEwen, B.S.; Brake, W.G. Anxiety and Fear Behaviors in Adult Male and Female C57BL/6 Mice Are Modulated by Maternal Separation. Horm. Behav. 2003, 43, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Brake, W.G.; Romeo, R.D.; Dunlop, J.C.; Gordon, M.; Buzescu, R.; Magarinos, A.M.; Allen, P.B.; Greengard, P.; Luine, V.; et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. USA. 2004, 101, 2185–2190. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, E.; Yamasaki, R.; Saitoh, B.-y.; Abdelhadi, A.; Nagata, S.; Yoshidomi, S.; Inoue, Y.; Matsumoto, K.; Kira, J.-i.; Isobe, N. Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice. Int. J. Mol. Sci. 2024, 25, 10464. https://doi.org/10.3390/ijms251910464
Tanaka E, Yamasaki R, Saitoh B-y, Abdelhadi A, Nagata S, Yoshidomi S, Inoue Y, Matsumoto K, Kira J-i, Isobe N. Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice. International Journal of Molecular Sciences. 2024; 25(19):10464. https://doi.org/10.3390/ijms251910464
Chicago/Turabian StyleTanaka, Eizo, Ryo Yamasaki, Ban-yu Saitoh, Amina Abdelhadi, Satoshi Nagata, Sato Yoshidomi, Yuka Inoue, Koichiro Matsumoto, Jun-ichi Kira, and Noriko Isobe. 2024. "Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice" International Journal of Molecular Sciences 25, no. 19: 10464. https://doi.org/10.3390/ijms251910464
APA StyleTanaka, E., Yamasaki, R., Saitoh, B.-y., Abdelhadi, A., Nagata, S., Yoshidomi, S., Inoue, Y., Matsumoto, K., Kira, J.-i., & Isobe, N. (2024). Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice. International Journal of Molecular Sciences, 25(19), 10464. https://doi.org/10.3390/ijms251910464





