Iron Metabolism in Aminolevulinic Acid-Photodynamic Therapy with Iron Chelators from the Thiosemicarbazone Group
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. The Antiproliferative Activity of TSC
2.3. An Accumulation of PPIX
2.4. An Ability to Chelate Iron Ions
2.5. The Phototoxic Effect
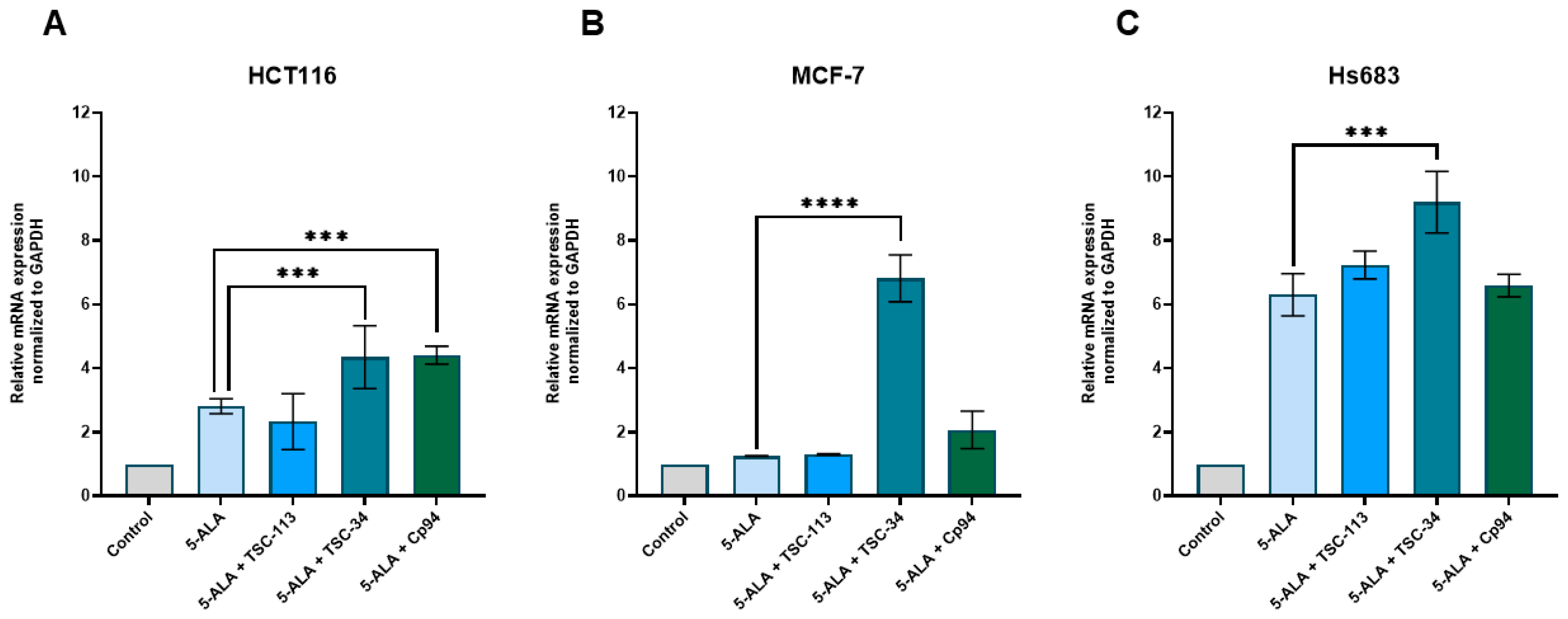
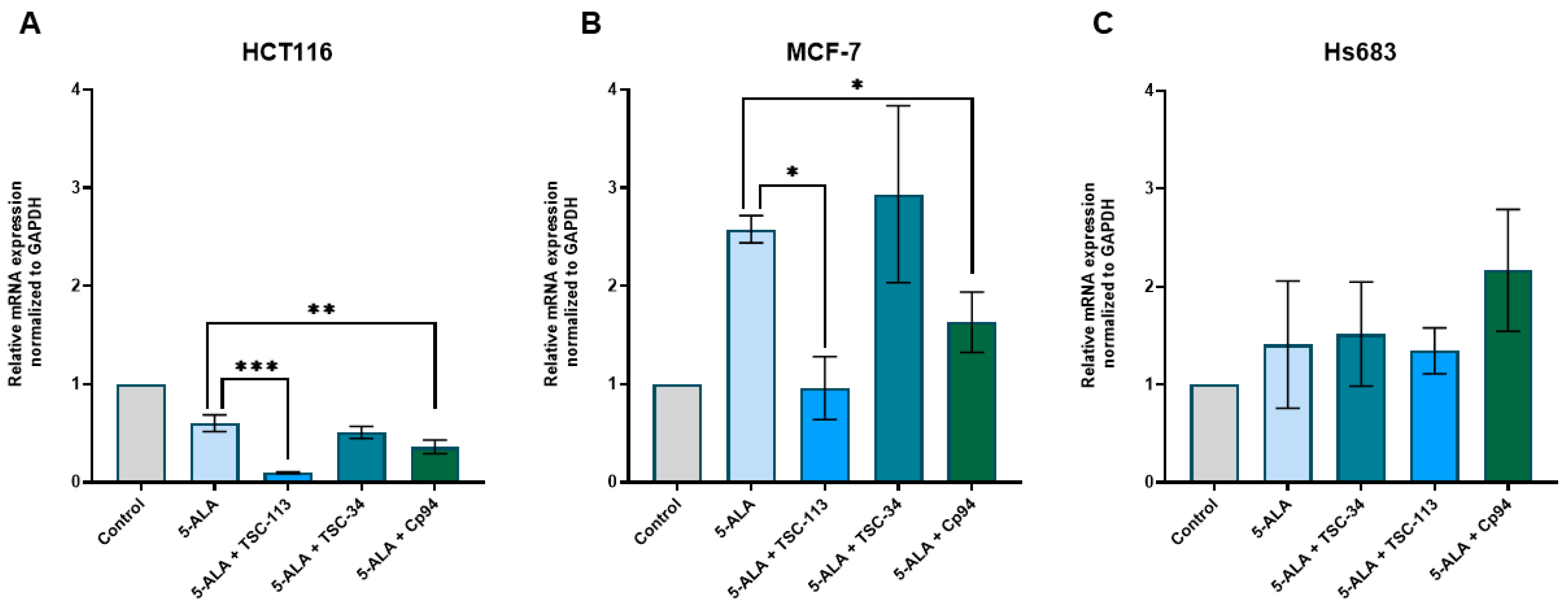
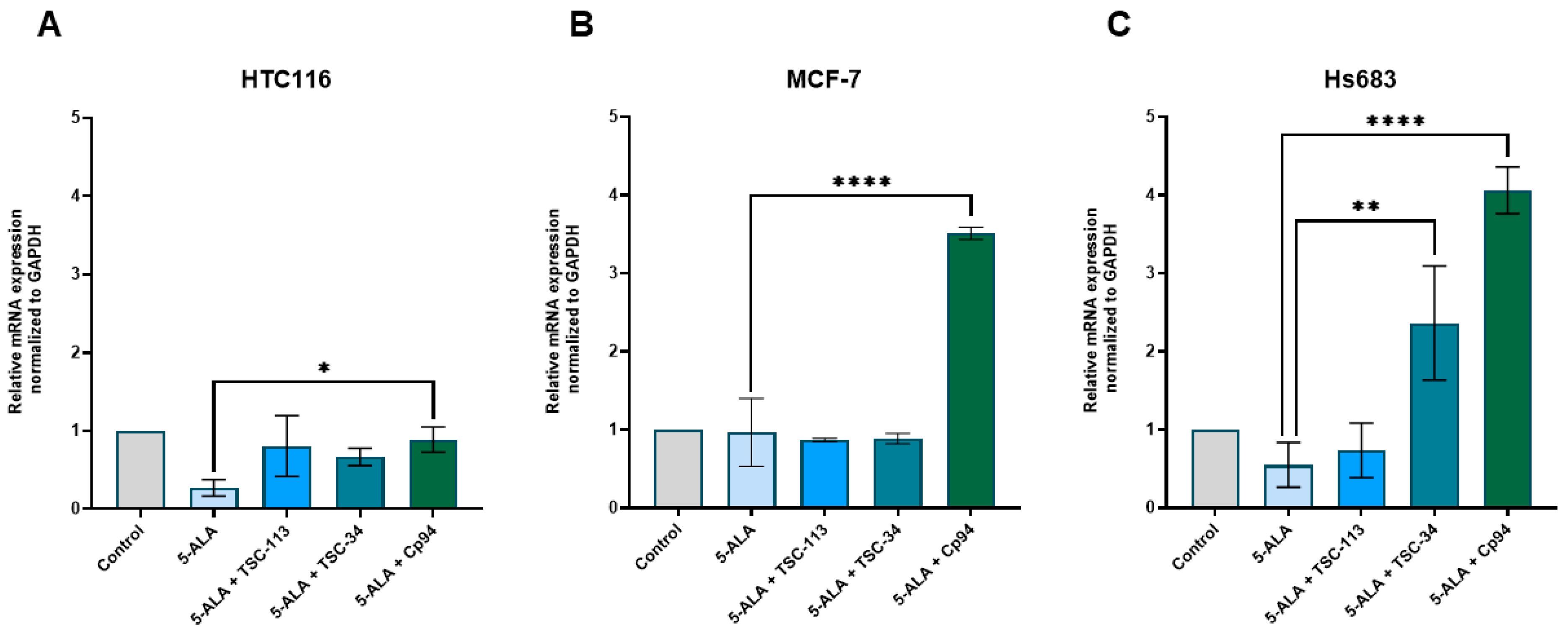
2.6. Iron Metabolism
3. Materials and Methods
3.1. Synthesis–Reagents and Equipment
3.2. General Synthetic Protocol for the Creation of Aromatic TSCs
3.3. Cell Lines and Cell Culture
3.4. Cytotoxicity Studies
3.5. Chelating Abilities
3.6. Accumulation of PPIX
3.7. Phototoxic Effect
3.8. Genes Expression
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nomoto, T.; Komoto, K.; Nagano, T.; Ishii, T.; Guo, H.; Honda, Y.; Ogura, S.I.; Ishizuka, M.; Nishiyama, N. Polymeric iron chelators for enhancing 5-aminolevulinic acid-induced photodynamic therapy. Cancer Sci. 2023, 114, 1086–1094. [Google Scholar] [CrossRef]
- Qin, J.; Zhou, C.; Zhu, M.; Shi, S.; Zhang, L.; Zhao, Y.; Li, C.; Wang, Y.; Wang, Y. Iron chelation promotes 5-aminolaevulinic acid-based photodynamic therapy against oral tongue squamous cell carcinoma. Photodiagn. Photodyn. Ther. 2020, 31, 101907. [Google Scholar] [CrossRef]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.E.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Curnow, A.; Pye, A. The importance of iron chelation and iron availability during PpIX-induced photodynamic therapy. Photonics Lasers Med. 2015, 4, 39–58. [Google Scholar] [CrossRef]
- Uehlinger, P.; Ballini, J.P.; van den Bergh, H.; Wagnieres, G. On the role of iron and one of its chelating agents in the production of protoporphyrin IX generated by 5-aminolevulinic acid and its hexyl ester derivative tested on an epidermal equivalent of human skin. Photochem. Photobiol. 2006, 82, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liang, C.; Xu, L.; Wang, Y.; Liu, W.; Zhang, K.; Liu, J.; Shi, J. Boosting 5-ALA-based photodynamic therapy by a liposomal nanomedicine through intracellular iron ion regulation. Acta Pharm. Sin. B 2021, 11, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.S.; Marchetti, J.M.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V. A vehicle for photodynamic therapy of skin cancer: Influence of dimethylsulphoxide on 5-aminolevulinic acid in vitro cutaneous permeation and in vivo protoporphyrin IX accumulation determined by confocal microscopy. J. Control. Release 2000, 65, 359–366. [Google Scholar] [CrossRef]
- Magnussen, A.; Reburn, C.; Perry, A.; Wood, M.; Curnow, A. Experimental investigation of a combinational iron chelating protoporphyrin IX prodrug for fluorescence detection and photodynamic therapy. Lasers Med. Sci. 2022, 37, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; MacRobert, A.J.; Porter, J.B.; Bown, S.G. The efficacy of an iron chelator (CP94) in increasing cellular protoporphyrin IX following intravesical 5-aminolaevulinic acid administration: An in vivo study. J. Photochem. Photobiol. B 1997, 38, 114–122. [Google Scholar] [CrossRef]
- Feuerstein, T.; Berkovitch-Luria, G.; Nudelman, A.; Rephaeli, A.; Malik, Z. Modulating ALA-PDT efficacy of mutlidrug resistant MCF-7 breast cancer cells using ALA prodrug. Photochem. Photobiol. Sci. 2011, 10, 1926–1933. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The inhibition of ferrochelatase enhances 5-aminolevulinic acid-based photodynamic action for prostate cancer. Photodiagn. Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelopoulou, M.; Grigalavicius, M.; Raabe, T.H.; Skarpen, E.; Juzenas, P.; Peng, Q.; Berg, K.; Theodossiou, T.A. Predictive biomarkers for 5-ALA-PDT can lead to personalized treatments and overcome tumor-specific resistances. Cancer Rep. 2022, 5, e1278. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Ripamonti, M.; Dardi, M.; Cozzi, A.; Santambrogio, P. Mitochondrial Ferritin: Its Role in Physiological and Pathological Conditions. Cells 2021, 10, 1969. [Google Scholar] [CrossRef]
- Martelli, A.; Wattenhofer-Donze, M.; Schmucker, S.; Bouvet, S.; Reutenauer, L.; Puccio, H. Frataxin is essential for extramitochondrial Fe-S cluster proteins in mammalian tissues. Hum. Mol. Genet. 2007, 16, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, S.; Martelli, A.; Colin, F.; Page, A.; Wattenhofer-Donze, M.; Reutenauer, L.; Puccio, H. Mammalian frataxin: An essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS ONE 2011, 6, e16199. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Oliva, C.R.; Flor, S.; Griguer, C.E. Mitoferrin, Cellular and Mitochondrial Iron Homeostasis. Cells 2022, 11, 3464. [Google Scholar] [CrossRef]
- Yien, Y.Y.; Perfetto, M. Regulation of Heme Synthesis by Mitochondrial Homeostasis Proteins. Front. Cell Dev. Biol. 2022, 10, 895521. [Google Scholar] [CrossRef]
- Barra, J.; Crosbourne, I.; Roberge, C.L.; Bossardi-Ramos, R.; Warren, J.S.A.; Matteson, K.; Wang, L.; Jourd’heuil, F.; Borisov, S.M.; Bresnahan, E. DMT1-dependent endosome-mitochondria interactions regulate mitochondrial iron translocation and metastatic outgrowth. Oncogene 2024, 43, 650–667. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, X.; Li, S.; Liu, W.; Yan, J.; Wang, S.; Cui, F.; Li, D.; Li, J. DMT1 differentially regulates mitochondrial complex activities to reduce glutathione loss and mitigate ferroptosis. Free Radic. Biol. Med. 2023, 207, 32–44. [Google Scholar] [CrossRef]
- Wolff, N.A.; Garrick, M.D.; Zhao, L.; Garrick, L.M.; Ghio, A.J.; Thevenod, F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 2018, 8, 211. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Bayeva, M.; Ghanefar, M.; Potini, V.; Sun, L.; Mutharasan, R.K.; Wu, R.; Khechaduri, A.; Jairaj Naik, T.; Ardehali, H. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc. Natl. Acad. Sci. USA 2012, 109, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Gawecki, R.; Malarz, K.; Rejmund, M.; Polanski, J.; Mrozek-Wilczkiewicz, A. Impact of thiosemicarbazones on the accumulation of PpIX and the expression of the associated genes. J. Photochem. Photobiol. B 2019, 199, 111585. [Google Scholar] [CrossRef]
- Gawecki, R.; Polanski, J.; Mrozek-Wilczkiewicz, A. A Thiosemicarbazone Derivative as a Booster in Photodynamic Therapy-A Way to Improve the Therapeutic Effect. Int. J. Mol. Sci. 2022, 23, 15370. [Google Scholar] [CrossRef]
- Serda, M.; Kalinowski, D.S.; Rasko, N.; Potuckova, E.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Malecki, J.G.; Sajewicz, M.; Ratuszna, A.; Muchowicz, A. Exploring the anti-cancer activity of novel thiosemicarbazones generated through the combination of retro-fragments: Dissection of critical structure-activity relationships. PLoS ONE 2014, 9, e110291. [Google Scholar] [CrossRef] [PubMed]
- Rzycka-Korzec, R.; Malarz, K.; Gawecki, R.; Mrozek-Wilczkiewicz, A.; Małecki, J.G.; Schab-Balcerzak, E.; Korzec, M.; Polanski, J. Effect of the complex-formation ability of thiosemicarbazones containing (aza)benzene or 3-nitro-1,8-naphthalimide unit towards Cu(II) and Fe(III) ions on their anticancer activity. J. Photochem. Photobiol. A Chem. 2021, 415, 113314. [Google Scholar] [CrossRef]
- Hashmi, K.; Satya; Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal applications of vanadium complexes with Schiff bases. J. Trace Elem. Med. Biol. 2023, 79, 127245. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; Gonzalez-Sarrias, A.; Fronczek, F.R. Synthesis, characterization, and preliminary in vitro studies of vanadium(IV) complexes with a Schiff base and thiosemicarbazones as mixed-ligands. Eur. J. Inorg. Chem. 2012, 2012, 664–677. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Serda, M.; Musiol, R.; Malecki, G.; Szurko, A.; Muchowicz, A.; Golab, J.; Ratuszna, A.; Polanski, J. Iron chelators in photodynamic therapy revisited: Synergistic effect by novel highly active thiosemicarbazones. ACS Med. Chem. Lett. 2014, 5, 336–339. [Google Scholar] [CrossRef]
- Curnow, A.; McIlroy, B.W.; Postle-Hacon, M.J.; Porter, J.B.; MacRobert, A.J.; Bown, S.G. Enhancement of 5-aminolaevulinic acid-induced photodynamic therapy in normal rat colon using hydroxypyridinone iron-chelating agents. Br. J. Cancer 1998, 78, 1278–1282. [Google Scholar] [CrossRef]
- Palasuberniam, P.; Kraus, D.; Mansi, M.; Braun, A.; Howley, R.; Myers, K.A.; Chen, B. Ferrochelatase Deficiency Abrogated the Enhancement of Aminolevulinic Acid-mediated Protoporphyrin IX by Iron Chelator Deferoxamine. Photochem. Photobiol. 2019, 95, 1052–1059. [Google Scholar] [CrossRef]
- Blake, E.; Curnow, A. The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Patel, D. The iron chelator Dp44mT does not protect myocytes against doxorubicin. J. Inorg. Biochem. 2009, 103, 1093–1101. [Google Scholar] [CrossRef]
- Santoro, A.; Vileno, B.; Palacios, O.; Peris-Diaz, M.D.; Riegel, G.; Gaiddon, C.; Krezel, A.; Faller, P. Reactivity of Cu(ii)-, Zn(ii)- and Fe(ii)-thiosemicarbazone complexes with glutathione and metallothionein: From stability to dissociation to transmetallation. Metallomics 2019, 11, 994–1004. [Google Scholar] [CrossRef]
- Serda, M.; Kalinowski, D.S.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Szurko, A.; Ratuszna, A.; Pantarat, N.; Kovacevic, Z.; Merlot, A.M.; Richardson, D.R. Synthesis and characterization of quinoline-based thiosemicarbazones and correlation of cellular iron-binding efficacy to anti-tumor efficacy. Bioorg. Med. Chem. Lett. 2012, 22, 5527–5531. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, W.; Palasuberniam, P.; Myers, K.A.; Wang, C.; Chen, B. Effects of Silencing Heme Biosynthesis Enzymes on 5-Aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy. Photochem. Photobiol. 2015, 91, 923–930. [Google Scholar] [CrossRef]
- Hara, Y.; Yanatori, I.; Tanaka, A.; Kishi, F.; Lemasters, J.J.; Nishina, S.; Sasaki, K.; Hino, K. Iron loss triggers mitophagy through induction of mitochondrial ferritin. EMBO Rep. 2020, 21, e50202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yin, R.; Wang, Y.; Lee, Y.H.; Luo, T.; Zhang, J.; Qiu, H.; Ambrose, S.; Wang, L.; Ren, J. Modulating mitochondrial morphology enhances antitumor effect of 5-ALA-mediated photodynamic therapy both in vitro and in vivo. J. Photochem. Photobiol. B 2017, 176, 81–91. [Google Scholar] [CrossRef]
- Dietz, J.V.; Willoughby, M.M.; Piel, R.B., 3rd; Ross, T.A.; Bohovych, I.; Addis, H.G.; Fox, J.L.; Lanzilotta, W.N.; Dailey, H.A.; Wohlschlegel, J.A. Mitochondrial contact site and cristae organizing system (MICOS) machinery supports heme biosynthesis by enabling optimal performance of ferrochelatase. Redox Biol. 2021, 46, 102125. [Google Scholar] [CrossRef]
- Lu, C.; Cortopassi, G. Frataxin knockdown causes loss of cytoplasmic iron-sulfur cluster functions, redox alterations and induction of heme transcripts. Arch. Biochem. Biophys. 2007, 457, 111–122. [Google Scholar] [CrossRef]
- Weerth, R.S.; Medlock, A.E.; Dailey, H.A. Ironing out the distribution of [2Fe-2S] motifs in ferrochelatases. J. Biol. Chem. 2021, 297, 101017. [Google Scholar] [CrossRef]
- Crooks, D.R.; Ghosh, M.C.; Haller, R.G.; Tong, W.H.; Rouault, T.A. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 2010, 115, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T.; Ishigaki, M.; Kataoka, T.; Taketani, S. Porcine ferrochelatase: The relationship between iron-removal reaction and the conversion of heme to Zn-protoporphyrin. Biosci. Biotechnol. Biochem. 2010, 74, 1415–1420. [Google Scholar] [CrossRef]
- Hayashi, M.; Fukuhara, H.; Inoue, K.; Shuin, T.; Hagiya, Y.; Nakajima, M.; Tanaka, T.; Ogura, S. The effect of iron ion on the specificity of photodynamic therapy with 5-aminolevulinic acid. PLoS ONE 2015, 10, e0122351. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Griguer, C.E.; Flor, S.; Oliva, C.R. Mitoferrin-1 Promotes Proliferation and Abrogates Protein Oxidation via the Glutathione Pathway in Glioblastoma. Antioxidants 2023, 12, 349. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Liu, P. Deferoxamine Enhanced Mitochondrial Iron Accumulation and Promoted Cell Migration in Triple-Negative MDA-MB-231 Breast Cancer Cells Via a ROS-Dependent Mechanism. Int. J. Mol. Sci. 2019, 20, 4952. [Google Scholar] [CrossRef] [PubMed]
- Blajan, I.; Miersch, H.; Schmidt, D.; Kristiansen, G.; Perner, S.; Ritter, M.; Ellinger, J.; Klumper, N. Comprehensive Analysis of the ATP-binding Cassette Subfamily B Across Renal Cancers Identifies ABCB8 Overexpression in Phenotypically Aggressive Clear Cell Renal Cell Carcinoma. Eur. Urol. Focus 2021, 7, 1121–1129. [Google Scholar] [CrossRef]


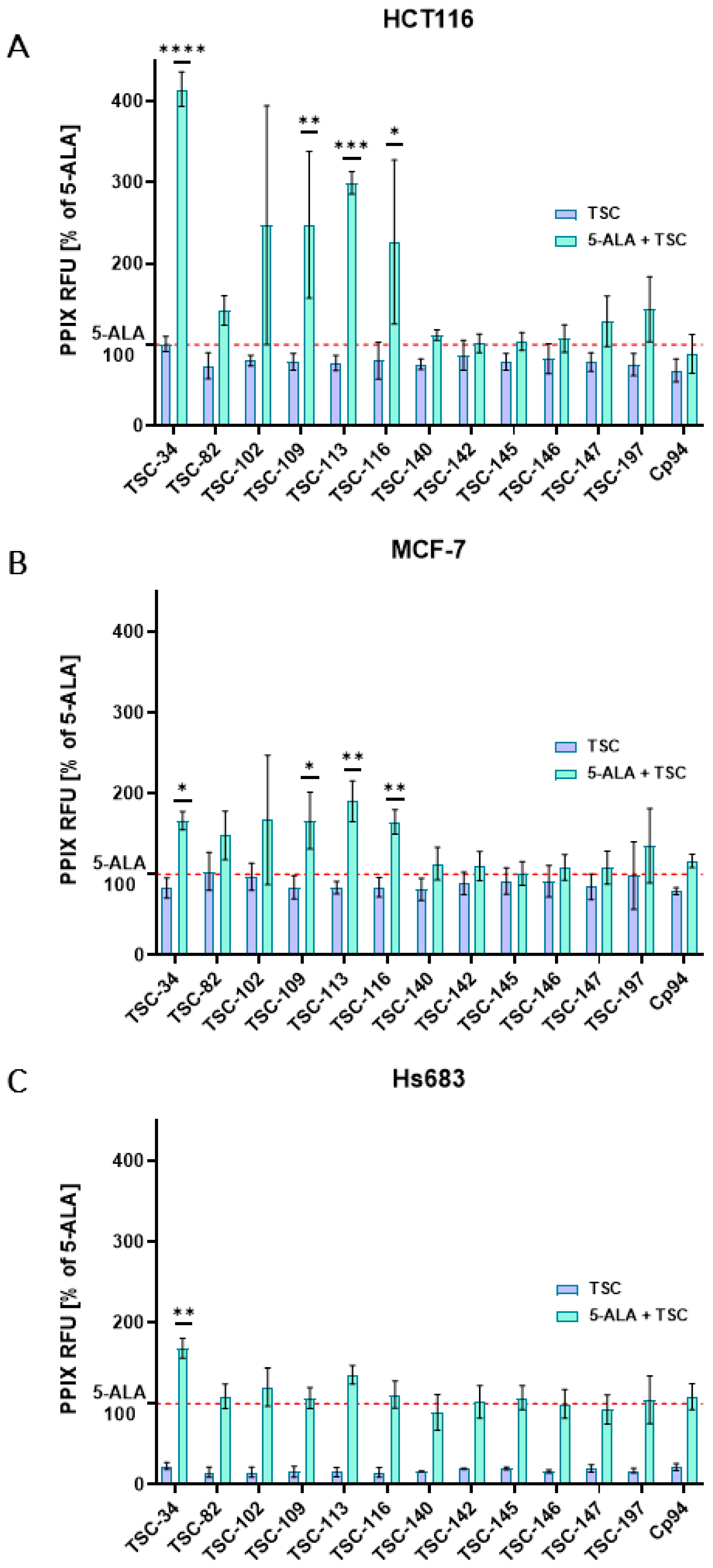

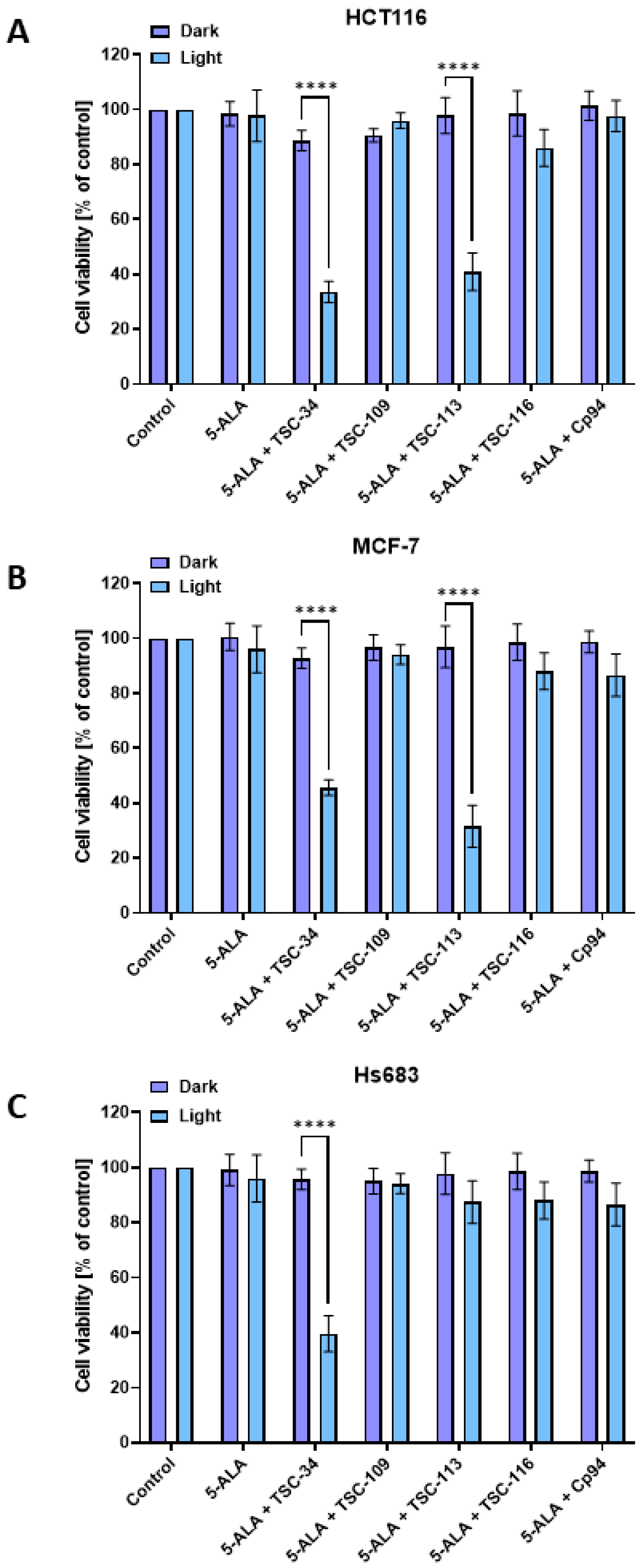
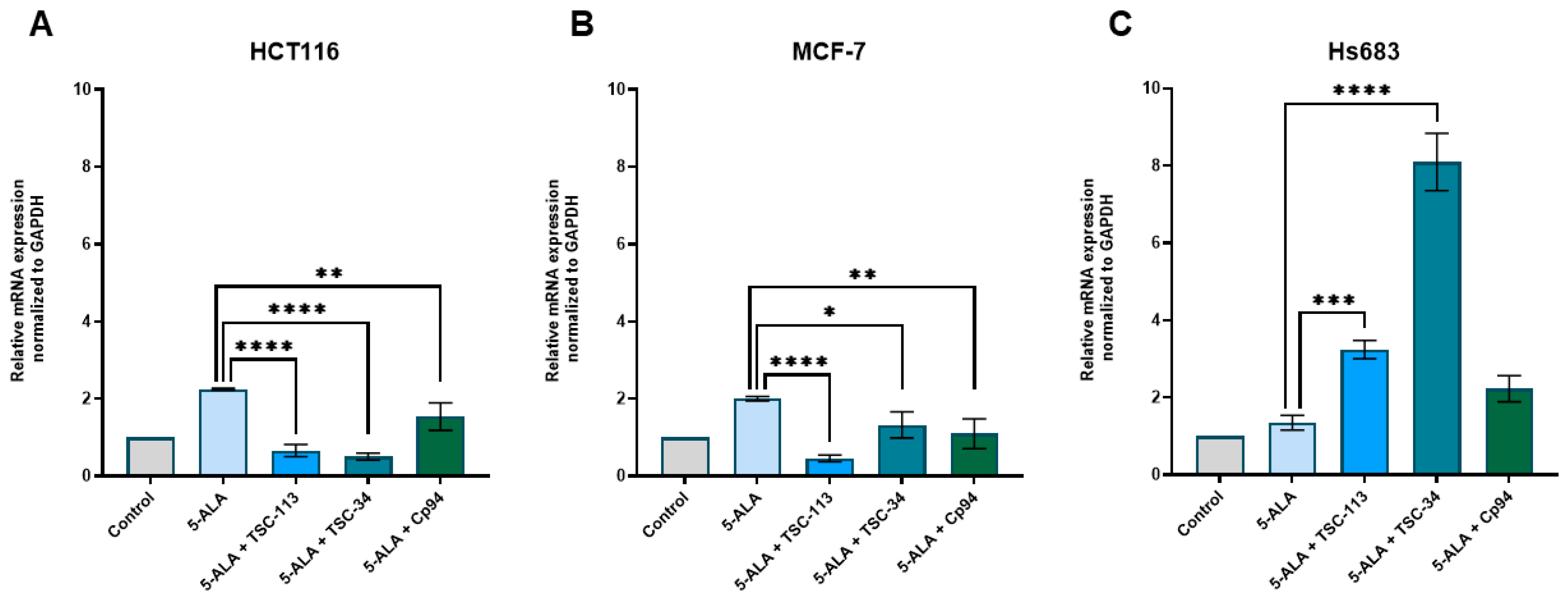
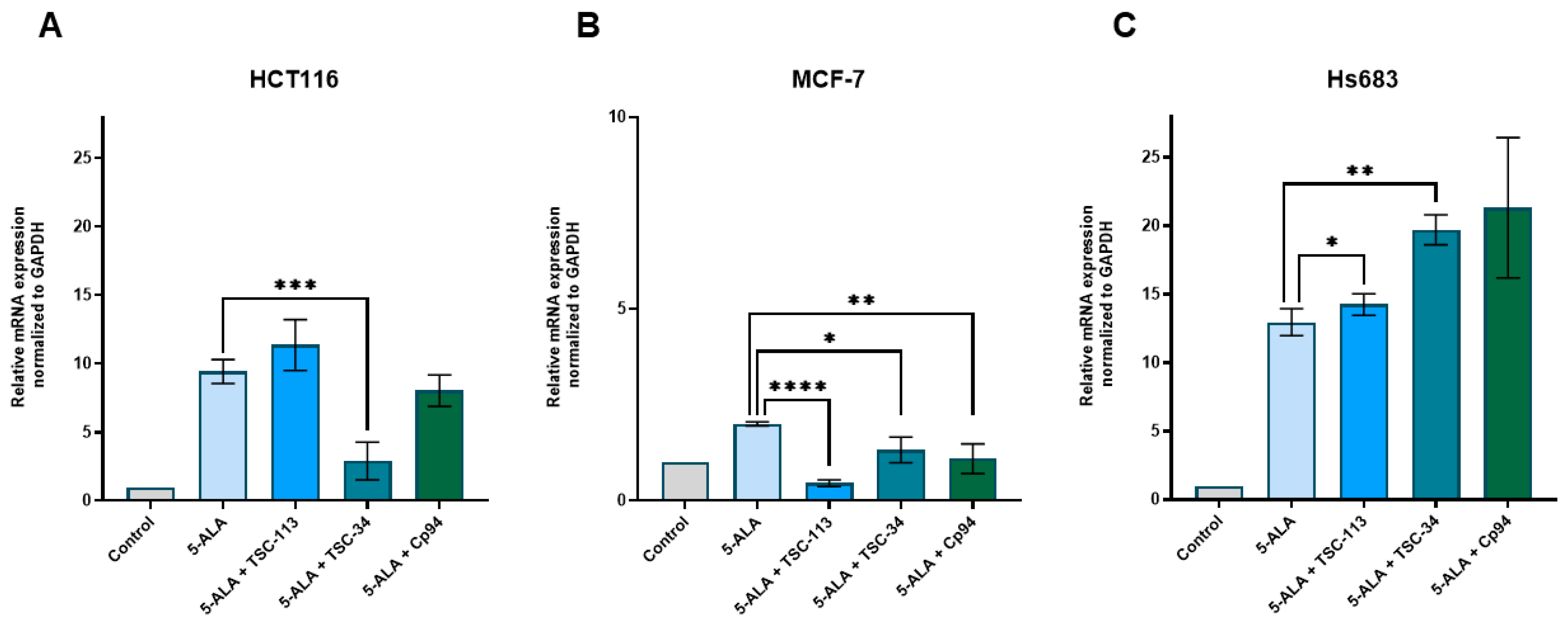


| No. | TSC | IC50 [µM] | |||
|---|---|---|---|---|---|
| HCT116 | MCF-7 | Hs683 | NHDF | ||
| 1 | TSC-34 | 18.31 ± 0.92 | >50 | >50 | 13.06 ± 1.90 |
| 2 | TSC-82 | 47.56 ± 1.99 | >50 | >50 | >50 |
| 3 | TSC-102 | >50 | >50 | >50 | >50 |
| 4 | TSC-109 | 30.87 ± 0.64 | 42.97 ± 1.33 | >50 | >50 |
| 5 | TSC-113 | >50 | >50 | >50 | >50 |
| 6 | TSC-116 | 34.88 ± 0.96 | >50 | >50 | >50 |
| 7 | TSC-140 | >50 | >50 | >50 | >50 |
| 8 | TSC-142 | >50 | >50 | >50 | >50 |
| 9 | TSC-145 | >50 | >50 | >50 | >50 |
| 10 | TSC-146 | >50 | >50 | >50 | >50 |
| 11 | TSC-147 | >50 | >50 | >50 | >50 |
| 12 | TSC-197 | >50 | >50 | >50 | >50 |
| 13 | Cp94 | >50 | >50 | >50 | >50 |
| Sample | Compound | Isosbestic Point [nm] | Complex with Fe3+ |
|---|---|---|---|
| λmax [nm] | λmax [nm] | ||
| Cp-94 | 280 | 290 | 510 |
| TSC-34 | 337 | 366 | 415 |
| TSC-109 | 340 | 378 | 449 |
| TSC-113 | 305 | 342 | 362 |
| TSC-116 | 308 | 345 | 385 |
| Gene Name | Sequence 5→3 (F—Forward, R—Reverse) | |
|---|---|---|
| DMT1 | F | GGTCACGCTTTGCCCGA |
| R | CAATCCGCCAGCCTAGTCC | |
| MFRN1 | F | ACTCGGTGAAGACACGAATGC |
| R | CAGCTATCCCGTTGGCTAGG | |
| MFRN2 | F | CACTGCGTGATGTACCCCAT |
| R | CAACACATTGCGATAGCGGG | |
| ABCB8 | F | TATTTCGGGTCGGGATTCGG |
| R | TCCAGTTTTCCCATTACGCCA | |
| FNX | F | TGGACCTAAGCGTTATGACTGG |
| R | TCTTCCCGTGTGAGTTGCTT | |
| FTMT | F | CCATCAACCGCCAGATCAAC |
| R | GTGCAATTCCAGCAACGACT | |
| FECH | F | GATGAATTGTCCCCCAACAC |
| R | GCTTCCGTCCCACTTGATTA | |
| HO-1 | F | CATCCCCTACACACCAGCCA |
| R | ATGTTGGGGAAGGTGAAGAAGG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawecki, R.; Rawicka, P.; Rogalska, M.; Serda, M.; Mrozek-Wilczkiewicz, A. Iron Metabolism in Aminolevulinic Acid-Photodynamic Therapy with Iron Chelators from the Thiosemicarbazone Group. Int. J. Mol. Sci. 2024, 25, 10468. https://doi.org/10.3390/ijms251910468
Gawecki R, Rawicka P, Rogalska M, Serda M, Mrozek-Wilczkiewicz A. Iron Metabolism in Aminolevulinic Acid-Photodynamic Therapy with Iron Chelators from the Thiosemicarbazone Group. International Journal of Molecular Sciences. 2024; 25(19):10468. https://doi.org/10.3390/ijms251910468
Chicago/Turabian StyleGawecki, Robert, Patrycja Rawicka, Marta Rogalska, Maciej Serda, and Anna Mrozek-Wilczkiewicz. 2024. "Iron Metabolism in Aminolevulinic Acid-Photodynamic Therapy with Iron Chelators from the Thiosemicarbazone Group" International Journal of Molecular Sciences 25, no. 19: 10468. https://doi.org/10.3390/ijms251910468
APA StyleGawecki, R., Rawicka, P., Rogalska, M., Serda, M., & Mrozek-Wilczkiewicz, A. (2024). Iron Metabolism in Aminolevulinic Acid-Photodynamic Therapy with Iron Chelators from the Thiosemicarbazone Group. International Journal of Molecular Sciences, 25(19), 10468. https://doi.org/10.3390/ijms251910468







