Genomic and Transcriptomic Profile of HNF1A-Mutated Liver Adenomas Highlights Molecular Signature and Potential Therapeutic Implications
Abstract
:1. Introduction
2. Results
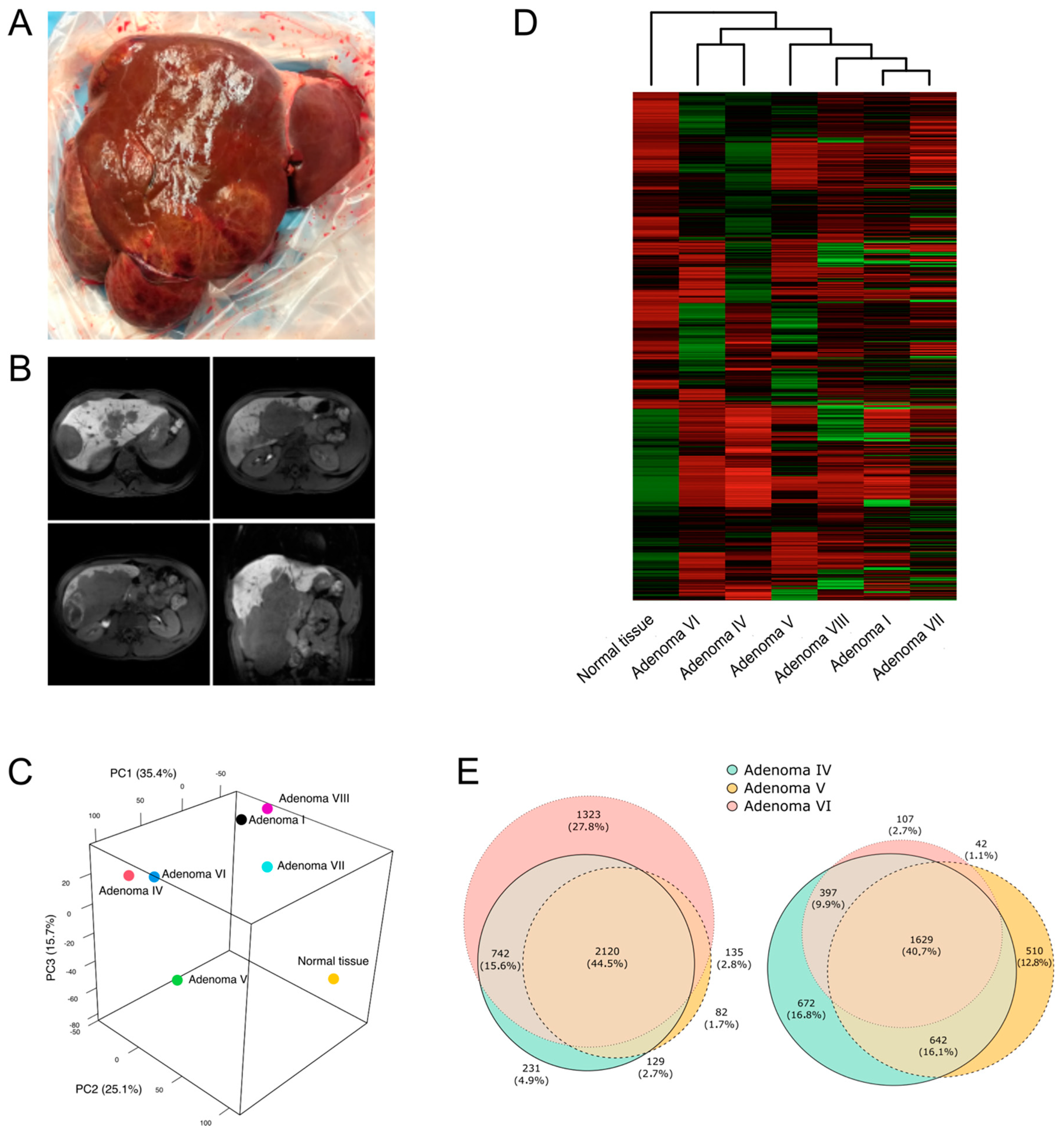
2.1. Hepatic Adenomas Sampling
2.2. Adenoma Samples Showed the Presence of a Second Somatic Variant
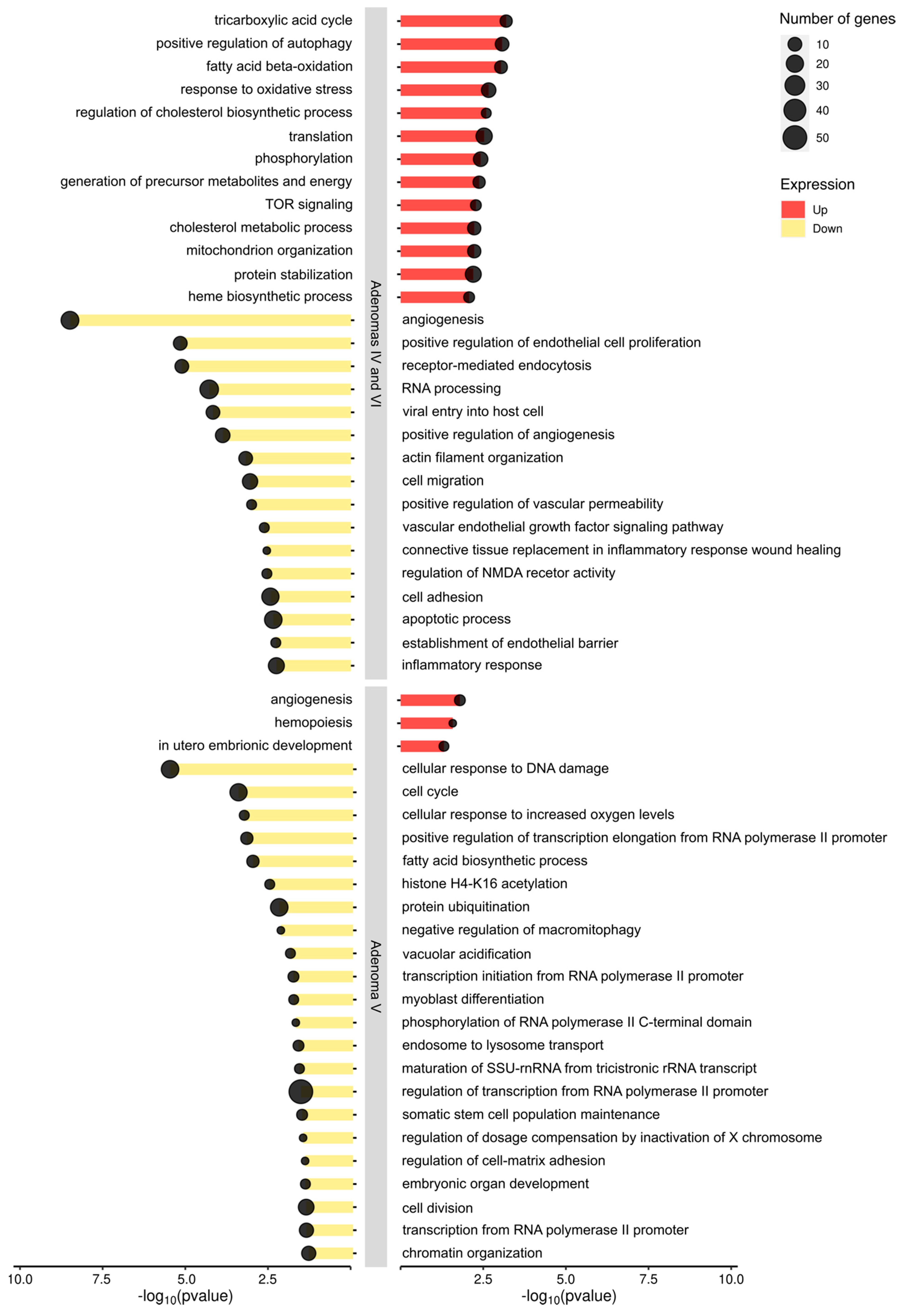
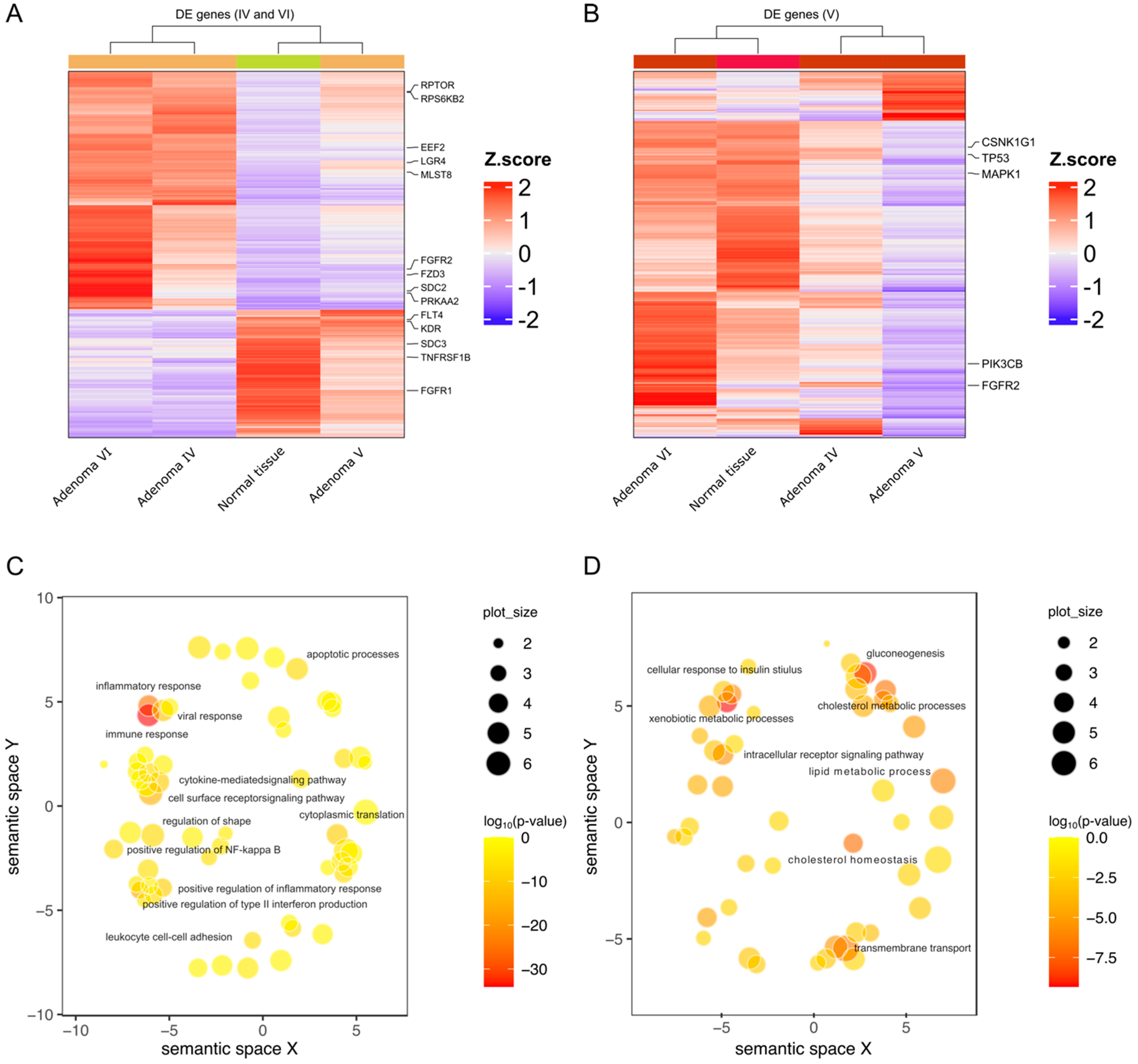
2.3. HNF1A Genetic Variants in Adenomas Control Different Molecular Pathways
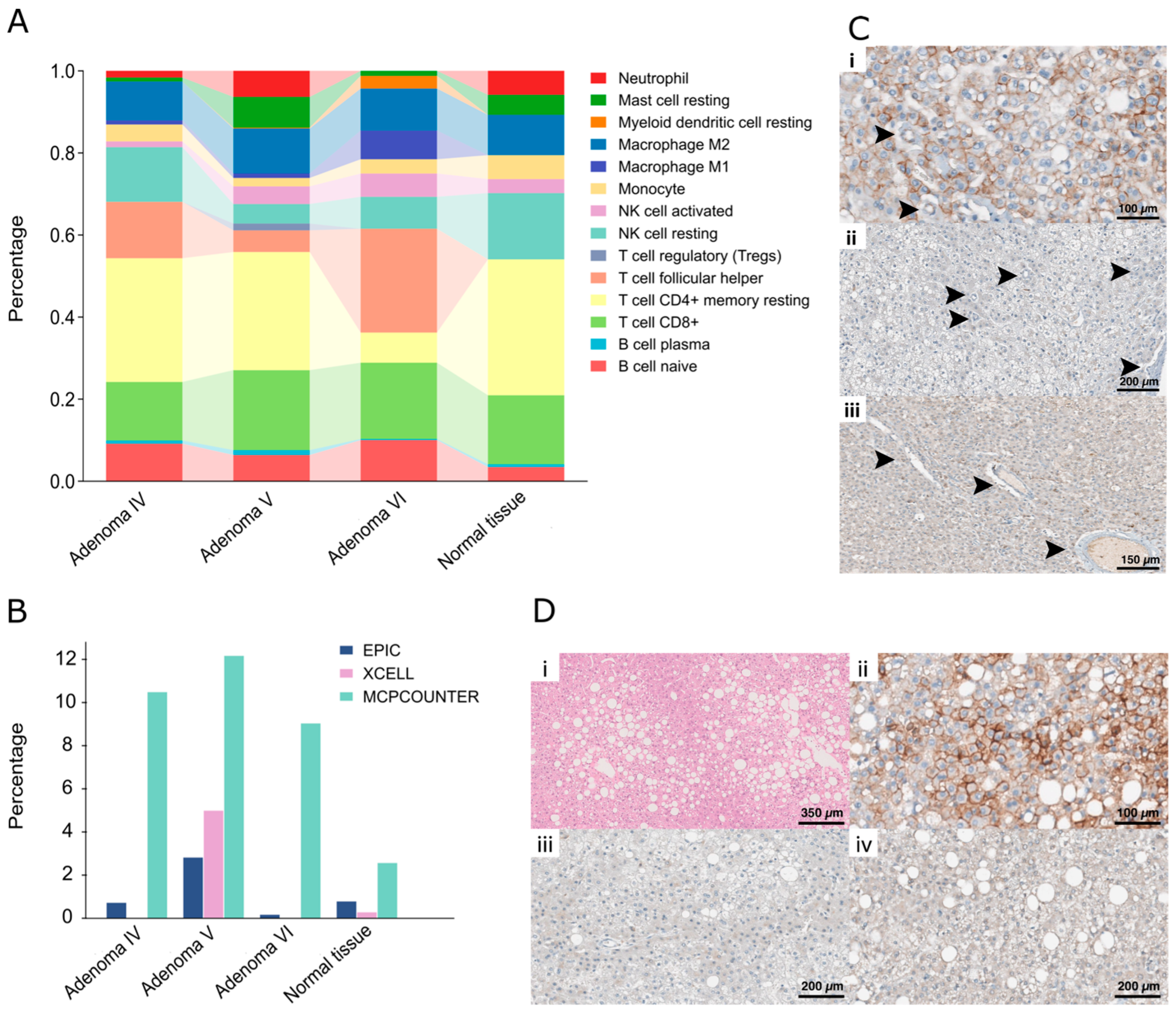
2.4. Histopathological Analyses Reflect the Molecular Characteristics of Each Adenoma
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Clinical Exome Sequencing (CES)
4.3. Sanger Sequencing
4.4. Multiplex Ligation-Dependent Probe Amplification (MLPA)
4.5. Digital Droplet PCR
4.6. RNA Sequencing
4.7. Real-Time PCR
4.8. Histopathological Analysis and Immunohistochemical Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjørkhaug, L.; Bratland, A.; Njølstad, P.R.; Molven, A. Functional Dissection of the HNF-1alpha Transcription Factor: A Study on Nuclear Localization and Transcriptional Activation. DNA Cell Biol. 2005, 24, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Soutoglou, E.; Papafotiou, G.; Katrakili, N.; Talianidis, I. Transcriptional Activation by Hepatocyte Nuclear Factor-1 Requires Synergism between Multiple Coactivator Proteins. J. Biol. Chem. 2000, 275, 12515–12520. [Google Scholar] [CrossRef] [PubMed]
- Soutoglou, E.; Viollet, B.; Vaxillaire, M.; Yaniv, M.; Pontoglio, M.; Talianidis, I. Transcription Factor-Dependent Regulation of CBP and P/CAF Histone Acetyltransferase Activity. EMBO J. 2001, 20, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Dohda, T.; Kaneoka, H.; Inayoshi, Y.; Kamihira, M.; Miyake, K.; Iijima, S. Transcriptional Coactivators CBP and P300 Cooperatively Enhance HNF-1alpha-Mediated Expression of the Albumin Gene in Hepatocytes. J. Biochem. 2004, 136, 313–319. [Google Scholar] [CrossRef]
- Ban, N.; Yamada, Y.; Someya, Y.; Miyawaki, K.; Ihara, Y.; Hosokawa, M.; Toyokuni, S.; Tsuda, K.; Seino, Y. Hepatocyte Nuclear Factor-1alpha Recruits the Transcriptional Co-Activator P300 on the GLUT2 Gene Promoter. Diabetes 2002, 51, 1409–1418. [Google Scholar] [CrossRef]
- Sato, Y.; Rahman, M.M.; Haneda, M.; Tsuyama, T.; Mizumoto, T.; Yoshizawa, T.; Kitamura, T.; Gonzalez, F.J.; Yamamura, K.-I.; Yamagata, K. HNF1α Controls Glucagon Secretion in Pancreatic α-Cells through Modulation of SGLT1. Biochim. Biophys Acta Mol. Basis Dis. 2020, 1866, 165898. [Google Scholar] [CrossRef] [PubMed]
- Sneha, P.; Kumar, D.T.; Lijo, J.; Megha, M.; Siva, R.; George Priya Doss, C. Probing the Protein-Protein Interaction Network of Proteins Causing Maturity Onset Diabetes of the Young. Adv. Protein Chem. Struct. Biol. 2018, 110, 167–202. [Google Scholar] [CrossRef]
- Vaxillaire, M.; Boccio, V.; Philippi, A.; Vigouroux, C.; Terwilliger, J.; Passa, P.; Beckmann, J.S.; Velho, G.; Lathrop, G.M.; Froguel, P. A Gene for Maturity Onset Diabetes of the Young (MODY) Maps to Chromosome 12q. Nat. Genet. 1995, 9, 418–423. [Google Scholar] [CrossRef]
- Nault, J.-C.; Couchy, G.; Balabaud, C.; Morcrette, G.; Caruso, S.; Blanc, J.-F.; Bacq, Y.; Calderaro, J.; Paradis, V.; Ramos, J.; et al. Molecular Classification of Hepatocellular Adenoma Associates with Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology 2017, 152, 880–894.e6. [Google Scholar] [CrossRef]
- Védie, A.-L.; Sutter, O.; Ziol, M.; Nault, J.-C. Molecular Classification of Hepatocellular Adenomas: Impact on Clinical Practice. Hepatic Oncol. 2018, 5, HEP04. [Google Scholar] [CrossRef]
- Akiyama, T.E.; Ward, J.M.; Gonzalez, F.J. Regulation of the Liver Fatty Acid-Binding Protein Gene by Hepatocyte Nuclear Factor 1alpha (HNF1alpha). Alterations in Fatty Acid Homeostasis in HNF1alpha-Deficient Mice. J. Biol. Chem. 2000, 275, 27117–27122. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Guo, H.; Fan, X.; Liu, T.; Xu, C.; He, Z.; Song, Y.; Gao, L.; Shao, S.; et al. Dominant-Negative HNF1α Mutant Promotes Liver Steatosis and Inflammation by Regulating Hepatic Complement Factor D. iScience 2023, 26, 108018. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Adachi, T.; Matsunaga, T.; Takeda, J.; Tsujimoto, G.; Ishihara, A.; Yasuda, K.; Tsuda, K. Mutant HNF-1alpha and Mutant HNF-1beta Identified in MODY3 and MODY5 Downregulate DPP-IV Gene Expression in Caco-2 Cells. Biochem. Biophys. Res. Commun. 2006, 346, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Avila Cobos, F.; Alquicira-Hernandez, J.; Powell, J.E.; Mestdagh, P.; De Preter, K. Benchmarking of Cell Type Deconvolution Pipelines for Transcriptomics Data. Nat. Commun. 2020, 11, 5650. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, T.; Zhai, X.; Xiao, X. Primary Hepatocellular Adenoma Due to Biallelic HNF1A Mutations and Its Co-Occurrence with MODY 3: Case-Report and Review of the Literature. Endocrine 2020, 67, 544–551. [Google Scholar] [CrossRef]
- Jeannot, E.; Lacape, G.; Gin, H.; Couchy, G.; Saric, J.; Laumonier, H.; Le Bail, B.; Bioulac-Sage, P.; Balabaud, C.; Zucman-Rossi, J. Double Heterozygous Germline HNF1A Mutations in a Patient with Liver Adenomatosis. Diabetes Care. 2012, 35, e35. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Sempoux, C.; Balabaud, C. Hepatocellular Adenomas: Morphology and Genomics. Gastroenterol Clin. N. Am. 2017, 46, 253–272. [Google Scholar] [CrossRef]
- Gu, N.; Suzuki, N.; Takeda, J.; Adachi, T.; Tsujimoto, G.; Aoki, N.; Ishihara, A.; Tsuda, K.; Yasuda, K. Effect of Mutations in HNF-1alpha and HNF-1beta on the Transcriptional Regulation of Human Sucrase-Isomaltase in Caco-2 Cells. Biochem. Biophys. Res. Commun. 2004, 325, 308–313. [Google Scholar] [CrossRef]
- Miles, D.A.; Holmes, S.; Minuk, G.Y. Hepatic Adenomatosis in a Young Woman with Non-Familial Maturity-Onset Diabetes of the Young Type 3. Can. Liver J. 2021, 4, 328–331. [Google Scholar] [CrossRef]
- Harryvan, T.J.; Tushuizen, M.E. A Young Patient with Diabetes and Liver Tumors. Gastroenterology 2018, 155, 25–26. [Google Scholar] [CrossRef]
- Willson, J.S.; Godwin, T.D.; Wiggins, G.A.; Guilford, P.J.; McCall, J.L. Primary Hepatocellular Neoplasms in a MODY3 Family with a Novel HNF1A Germline Mutation. J. Hepatol. 2013, 59, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Iwen, K.A.; Klein, J.; Hubold, C.; Lehnert, H.; Weitzel, J.M. Maturity-Onset Diabetes of the Young and Hepatic Adenomatosis—Characterisation of a New Mutation. Exp. Clin. Endocrinol. Diabetes 2013, 121, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Bacq, Y.; Jacquemin, E.; Balabaud, C.; Jeannot, E.; Scotto, B.; Branchereau, S.; Laurent, C.; Bourlier, P.; Pariente, D.; de Muret, A.; et al. Familial Liver Adenomatosis Associated with Hepatocyte Nuclear Factor 1alpha Inactivation. Gastroenterology 2003, 125, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Haring, M.P.D.; Vriesendorp, T.M.; Klein Wassink-Ruiter, J.S.; de Haas, R.J.; Gouw, A.S.H.; de Meijer, V.E. Diagnosis of Hepatocellular Adenoma in Men before Onset of Diabetes in HNF1A-MODY: Watch out for Winkers. Liver Int. 2019, 39, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, E.; Mellottee, L.; Bioulac-Sage, P.; Balabaud, C.; Scoazec, J.-Y.; Tran Van Nhieu, J.; Bacq, Y.; Michalak, S.; Buob, D.; Groupe d’étude Génétique des Tumeurs Hépatiques (INSERM Network); et al. Spectrum of HNF1A Somatic Mutations in Hepatocellular Adenoma Differs from That in Patients with MODY3 and Suggests Genotoxic Damage. Diabetes 2010, 59, 1836–1844. [Google Scholar] [CrossRef]
- Pelletier, L.; Rebouissou, S.; Paris, A.; Rathahao-Paris, E.; Perdu, E.; Bioulac-Sage, P.; Imbeaud, S.; Zucman-Rossi, J. Loss of Hepatocyte Nuclear Factor 1alpha Function in Human Hepatocellular Adenomas Leads to Aberrant Activation of Signaling Pathways Involved in Tumorigenesis. Hepatology 2010, 51, 557–566. [Google Scholar] [CrossRef]
- Rebouissou, S.; Imbeaud, S.; Balabaud, C.; Boulanger, V.; Bertrand-Michel, J.; Tercé, F.; Auffray, C.; Bioulac-Sage, P.; Zucman-Rossi, J. HNF1alpha Inactivation Promotes Lipogenesis in Human Hepatocellular Adenoma Independently of SREBP-1 and Carbohydrate-Response Element-Binding Protein (ChREBP) Activation. J. Biol. Chem. 2007, 282, 14437–14446. [Google Scholar] [CrossRef]
- Cappellen, D.; Catry-Thomas, I.; Castain, C.; Bioulac-Sage, P. Hepatocellular Adenoma with a Double Mutation HNF1A and IDH1 in a Patient with Ollier Disease. Liver Int. 2021, 41, 3009–3010. [Google Scholar] [CrossRef]
- Ding, C.-H.; Deng, L.-F.; Chen, F.; Ding, K.; Chen, W.-S.; Xie, W.-F.; Zhang, X.P. Q511L Mutation of HNF1α in Hepatocellular Carcinoma Suppresses the Transcriptional Activity and the Anti-Tumor Effect of HNF1α. Biochem. Biophys. Res. Commun. 2018, 495, 86–91. [Google Scholar] [CrossRef]
- Yasir, S.; Chen, Z.E.; Jain, D.; Kakar, S.; Wu, T.-T.; Yeh, M.M.; Torbenson, M.S. Hepatic Adenomas in Patients 60 and Older Are Enriched for HNF1A Inactivation and Malignant Transformation. Am. J. Surg. Pathol. 2022, 46, 786–792. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Abou-Alfa, G.K.; Stadler, Z.K.; Mandelker, D.L.; Roehrl, M.H.A.; Zehir, A.; Vakiani, E.; Middha, S.; Klimstra, D.S.; Shia, J. Somatic HNF1A Mutations in the Malignant Transformation of Hepatocellular Adenomas: A Retrospective Analysis of Data from MSK-IMPACT and TCGA. Hum. Pathol. 2019, 83, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hepkema, J.T.; Poelmann, F.B.; Gouw, A.S.H.; de Haas, R.J.; Duiker, E.W.; Blokzijl, H.; Klaase, J.M. Malignant Transformation of an HNF1a-Inactivated Hepatocellular Adenoma to Hepatocellular Carcinoma. Case Rep. Gastroenterol. 2020, 14, 577–585. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Singh, A.B.; Cao, A.; Liu, J. Inhibition of PCSK9 Transcription by Berberine Involves Down-Regulation of Hepatic HNF1α Protein Expression through the Ubiquitin-Proteasome Degradation Pathway. J. Biol. Chem. 2015, 290, 4047–4058. [Google Scholar] [CrossRef]
- DeForest, N.; Kavitha, B.; Hu, S.; Isaac, R.; Krohn, L.; Wang, M.; Du, X.; De Arruda Saldanha, C.; Gylys, J.; Merli, E.; et al. Human Gain-of-Function Variants in HNF1A Confer Protection from Diabetes but Independently Increase Hepatic Secretion of Atherogenic Lipoproteins. Cell Genom. 2023, 3, 100339. [Google Scholar] [CrossRef]
- He, J.; Du, C.; Peng, X.; Hong, W.; Qiu, D.; Qiu, X.; Zhang, X.; Qin, Y.; Zhang, Q. Hepatocyte Nuclear Factor 1A Suppresses Innate Immune Response by Inducing Degradation of TBK1 to Inhibit Steatohepatitis. Genes Dis. 2023, 10, 1596–1612. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Qiu, D.; Zhang, Q. HNF1A Regulates the Crosstalk between Innate Immune Responses and MAFLD by Mediating Autophagic Degradation of TBK1. Autophagy 2023, 19, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, A.M.; Wobser, H.; Bonner, C.; Anguissola, S.; Rehm, M.; Concannon, C.G.; Prehn, J.H.M.; Byrne, M.M. Early Loss of Mammalian Target of Rapamycin Complex 1 (mTORC1) Signalling and Reduction in Cell Size during Dominant-Negative Suppression of Hepatic Nuclear Factor 1-Alpha (HNF1A) Function in INS-1 Insulinoma Cells. Diabetologia 2009, 52, 136–144. [Google Scholar] [CrossRef]
- Farrelly, A.M.; Kilbride, S.M.; Bonner, C.; Prehn, J.H.M.; Byrne, M.M. Rapamycin Protects against Dominant Negative-HNF1A-Induced Apoptosis in INS-1 Cells. Apoptosis 2011, 16, 1128–1137. [Google Scholar] [CrossRef]
- Migliorero, M.; Kalantari, S.; Bracciamà, V.; Sorbini, M.; Arruga, F.; Peruzzi, L.; Biamino, E.; Amoroso, A.; Vaisitti, T.; Deaglio, S. A Novel COLEC10 Mutation in a Child with 3MC Syndrome. Eur. J. Med. Genet. 2021, 64, 104374. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A Novel Biological Module-Centric Algorithm to Functionally Analyze Large Gene Lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [PubMed]
- Le Cao, K.-A.; Rohart, F.; Gonzalez, I.; Dejean, S.; Bartolo, F.; Monget, P.; Coquery, J.; Yao, F.; Liquet, B. mixOmics: Omics Data Integration Project; R Package Version 6.1.1.; mixOmics: Melbourne, Australia, 2016. [Google Scholar]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Erratum to: Estimating the Population Abundance of Tissue-Infiltrating Immune and Stromal Cell Populations Using Gene Expression. Genome. Biol. 2016, 17, 249. [Google Scholar] [CrossRef]
- Racle, J.; Gfeller, D. EPIC: A Tool to Estimate the Proportions of Different Cell Types from Bulk Gene Expression Data. Methods Mol. Biol. 2020, 2120, 233–248. [Google Scholar] [CrossRef]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally Portraying the Tissue Cellular Heterogeneity Landscape. Genome. Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
| Sample | Gene | Additional Somatic Mutation | Chromosomic Position (hg37) | Variant Frequency | ACMG Classification |
|---|---|---|---|---|---|
| Normal parenchyma | / | / | / | / | / |
| Adenoma I | / | / | / | / | / |
| Adenoma IV | HNF1A | Deletion of exons 3–10 | chr12:121431312-121613291del | 0.05 | C4 |
| Adenoma V | ARID1A | c.3575_3599del, p.(Asn1192Argfs*6) | chr1:27099336 | 0.15 | C4 |
| Adenoma VI | HNF1A | c.406dup, p.(Thr136Asnfs*52) | chr12:121426714 | 0.35 | C4 |
| Adenoma VII | HNF1A | c.1226del, p.(Pro409Leufs*4) | chr12:121434460 | 0.16 | C4 |
| Adenoma VIII | HNF1A | c.714-1_724del | chr12:121431966-121431977 | 0.07 | C4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faini, A.C.; Arruga, F.; Pinon, M.; Bracciamà, V.; Vallone, F.E.; Mioli, F.; Sorbini, M.; Migliorero, M.; Gambella, A.; Carota, D.; et al. Genomic and Transcriptomic Profile of HNF1A-Mutated Liver Adenomas Highlights Molecular Signature and Potential Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 10483. https://doi.org/10.3390/ijms251910483
Faini AC, Arruga F, Pinon M, Bracciamà V, Vallone FE, Mioli F, Sorbini M, Migliorero M, Gambella A, Carota D, et al. Genomic and Transcriptomic Profile of HNF1A-Mutated Liver Adenomas Highlights Molecular Signature and Potential Therapeutic Implications. International Journal of Molecular Sciences. 2024; 25(19):10483. https://doi.org/10.3390/ijms251910483
Chicago/Turabian StyleFaini, Angelo Corso, Francesca Arruga, Michele Pinon, Valeria Bracciamà, Francesco Edoardo Vallone, Fiorenza Mioli, Monica Sorbini, Martina Migliorero, Alessandro Gambella, Damiano Carota, and et al. 2024. "Genomic and Transcriptomic Profile of HNF1A-Mutated Liver Adenomas Highlights Molecular Signature and Potential Therapeutic Implications" International Journal of Molecular Sciences 25, no. 19: 10483. https://doi.org/10.3390/ijms251910483








