Extraction Condition Optimization, Quantitative Analysis, and Anti-AD Bioactivity Evaluation of Acorn Polyphenols from Quercus variabilis, Quercus aliena, and Quercus dentata
Abstract
:1. Introduction
2. Results
2.1. Determination of Polyphenols in Main Acorns
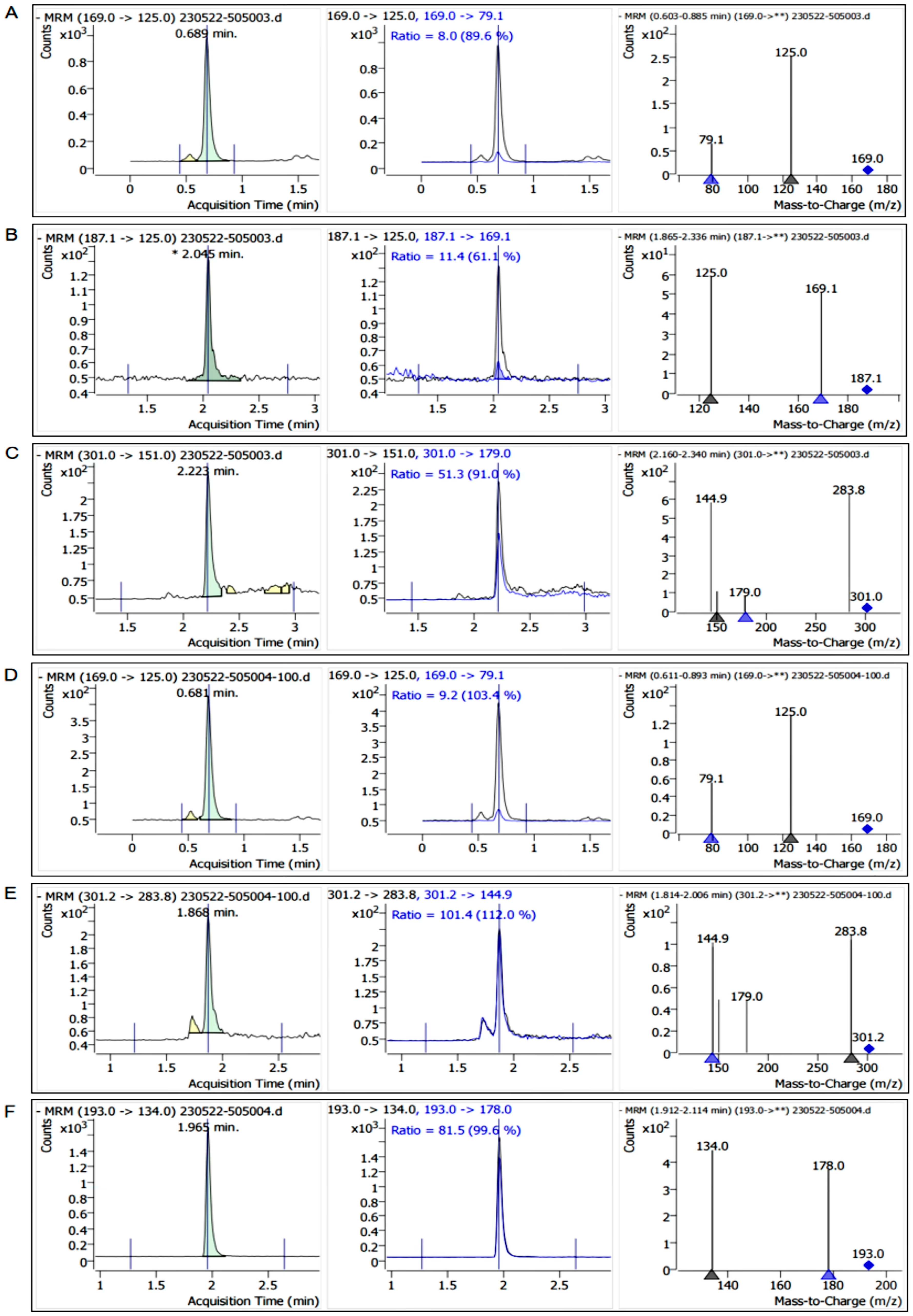
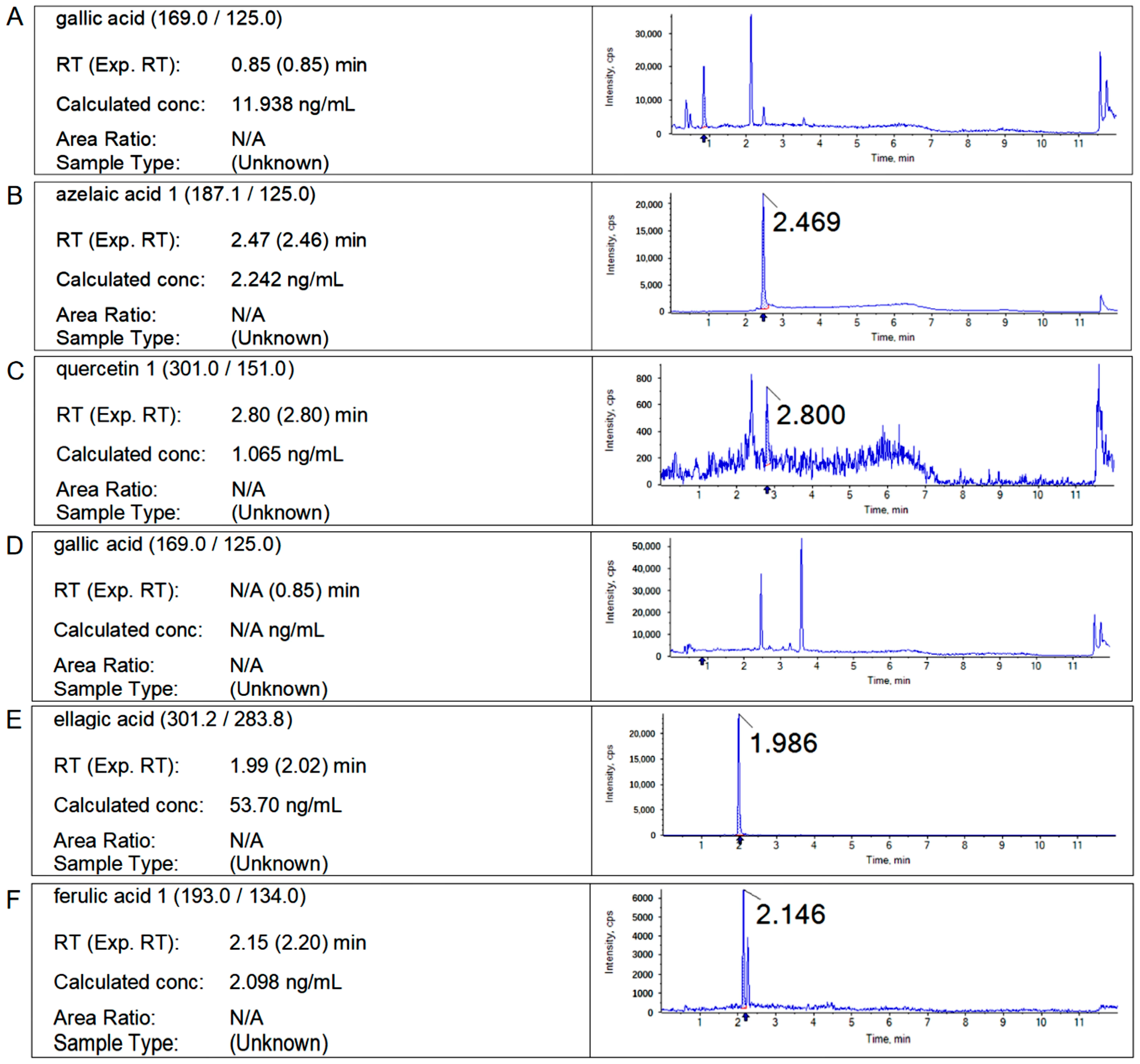
2.2. Results of Liquid Chromatography
2.3. Content Analysis of Main Polyphenols
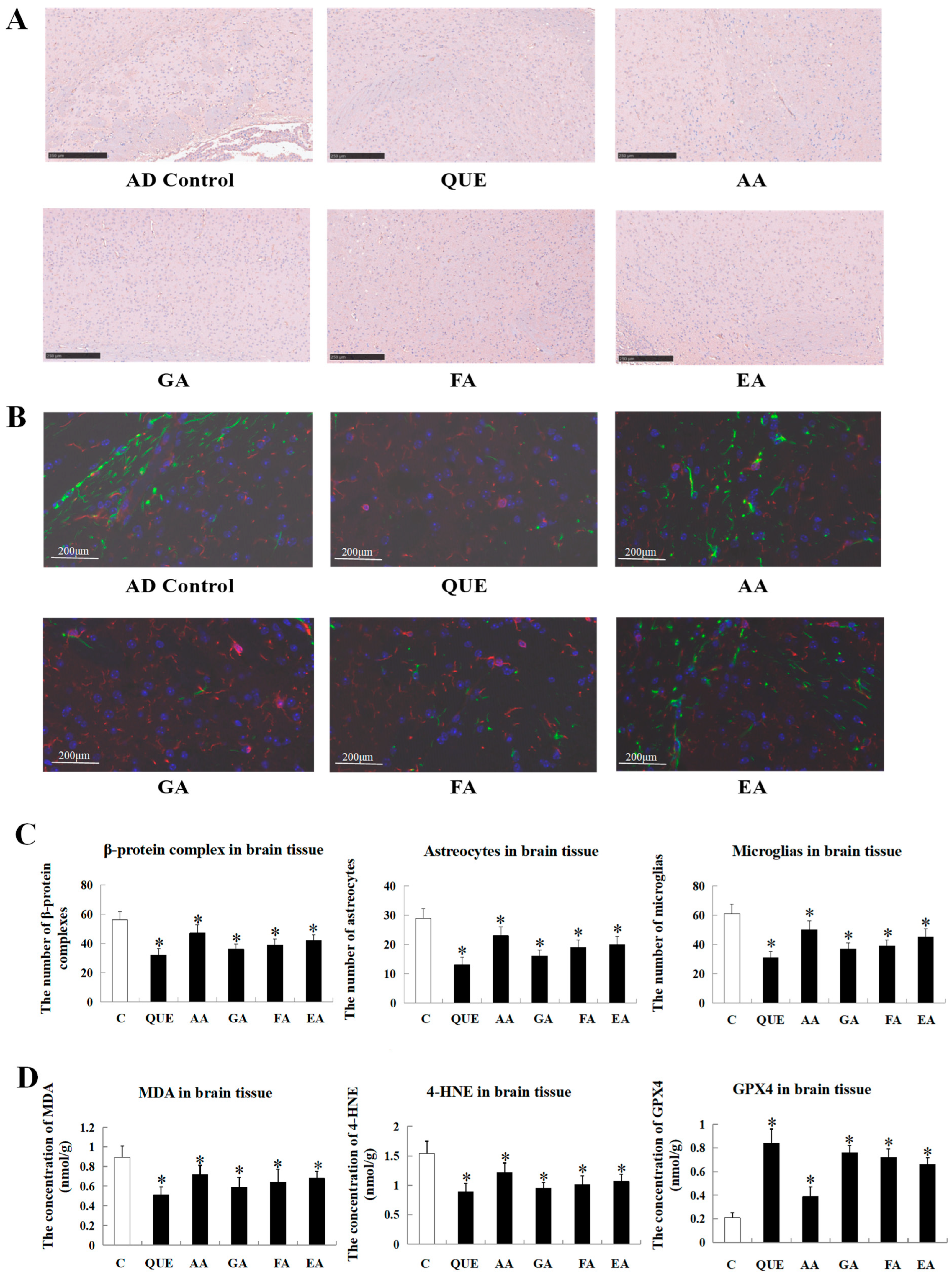
2.4. Comparison of the Effects of Main Acorn Polyphenols on the Progress of the Disease Course in AD Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction and Purification of Free and Bound Polyphenols from Acorns
4.3. Analysis of Free or Bound Polyphenols by HPLC-MC/MS
4.4. Animal Model Construction
4.5. Specific Marker Staining of the Brain Tissue (GFAP/Iba-1 Fluorescence Double Staining and Aβ)
4.6. Detection of MDA, 4-HNE, and GPX4 in Brain Tissue of AD Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Lu, Y.; Song, Z.; Huang, D. Characterizations of the endogenous starch hydrolase inhibitors in Acorns of Quercus fabri Hance. Food Chem. 2018, 258, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł.; Adamczak, A.; Duda, M. Tannin content in acorns (Quercus spp.) from Poland. Dendrobiology 2014, 72, 103–111. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Q.; Wu, Y.; Ouyang, J. Inhibitory effects of acorn (Quercus variabilis Blume) kernel-derived polyphenols on the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase IV. Food Biosci. 2021, 43, 101224. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Morales, D. Oak trees (Quercus spp.) as a source of extracts with biological activities: A narrative review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira JM, F.; Romano, A. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind. Crops Prod. 2013, 45, 114–120. [Google Scholar] [CrossRef]
- Askari, S.F.; Jahromi, B.N.; Dehghanian, A.; Zarei, A.; Tansaz, M.; Badr, P.; Azadi, A.; Mohagheghzadeh, A. Effect of a novel herbal vaginal suppository containing myrtle and oak gall in the treatment of vaginitis: A randomized clinical trial. DARU J. Pharm. Sci. 2020, 28, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jegal, H.; Bong, S.K.; Yoon, K.N.; Park, N.J.; Shin, M.S.; Yang, M.H.; Kim, Y.K.; Kim, S.N. Anti-Atopic Effect of Acorn Shell Extract on Atopic Dermatitis-Like Lesions in Mice and Its Active Phytochemicals. Biomolecules 2020, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mezni, F.; Stiti, B.; Fkiri, S.; Ayari, F.; Ben Slimane, L.; Ksouri, R.; Khaldi, A. Phenolic profile and in vitro anti-diabetic activity of acorn from four African Quercus species (Q. suber, Q. canariensis, Q. coccifera and Q. ilex). S. Afr. J. Bot. 2022, 146, 771–775. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shon, M.S.; Kim, G.N.; Lee, S.C. Antioxidant and anti-adipogenic activities of persimmon tannins. Food Sci. Biotechnol. 2014, 23, 1689–1694. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.H.; Douglas, T.G.; Eicke, L. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free. Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free. Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging: Exp. Clin. Res. 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.U.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef]
- Döner, D.; Icier, F. Exergoeconomic analysis of ultrasound-assisted extraction of tannins from acorn fruit. J. Food Eng. 2023, 367, 111851. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Wang, C.; Chang, T.; Jiang, H. Ultrasound-Homogenization-Assisted Extraction of polyphenols from coconut mesocarp: Optimization study. Ultrason. Sonochemistry 2021, 78, 105739. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2019, 49, 102294. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; Penna, G.L.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 82, S335–S357. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, A.J.; Manivasagam, T.; Essa, M.M. Role of Plant Polyphenols in Alzheimer’s Disease. Adv. Neurobiol. 2016, 12, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Yan, J.; Zhou, Q.; Wang, X. Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols. Int. J. Mol. Sci. 2022, 23, 13886. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Aβ(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Rahaman, M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Prottay, A.A.S.; Rokonuzzman; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 64, 408–419. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Miao, Z.; Jiang, X.; Zheng, Y.; Yang, G. Quercitrin improved cognitive impairment through inhibiting inflammation induced by microglia in Alzheimer’s disease mice. NeuroReport 2022, 33, 327–335. [Google Scholar] [CrossRef]
- Wang, N.Y.; Li, J.N.; Liu, W.L.; Huang, Q.; Li, W.X.; Tan, Y.H.; Liu, F.; Song, Z.H.; Wang, M.Y.; Xie, N.; et al. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2020, 33, 722–732. [Google Scholar] [CrossRef]
- Ahmed, T.; Setzer, W.N.; Fazel Nabavi, S.; Erdogan Orhan, I.; Braidy, N.; Sobarzo-Sanchez, E.; Mohammad Nabavi, S. Insights Into Effects of Ellagic Acid on the Nervous System: A Mini Review. Curr. Pharm. Des. 2016, 22, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]



| Total Phenolic Content (TPC) | Q. variabilis (mg GAE/g d.w.) | Q. aliena (mg GAE/g d.w.) | Q. dentata (mg GAE/g d.w.) |
|---|---|---|---|
| Free polyphenol | 1.32 | 1.07 | 1.21 |
| Bound polyphenol | 133.70 | 64.38 | 8.45 |
| Chemical Compound | Extract of Quercus variabilis Acorn Nutlet (mg/kg) | Extract of Quercus aliena Acorn Kernel (mg/kg) | Extract of Quercus dentata Acorn Kernel (mg/kg) |
|---|---|---|---|
| Free gallic acid | 134.28 | 105.86 | 119.38 |
| Quercetin | 5.63 | 10.74 | 10.65 |
| Azelaic acid | 51.84 | 2.05 | 22.42 |
| Bound gallic acid | 2098.33 | 3971.75 | 0 |
| Ferulic acid | 2217.52 | 620.05 | 2.098 |
| Ellagic acid | 67,537.04 | 29,471.77 | 53.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Han, Z.; Ran, L.; Zhang, T.; Li, J.; Li, H. Extraction Condition Optimization, Quantitative Analysis, and Anti-AD Bioactivity Evaluation of Acorn Polyphenols from Quercus variabilis, Quercus aliena, and Quercus dentata. Int. J. Mol. Sci. 2024, 25, 10536. https://doi.org/10.3390/ijms251910536
Du J, Han Z, Ran L, Zhang T, Li J, Li H. Extraction Condition Optimization, Quantitative Analysis, and Anti-AD Bioactivity Evaluation of Acorn Polyphenols from Quercus variabilis, Quercus aliena, and Quercus dentata. International Journal of Molecular Sciences. 2024; 25(19):10536. https://doi.org/10.3390/ijms251910536
Chicago/Turabian StyleDu, Jianing, Zhengkun Han, Longyi Ran, Taiyu Zhang, Junru Li, and Huiying Li. 2024. "Extraction Condition Optimization, Quantitative Analysis, and Anti-AD Bioactivity Evaluation of Acorn Polyphenols from Quercus variabilis, Quercus aliena, and Quercus dentata" International Journal of Molecular Sciences 25, no. 19: 10536. https://doi.org/10.3390/ijms251910536






