Circulating Matrix Metalloproteinases for Prediction of Aortic Dilatation in Children with Bicuspid Aortic Valve: A Single-Center, Observational Study
Abstract
:1. Introduction
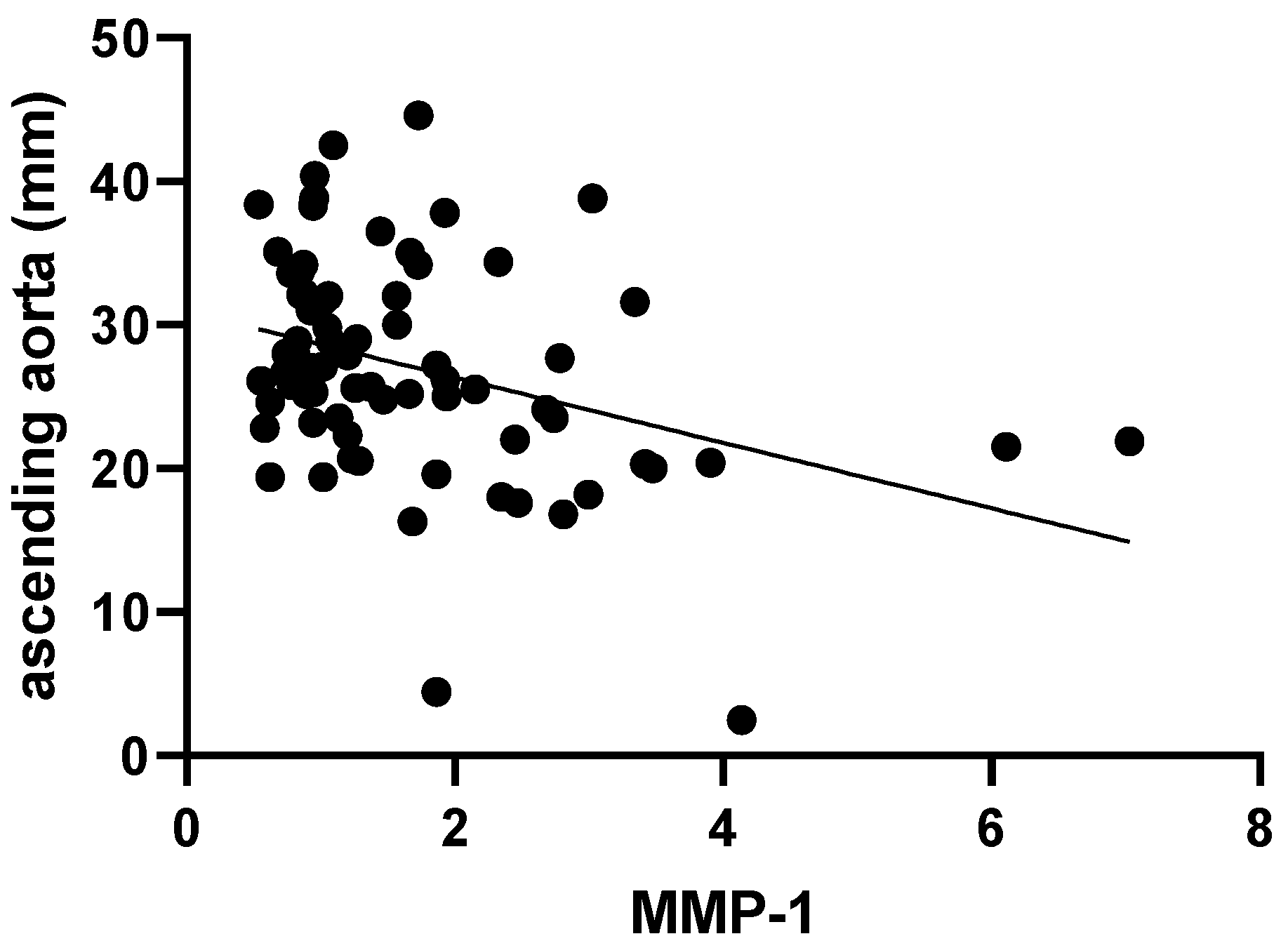
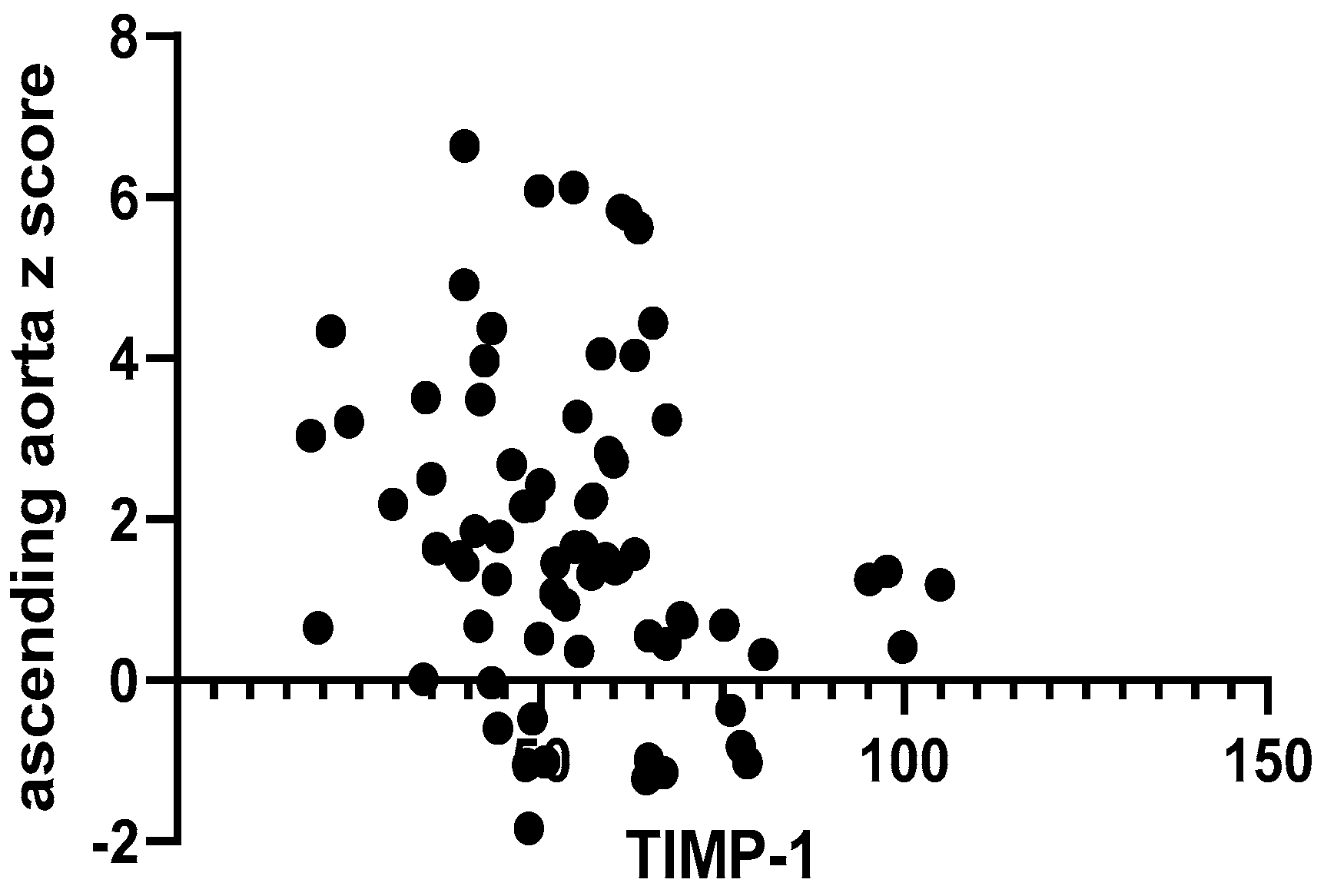
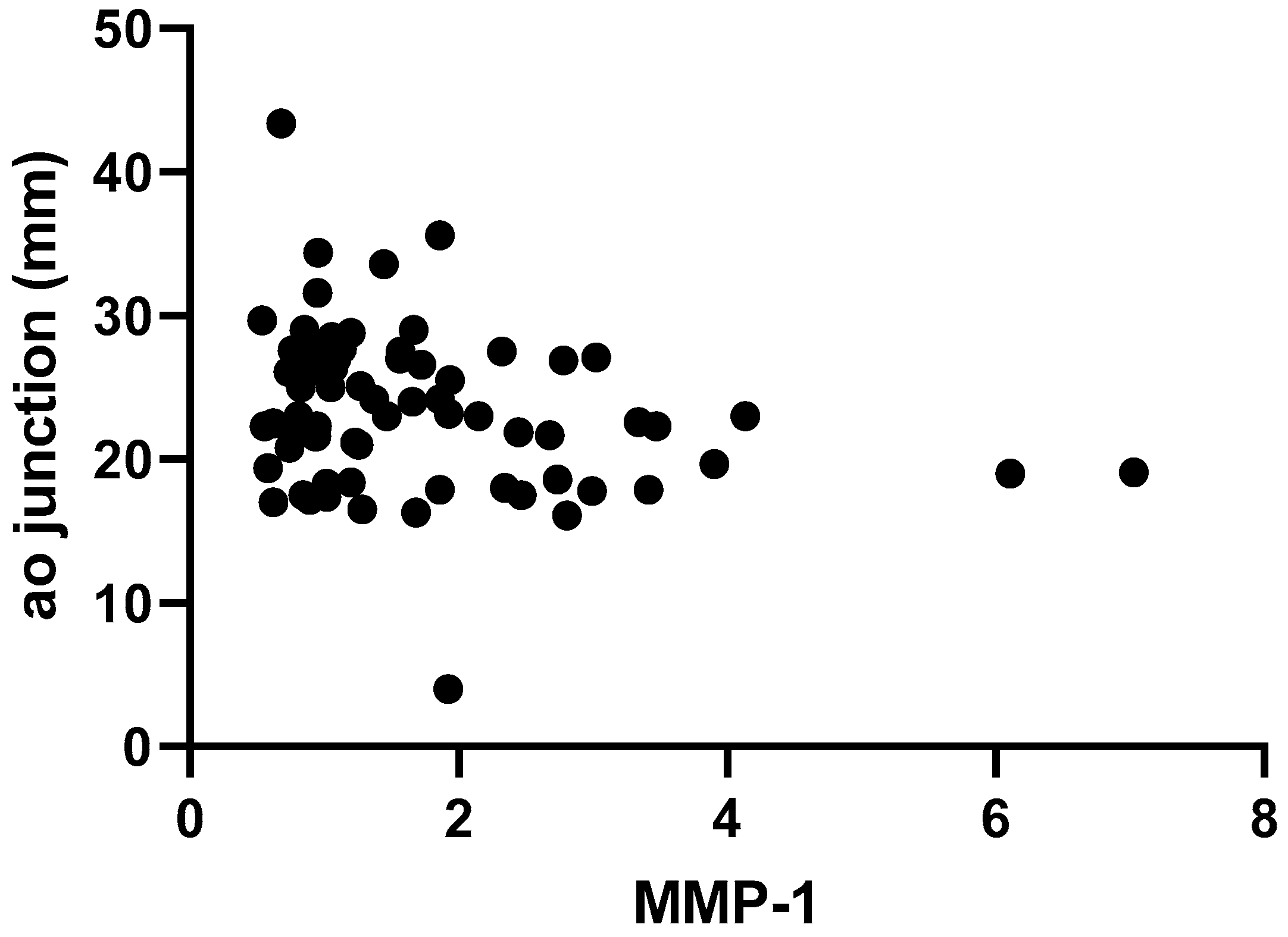
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Cardiac Ultrasound Evaluation
4.3. Serum Biomarker Quantification
4.4. Statistical Analysis
4.5. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaziani, G.; Girolami, F.; Arcieri, L.; Calabri, G.B.; Porcedda, G.; Di Filippo, C.; Surace, F.C.; Pozzi, M.; Favilli, S. Bicuspid Aortic Valve in Children and Adolescents: A Comprehensive Review. Diagnostics 2022, 12, 1751. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, A.-S.; Vøgg, O.; Pihl, C.; Raja, A.A.; Sundberg, K.; Vedel, C.; Zingenberg, H.; Jørgensen, F.S.; Vejlstrup, N.; Iversen, K.; et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA 2021, 325, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Neonatal (and Infant) Coarctation of the Aorta: Management Challenges. Res. Rep. Neonatol. 2020, 10, 11–22. [Google Scholar] [CrossRef]
- Joshi, G.; Skinner, G.; Shebani, S.O. Presentation of Coarctation of the Aorta in the Neonates and the Infant with Short and Long Term Implications. Paediatr. Child Health 2017, 27, 83–89. [Google Scholar] [CrossRef]
- Doshi, A.R.; Chikkabyrappa, S. Coarctation of Aorta in Children. Cureus 2018, 10, e3690. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Klautz, R.J.M.; Poelmann, R.E. Intrinsic Histological and Morphological Abnormalities of the Pediatric Thoracic Aorta in Bicuspid Aortic Valve Patients Are Predictive for Future Aortopathy. Pathol. Res. Pract. 2023, 248, 154620. [Google Scholar] [CrossRef]
- De Cario, R.; Giannini, M.; Cassioli, G.; Kura, A.; Gori, A.M.; Marcucci, R.; Nistri, S.; Pepe, G.; Giusti, B.; Sticchi, E. Tracking an Elusive Killer: State of the Art of Molecular-Genetic Knowledge and Laboratory Role in Diagnosis and Risk Stratification of Thoracic Aortic Aneurysm and Dissection. Diagnostics 2022, 12, 1785. [Google Scholar] [CrossRef] [PubMed]
- Grattan, M.; Prince, A.; Rumman, R.K.; Morgan, C.; Petrovic, M.; Hauck, A.; Young, L.; Franco-Cereceda, A.; Loeys, B.; Mohamed, S.A.; et al. Predictors of Bicuspid Aortic Valve-Associated Aortopathy in Childhood: A Report from the MIBAVA Consortium. Circ. Cardiovasc. Imaging 2020, 13, e009717. [Google Scholar] [CrossRef] [PubMed]
- Padang, R.; Bannon, P.G.; Jeremy, R.; Richmond, D.R.; Semsarian, C.; Vallely, M.; Wilson, M.; Yan, T.D. The Genetic and Molecular Basis of Bicuspid Aortic Valve Associated Thoracic Aortopathy: A Link to Phenotype Heterogeneity. Ann. Cardiothorac. Surg. 2013, 2, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Pugh, N.L.; Devereux, R.B.; Eagle, K.A.; Holmes, K.; LeMaire, S.A.; Milewski, R.K.; Morris, S.A.; Prakash, S.K.; Pyeritz, R.E.; et al. Aortic Dilatation Associated with Bicuspid Aortic Valve: Relation to Sex, Hemodynamics, and Valve Morphology (the National Heart Lung and Blood Institute-Sponsored National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions). Am. J. Cardiol. 2017, 120, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Bunton, T.E.; Biery, N.J.; Myers, L.; Gayraud, B.; Ramirez, F.; Dietz, H.C. Phenotypic Alteration of Vascular Smooth Muscle Cells Precedes Elastolysis in a Mouse Model of Marfan Syndrome. Circ. Res. 2001, 88, 37–43. [Google Scholar] [CrossRef]
- Fedak, P.W.M.; de Sa, M.P.L.; Verma, S.; Nili, N.; Kazemian, P.; Butany, J.; Strauss, B.H.; Weisel, R.D.; David, T.E. Vascular Matrix Remodeling in Patients with Bicuspid Aortic Valve Malformations: Implications for Aortic Dilatation. J. Thorac. Cardiovasc. Surg. 2003, 126, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Braverman, A.C. Bicuspid Aortic Valve and Associated Aortic Wall Abnormalities. Curr. Opin. Cardiol. 1996, 11, 501–503. [Google Scholar] [CrossRef]
- Della Corte, A.; Quarto, C.; Bancone, C.; Castaldo, C.; Di Meglio, F.; Nurzynska, D.; De Santo, L.S.; De Feo, M.; Scardone, M.; Montagnani, S.; et al. Spatiotemporal Patterns of Smooth Muscle Cell Changes in Ascending Aortic Dilatation with Bicuspid and Tricuspid Aortic Valve Stenosis: Focus on Cell-Matrix Signaling. J. Thorac. Cardiovasc. Surg. 2008, 135, 8–18.e2. [Google Scholar] [CrossRef]
- Grewal, N.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; Klautz, R.J.M.; Lindeman, J.H.N.; Goumans, M.-J.; Palmen, M.; Mohamed, S.A.; Sievers, H.-H.; Bogers, A.J.J.C.; et al. Ascending Aorta Dilation in Association with Bicuspid Aortic Valve: A Maturation Defect of the Aortic Wall. J. Thorac. Cardiovasc. Surg. 2014, 148, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Franken, R.; Mulder, B.J.M.; Goumans, M.-J.; Lindeman, J.H.N.; Jongbloed, M.R.M.; DeRuiter, M.C.; Klautz, R.J.M.; Bogers, A.J.J.C.; Poelmann, R.E.; et al. Histopathology of Aortic Complications in Bicuspid Aortic Valve versus Marfan Syndrome: Relevance for Therapy? Heart Vessel. 2016, 31, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Koullias, G.J.; Korkolis, D.P.; Ravichandran, P.; Psyrri, A.; Hatzaras, I.; Elefteriades, J.A. Tissue Microarray Detection of Matrix Metalloproteinases, in Diseased Tricuspid and Bicuspid Aortic Valves with or without Pathology of the Ascending Aorta. Eur. J. Cardiothorac. Surg. 2004, 26, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Rossé, C.; Lodillinsky, C.; Fuhrmann, L.; Nourieh, M.; Monteiro, P.; Irondelle, M.; Lagoutte, E.; Vacher, S.; Waharte, F.; Paul-Gilloteaux, P.; et al. Control of MT1-MMP Transport by Atypical PKC during Breast-Cancer Progression. Proc. Natl. Acad. Sci. USA 2014, 111, E1872–E1879. [Google Scholar] [CrossRef] [PubMed]
- Okusha, Y.; Eguchi, T.; Tran, M.T.; Sogawa, C.; Yoshida, K.; Itagaki, M.; Taha, E.A.; Ono, K.; Aoyama, E.; Okamura, H.; et al. Extracellular Vesicles Enriched with Moonlighting Metalloproteinase Are Highly Transmissive, Pro-Tumorigenic, and Trans-Activates Cellular Communication Network Factor (CCN2/CTGF): CRISPR against Cancer. Cancers 2020, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Membrane-Type Matrix Metalloproteinases: Their Functions and Regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Holmes, D.R.; Mertens, R.A.; Liao, S.; Botney, M.D.; Mecham, R.P.; Welgus, H.G.; Parks, W.C. Production and Localization of 92-Kilodalton Gelatinase in Abdominal Aortic Aneurysms. An Elastolytic Metalloproteinase Expressed by Aneurysm-Infiltrating Macrophages. J. Clin. Investig. 1995, 96, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Keiser, J.A. Expression of Membrane-Type Matrix Metalloproteinase in Rabbit Neointimal Tissue and Its Correlation with Matrix-Metalloproteinase-2 Activation. J. Vasc. Res. 1998, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, X.; Gardashova, G.; Yang, Y.; Zhang, Y.; Xu, L.; Zeng, Y. Molecular and Functional Extracellular Vesicle Analysis Using Nanopatterned Microchips Monitors Tumor Progression and Metastasis. Sci. Transl. Med. 2020, 12, eaaz2878. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Lee, J.; Basu, R.; Sakamuri, S.S.V.P.; Wang, X.; Fan, D.; Kassiri, Z. Divergent Roles of Matrix Metalloproteinase 2 in Pathogenesis of Thoracic Aortic Aneurysm. Arter. Thromb. Vasc. Biol. 2015, 35, 888–898. [Google Scholar] [CrossRef]
- Ravn, H.B.; Falk, E. Histopathology of Plaque Rupture. Cardiol. Clin. 1999, 17, 263–270. [Google Scholar] [CrossRef]
- Pasta, S.; Agnese, V.; Gallo, A.; Cosentino, F.; Di Giuseppe, M.; Gentile, G.; Raffa, G.M.; Maalouf, J.F.; Michelena, H.I.; Bellavia, D.; et al. Shear Stress and Aortic Strain Associations With Biomarkers of Ascending Thoracic Aortic Aneurysm. Ann. Thorac. Surg. 2020, 110, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix Metalloproteinases 2 and 9 Work in Concert to Produce Aortic Aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef]
- Soto-Navarrete, M.T.; Pozo-Vilumbrales, B.; López-Unzu, M.Á.; Rueda-Martínez, C.; Fernández, M.C.; Durán, A.C.; Pavón-Morón, F.J.; Rodríguez-Capitán, J.; Fernández, B. Experimental Evidence of the Genetic Hypothesis on the Etiology of Bicuspid Aortic Valve Aortopathy in the Hamster Model. Front. Cardiovasc. Med. 2022, 9, 928362. [Google Scholar] [CrossRef]
- Făgărășan, A.; Săsăran, M.O. The Predictive Role of Plasma Biomarkers in the Evolution of Aortopathies Associated with Congenital Heart Malformations. Int. J. Mol. Sci. 2022, 23, 4993. [Google Scholar] [CrossRef]
- DeCoux, A.; Lindsey, M.L.; Villarreal, F.; Garcia, R.A.; Schulz, R. Myocardial Matrix Metalloproteinase-2: Inside out and Upside Down. J. Mol. Cell. Cardiol. 2014, 77, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Pasipoularides, A. Clinical-Pathological Correlations of BAV and the Attendant Thoracic Aortopathies. Part 2: Pluridisciplinary Perspective on Their Genetic and Molecular Origins. J. Mol. Cell. Cardiol. 2019, 133, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Sticchi, E.; De Cario, R.; Magi, A.; Giglio, S.; Provenzano, A.; Nistri, S.; Pepe, G.; Giusti, B. Bicuspid Aortic Valve: Role of Multiple Gene Variants in Influencing the Clinical Phenotype. BioMed Res. Int. 2018, 2018, 8386123. [Google Scholar] [CrossRef] [PubMed]
- Giusti, B.; Sticchi, E.; De Cario, R.; Magi, A.; Nistri, S.; Pepe, G. Genetic Bases of Bicuspid Aortic Valve: The Contribution of Traditional and High-Throughput Sequencing Approaches on Research and Diagnosis. Front. Physiol. 2017, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.M.; Sanders, S.P.; Khairy, P.; Jenkins, K.J.; Gauvreau, K.; Lang, P.; Simonds, H.; Colan, S.D. Morphology of Bicuspid Aortic Valve in Children and Adolescents. J. Am. Coll. Cardiol. 2004, 44, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Khairy, P.; Graham, D.A.; Colan, S.D.; Galvin, T.C.; Sanders, S.P.; Singh, M.N.; Bhatt, A.; Lacro, R.V. Bicuspid Aortic Valve and Associated Aortic Dilation in the Young. Heart 2012, 98, 1014–1019. [Google Scholar] [CrossRef]
- Kong, W.K.F.; Regeer, M.V.; Poh, K.K.; Yip, J.W.; van Rosendael, P.J.; Yeo, T.C.; Tay, E.; Kamperidis, V.; van der Velde, E.T.; Mertens, B.; et al. Inter-Ethnic Differences in Valve Morphology, Valvular Dysfunction, and Aortopathy between Asian and European Patients with Bicuspid Aortic Valve. Eur. Heart J. 2018, 39, 1308–1313. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic Activity in Amphibian Tissues: A Tissue Culture Assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Maybee, D.V.; Ink, N.L.; Ali, M.A.M. Novel Roles of MT1-MMP and MMP-2: Beyond the Extracellular Milieu. Int. J. Mol. Sci. 2022, 23, 9513. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.M.; Khalil, R.A. Matrix Metalloproteinase Inhibitors as Investigative Tools in the Pathogenesis and Management of Vascular Disease. Exp. Suppl. 2012, 103, 209–279. [Google Scholar] [CrossRef] [PubMed]
- Tamarina, N.A.; McMillan, W.D.; Shively, V.P.; Pearce, W.H. Expression of Matrix Metalloproteinases and Their Inhibitors in Aneurysms and Normal Aorta. Surgery 1997, 122, 264–271, discussion 271–272. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, J.S.; Ivey, C.R.; Wheeler, J.B.; Akerman, A.W.; Rice, A.; Patel, R.K.; Stroud, R.E.; Shah, A.A.; Hughes, C.G.; Ferrari, G.; et al. Plasma Biomarkers for Distinguishing Etiological Subtypes of Thoracic Aortic Aneurysm Disease. J. Thorac. Cardiovasc. Surg. 2013, 145, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Liao, M.; Zou, S.; Bao, Y.; Jin, J.; Yang, J.; Liu, Y.; Green, M.; Yang, F.; Qu, L. Matrix Metalloproteinases Are Regulated by MicroRNA 320 in Macrophages and Are Associated with Aortic Dissection. Exp. Cell Res. 2018, 370, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Choi, J.C.; Minard, C.G.; Hou, X.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. Matrix Metalloproteinase Levels in Chronic Thoracic Aortic Dissection. J. Surg. Res. 2014, 189, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgi, G.; D’Averio, R.; Mauriello, A.; Bondio, M.; Pontillo, M.; Castelvecchio, S.; Trimarchi, S.; Tolva, V.; Nano, G.; Rampoldi, V.; et al. Plasma Levels of Metalloproteinases-3 and -9 as Markers of Successful Abdominal Aortic Aneurysm Exclusion after Endovascular Graft Treatment. Circulation 2001, 104, I288–I295. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, A.E.; Rubin, B.G.; Sanchez, L.A.; Geraghty, P.A.; Thompson, R.W.; Curci, J.A. A Randomized, Placebo-Controlled Trial of Doxycycline after Endoluminal Aneurysm Repair. J. Vasc. Surg. 2008, 48, 519–526, discussion 526. [Google Scholar] [CrossRef]
- Hanedan Onan, S.; Baykan, A.; Sezer, S.; Narin, F.; Mavili, E.; Baykan, Z.; Uzum, K.; Narin, N. Evaluation of Cardiovascular Changes in Children with BAVs. Pediatr. Cardiol. 2016, 37, 472–481. [Google Scholar] [CrossRef]
- Irqsusi, M.; Dong, L.A.; Rodepeter, F.R.; Ramzan, R.; Talipov, I.; Ghazy, T.; Günther, M.; Vogt, S.; Rastan, A.J. The Role of Matrix Metalloproteinases in Thoracic Aortic Disease: Are They Indicators for the Pathogenesis of Dissections? Biomedicines 2024, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Tzemos, N.; Lyseggen, E.; Silversides, C.; Jamorski, M.; Tong, J.H.; Harvey, P.; Floras, J.; Siu, S. Endothelial Function, Carotid-Femoral Stiffness, and Plasma Matrix Metalloproteinase-2 in Men with Bicuspid Aortic Valve and Dilated Aorta. J. Am. Coll. Cardiol. 2010, 55, 660–668. [Google Scholar] [CrossRef]
- Spaziani, G.; Bonanni, F.; Girolami, F.; Bennati, E.; Calabri, G.B.; Di Filippo, C.; Porcedda, G.; Passantino, S.; Nistri, S.; Olivotto, I.; et al. Aortic Dilatation in Pediatric Patients with Bicuspid Aortic Valve: How the Choice of Nomograms May Change Prevalence. Diagnostics 2023, 13, 1490. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Enriquez-Sarano, M.; Bax, J.J.; Otto, C.M.; Schäfers, H.-J. Speaking a Common Language: Introduction to a Standard Terminology for the Bicuspid Aortic Valve and Its Aortopathy. Prog. Cardiovasc. Dis. 2020, 63, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Giordano, R.; Scalese, M.; Murzi, B.; Assanta, N.; Spadoni, I.; Maura, C.; Marco, M.; Molinaro, S.; Kutty, S.; et al. Nomograms for Two-Dimensional Echocardiography Derived Valvular and Arterial Dimensions in Caucasian Children. J. Cardiol. 2017, 69, 208–215. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23, quiz 101–102. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Group 1 (n = 43) | Group 2 (n = 30) | p Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 12.01 ± 3.54 | 13.42 ± 4.11 | 0.07 | |
| Gender | Male (%) | 38.35 | 36.98 | p = 0.01, OR = 0.2 |
| Female (%) | 20.54 | 4.10 | ||
| Background | Urban (%) | 35.61 | 26.02 | p = 0.80, OR = 0.88 |
| Rural (%) | 23.28 | 15.06 | ||
| BMI (mean ± SD) | 19.43 ± 4.49 | 20.02 ± 4.06 | 0.39 | |
| Body surface (mean ± SD) * | 1.44 ± 0.40 | 1.54 ± 0.40 | 0.30 | |
| Aortic stenosis (n = 38) | Mild (%) | 21.05 | 36.84 | 0.03 |
| Moderate (%) | 18.42 | 2.63 | ||
| Severe (%) | 7.89 | 13.15 | ||
| Aortic insufficiency (n = 61) | Mild (%) | 52.45 | 29.50 | 0.12 |
| Moderate (%) | 6.55 | 8.19 | ||
| Severe (%) | 0 | 3.27 | ||
| Aortic annulus (mm, mean ± SD) | 21.09 ± 3.97 | 23.83 ± 4.42 | <0.01 | |
| Aortic annulus (z score, mean ± SD) | 1.26 ± 1.10 | 2.28 ± 1.72 | <0.01 | |
| Aortic sinus (mm, mean ± SD) | 24.82 ± 4.90 | 26.84 ± 8.18 | 0.23 | |
| Aortic sinus (z score, mean ± SD) | 0.01 ± 1.43 | 1.24 ± 1.87 | <0.01 | |
| Aortic junction (mm, mean ± SD) | 22.01 ± 4.11 | 25.95 ± 6.87 | <0.01 | |
| Aortic junction (z score, mean ± SD) | 1.11 ± 1.05 | 2.81 ± 1.56 | <0.01 | |
| Ascending aorta (mm, mean ± SD) | 24.26 ± 5.95 | 31.09 ± 8.09 | <0.01 | |
| Ascending aorta (z score, mean ± SD) | 0.83 ± 1.23 | 3.36 ± 1.94 | <0.01 | |
| EF (%, mean ± SD) | 66.63 ± 7.78 | 68.17 ± 8.17 | 0.42 | |
| Left ventricle diameter (mean ± SD) | 4.12 ± 0.60 | 4.54 ± 0.84 | 0.03 | |
| Fractional shortening (%, mean ± SD) | 38.16 ± 7.71 | 38.52 ± 7.34 | 0.84 | |
| Aortic phenotype | 0 (%) | 17.80 | 15.06 | 0.61 |
| IB (%) | 34.24 | 19.17 | ||
| IA/IC/II (%) | 6.84 | 6.84 | ||
| Variable | Aortic Phenotype | p Value | |||
|---|---|---|---|---|---|
| 0 (n = 22) | IB (n = 39) | IA/IC/II (n = 12) | |||
| Gender | Female (%) | 8.21 | 15.06 | 1.36 | 0.49 |
| Male (%) | 24.65 | 38.35 | 12.32 | ||
| Aortic stenosis | Yes (%) | 20.54 | 20.54 | 10.95 | 0.04 |
| No (%) | 9.58 | 32.87 | 5.47 | ||
| 0 (n = 17) | IB (n = 15) | IA/IC/II (n = 6) | |||
| Aortic stenosis | Mild (%) | 21.05 | 23.68 | 13.15 | 0.31 |
| Moderate (%) | 7.89 | 10.52 | 2.63 | ||
| Severe (%) | 17.14 | 5.26 | 0 | ||
| Parameter | Female Patients (n = 18) | Male Patients (n = 55) | p Value |
|---|---|---|---|
| MMP-1 (pg/mL, mean ± SD) | 1.79 ± 1.74 | 1.73 ± 1.15 | 0.42 |
| MMP-2 (pg/mL, mean ± SD) | 143.1 ± 20.62 | 142.5 ± 30.31 | 0.49 |
| MMP-9 (pg/mL, mean ± SD) | 117.9 ± 52.21 | 128.2 ± 74.95 | 0.92 |
| TIMP-1 * (pg/mL, mean ± SD) | 57.53 ± 15.75 | 53.60 ± 18.12 | 0.41 |
| Parameter | Group 1 (n = 43) | Group 2 (n = 30) | p Value |
|---|---|---|---|
| NT-pro-BNP (ng/mL, mean ± SD) | 22.51 ± 19.16 | 39.66 ± 53.58 | 0.37 |
| MMP-1 (pg/mL, mean ± SD) | 1.86 ± 1.39 | 1.43 ± 0.78 | 0.27 |
| MMP-2 (pg/mL, mean ± SD) | 144.6 ± 24.23 | 139.9 ± 33.11 | 0.11 |
| MMP-9 (pg/mL, mean ± SD) | 132.5 ± 78.54 | 115.8 ± 54.83 | 0.31 |
| TIMP-1 * (pg/mL, mean ± SD) | 57.87 ± 18.29 | 49.84 ± 15.49 | 0.04 |
| Vitamin D (ng/L, mean ± SD) | 39.57 ± 21.89 | 39.29 ± 24.07 | 0.93 |
| CRP (mg/dL, mean ± SD) | 0.34 ± 0.20 | 0.42 ± 0.22 | 0.28 |
| Parameter | Group A (n = 18) | Group B (n = 20) | p Value |
|---|---|---|---|
| NT-pro-BNP (ng/mL, mean ± SD) | 14.87 ± 13.75 | 33.54 ± 54.44 | 0.22 |
| MMP-1 (pg/mL, mean ± SD) | 2.06 ± 1.84 | 1.50 ± 0.89 | 0.61 |
| MMP-2 (pg/mL, mean ± SD) | 144.1 ± 19.05 | 140 ± 37.97 | 0.15 |
| MMP-9 (pg/mL, mean ± SD) | 139.4 ± 73.78 | 109.1 ± 52.93 | 0.23 |
| TIMP-1 * (pg/mL, mean ± SD) | 53.90 ± 14.24 | 49.28 ± 13.73 | 0.31 |
| Vitamin D (ng/L, mean ± SD) | 42.25 ± 21.99 | 38.97 ± 22.92 | 0.87 |
| CRP (mg/dL, mean ± SD) | 0.28 ± 0.16 | 0.31 ± 0.24 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Făgărășan, A.; Săsăran, M.O.; Gozar, L.; Toma, D.; Șuteu, C.; Ghiragosian-Rusu, S.; Al-Akel, F.C.; Szabo, B.; Huțanu, A. Circulating Matrix Metalloproteinases for Prediction of Aortic Dilatation in Children with Bicuspid Aortic Valve: A Single-Center, Observational Study. Int. J. Mol. Sci. 2024, 25, 10538. https://doi.org/10.3390/ijms251910538
Făgărășan A, Săsăran MO, Gozar L, Toma D, Șuteu C, Ghiragosian-Rusu S, Al-Akel FC, Szabo B, Huțanu A. Circulating Matrix Metalloproteinases for Prediction of Aortic Dilatation in Children with Bicuspid Aortic Valve: A Single-Center, Observational Study. International Journal of Molecular Sciences. 2024; 25(19):10538. https://doi.org/10.3390/ijms251910538
Chicago/Turabian StyleFăgărășan, Amalia, Maria Oana Săsăran, Liliana Gozar, Daniela Toma, Carmen Șuteu, Simina Ghiragosian-Rusu, Flavia Cristina Al-Akel, Boglarka Szabo, and Adina Huțanu. 2024. "Circulating Matrix Metalloproteinases for Prediction of Aortic Dilatation in Children with Bicuspid Aortic Valve: A Single-Center, Observational Study" International Journal of Molecular Sciences 25, no. 19: 10538. https://doi.org/10.3390/ijms251910538






