Mesenchymal Stem Cell Therapy: Therapeutic Opportunities and Challenges for Diabetic Kidney Disease
Abstract
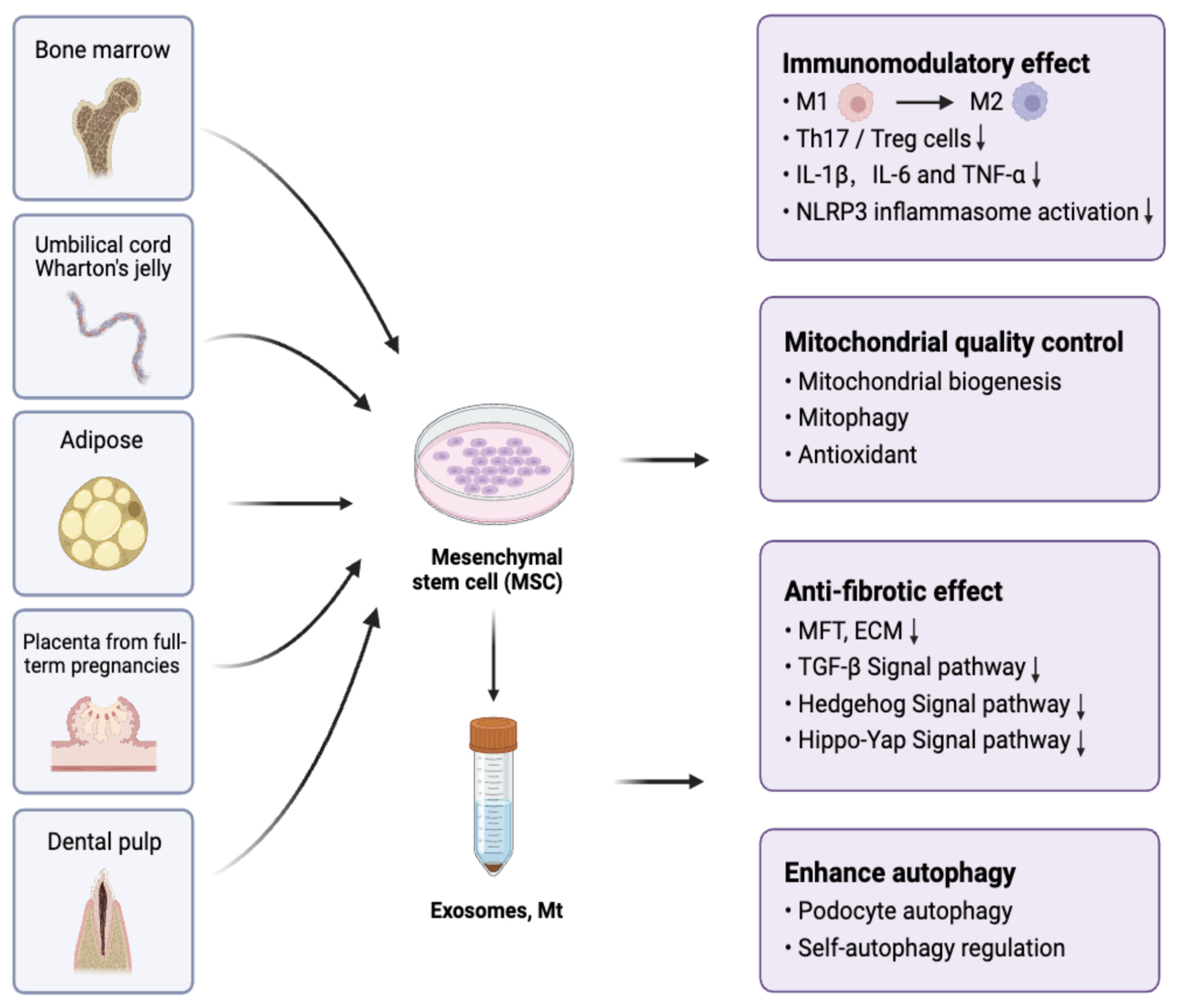
:1. Introduction
2. The Use of MSC Therapy in DKD
2.1. Preclinical Study
2.2. MSC Therapies Toward Clinical Translation
3. The Mechanism of MSC Therapy in Treating DKD
3.1. Immunomodulatory Effect
3.2. Mitochondrial Quality Control
3.3. Antifibrotic Effect
3.4. Enhance Autophagy
4. The Signaling Pathways Involved in MSC Treatment of DKD
5. MSC Therapy and Traditional Drug Treatment
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ricciardi, C.A.; Gnudi, L. Kidney disease in diabetes: From mechanisms to clinical presentation and treatment strategies. Metabolism 2021, 124, 154890. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell 2008, 2, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef] [PubMed]
- Plumas, J.; Chaperot, L.; Richard, M.J.; Molens, J.P.; Bensa, J.C.; Favrot, M.C. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia 2005, 19, 1597–1604. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur. Respir. J. 2021, 58, 2002978. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Little, M.H. Stromal cells in tissue homeostasis: Balancing regeneration and fibrosis. Nat. Rev. Nephrol. 2013, 9, 747–753. [Google Scholar] [CrossRef]
- Penn, P.E.; Jiang, D.Z.; Fei, R.G.; Sitnicka, E.; Wolf, N.S. Dissecting the hematopoietic microenvironment. IX. Further characterization of murine bone marrow stromal cells. Blood 1993, 81, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Panepucci, R.A.; Siufi, J.L.C.; Silva, W.A.; Proto-Siquiera, R.; Neder, L.; Orellana, M.; Rocha, V.; Covas, D.T.; Zago, M.A. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 2004, 22, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.-D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; AlTalabani, A.A.; Knawy, B.A. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. Rep. 2013, 9, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, I.; Hwang, S.H.; Han, H.; Ha, H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res. Clin. Pract. 2012, 98, 465–473. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 2006, 103, 17438–17443. [Google Scholar] [CrossRef]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.-H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, C.; Yang, C.; Zhang, J.; Dong, Q.; Wang, W. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018, 215, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-L.; Liu, Y.-J.; Yang, S.-G.; Zhao, Q.-J.; Wang, X.; Gong, W.; Han, Z.-B.; Xu, Z.-S.; Lu, Y.-X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar] [PubMed]
- Yuan, Y.; Li, L.; Zhu, L.; Liu, F.; Tang, X.; Liao, G.; Liu, J.; Cheng, J.; Chen, Y.; Lu, Y. Mesenchymal stem cells elicit macrophages into M2 phenotype via improving transcription factor EB-mediated autophagy to alleviate diabetic nephropathy. Stem Cells 2020, 38, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Sávio-Silva, C.; Soinski-Sousa, P.E.; Simplício-Filho, A.; Bastos, R.M.C.; Beyerstedt, S.; Rangel, É.B. Therapeutic Potential of Mesenchymal Stem Cells in a Pre-Clinical Model of Diabetic Kidney Disease and Obesity. Int. J. Mol. Sci. 2021, 22, 1546. [Google Scholar] [CrossRef] [PubMed]
- Konari, N.; Nagaishi, K.; Kikuchi, S.; Fujimiya, M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci. Rep. 2019, 9, 5184. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, L.; Fan, Y.; Li, Y. Marrow mesenchymal stem cell mediates diabetic nephropathy progression via modulation of Smad2/3/WTAP/m6A/ENO1 axis. FASEB J. 2024, 38, e23729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, C.; Wen, X.; Chen, Y.; Mao, R.; Cui, D.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; et al. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103+ DCs-mediated CD8+ T cell responses. J. Cell Mol. Med. 2020, 24, 5817–5831. [Google Scholar] [CrossRef]
- Lin, L.; Lin, H.; Wang, D.; Bao, Z.; Cai, H.; Zhang, X. Bone marrow mesenchymal stem cells ameliorated kidney fibrosis by attenuating TLR4/NF-κB in diabetic rats. Life Sci. 2020, 262, 118385. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, J.; He, Z.; Yang, M.; Li, L.; Jiang, H. Mesenchymal Stem Cells Reverse Diabetic Nephropathy Disease via Lipoxin A4 by Targeting Transforming Growth Factor β (TGF-β)/smad Pathway and Pro-Inflammatory Cytokines. Med. Sci. Monit. 2019, 25, 3069–3076. [Google Scholar] [CrossRef]
- Tang, L.-X.; Wei, B.; Jiang, L.-Y.; Ying, Y.-Y.; Li, K.; Chen, T.-X.; Huang, R.-F.; Shi, M.-J.; Xu, H. Intercellular mitochondrial transfer as a means of revitalizing injured glomerular endothelial cells. World J. Stem Cells 2022, 14, 729–743. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Zhao, X.; Yang, X.; Wan, X.; An, Z.; Zhang, H.; Tian, J.; Ge, C.; Song, X. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Diabetic Kidney Disease in Rats by Inhibiting Apoptosis and Inflammation. Front. Biosci. (Landmark Ed.) 2023, 28, 203. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N.; Ahmed, I.A.; Hussien, N.I.; Dessouky, A.A.; Farid, A.S.; Elshazly, A.M.; Mostafa, O.; Gazzar, W.B.E.; Sorour, S.M.; Seleem, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells 2018, 7, 226. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Liao, G.; Chen, Y.; Luo, A.; Liu, J.; Yuan, Y.; Li, L.; Yang, L.; Wang, H.; Liu, F.; et al. Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem Cell Res. Ther. 2019, 10, 363. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, Q.; Wang, Y.; Ma, Y.; Chen, F.; Zhu, X.; Shi, J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020, 295, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef]
- Hao, Y.; Miao, J.; Liu, W.; Cai, K.; Huang, X.; Peng, L. Mesenchymal Stem Cell-Derived Exosomes Carry MicroRNA-125a to Protect Against Diabetic Nephropathy by Targeting Histone Deacetylase 1 and Downregulating Endothelin-1. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Jin, Q.; Kong, L.; Zhang, D.; Teng, Y.; Lin, L.; Yao, X.; Jin, Y.; Li, M. microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis. J. Bioenerg. Biomembr. 2022, 54, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Takemura, S.; Shimizu, T.; Oka, M.; Sekiya, S.; Babazono, T. Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model. J. Diabetes Investig. 2020, 11, 545–553. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, J.E.; Kim, H.S.; Jung, M.K.; Ko, M.S.; Kim, M.-O.; Park, H.S.; Oh, W.; Choi, S.J.; Jin, H.J.; et al. Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, E.; Li, C.; Han, B.; Zhang, Q.; Rao, W.; Xiao, C.; Wu, D. Umbilical Cord-Derived Mesenchymal Stem Cells Ameliorate Nephrocyte Injury and Proteinuria in a Diabetic Nephropathy Rat Model. J. Diabetes Res. 2020, 2020, 8035853. [Google Scholar] [CrossRef] [PubMed]
- Xiang, E.; Han, B.; Zhang, Q.; Rao, W.; Wang, Z.; Chang, C.; Zhang, Y.; Tu, C.; Li, C.; Wu, D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res. Ther. 2020, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Le, X.; Zheng, S.; Zhang, K.; He, J.; Liu, M.; Tu, C.; Rao, W.; Du, H.; Ouyang, Y.; et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res. Ther. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Bai, X.; Lou, Y.; Zhu, Y.; Jiang, S.; Zhang, L.; Tian, N.; Luo, P.; Li, B. Human umbilical cord mesenchymal stem cells reduce oxidative damage and apoptosis in diabetic nephropathy by activating Nrf2. Stem Cell Res. Ther. 2021, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yeh, J.-N.; Chiang, J.Y.; Sung, P.-H.; Chen, Y.-L.; Liu, F.; Yip, H.-K. Intrarenal arterial administration of human umbilical cord-derived mesenchymal stem cells effectively preserved the residual renal function of diabetic kidney disease in rat. Stem Cell Res. Ther. 2022, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Chen, J.; Liang, H.; Liu, Y.; Chen, W.; Zhou, L.; Shan, L.; Wang, H. Intravenous injection of human umbilical cord-derived mesenchymal stem cells ameliorates not only blood glucose but also nephrotic complication of diabetic rats through autophagy-mediated anti-senescent mechanism. Stem Cell Res. Ther. 2023, 14, 146. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Hu, B.; Li, P.; Abuduaini, Y.; Zhao, H.; Jieensihan, A.; Chen, X.; Wang, S.; Guo, N.; et al. Human umbilical cord mesenchymal stem cells attenuate diabetic nephropathy through the IGF1R-CHK2-p53 signalling axis in male rats with type 2 diabetes mellitus. J. Zhejiang Univ. Sci. B 2024, 25, 568–580. [Google Scholar] [CrossRef]
- He, J.; Liu, B.; Du, X.; Wei, Y.; Kong, D.; Feng, B.; Guo, R.; Asiamah, E.A.; Griffin, M.D.; Hynes, S.O.; et al. Amelioration of diabetic nephropathy in mice by a single intravenous injection of human mesenchymal stromal cells at early and later disease stages is associated with restoration of autophagy. Stem Cell Res. Ther. 2024, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, Y.; Zhang, J.; Dong, Q.; Yang, M.; Wang, W. Mouse Umbilical Cord Mesenchymal Stem Cell Paracrine Alleviates Renal Fibrosis in Diabetic Nephropathy by Reducing Myofibroblast Transdifferentiation and Cell Proliferation and Upregulating MMPs in Mesangial Cells. J. Diabetes Res. 2020, 2020, 3847171. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zang, N.; Song, J.; Guo, X.; He, Q.; Hu, H.; Yang, M.; Wang, Y.; Yang, J.; Zou, Y.; et al. Exosomes derived from mesenchymal stem cells attenuate diabetic kidney disease by inhibiting cell apoptosis and epithelial-to-mesenchymal transition via miR-424-5p. FASEB J. 2022, 36, e22517. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, H.; Lv, S.; Liu, Q.; Li, S.; Yang, X.; Liu, G. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Diabetic Kidney Disease Through the NLRP3 Signaling Pathway. Stem Cells 2023, 41, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, D.; Lv, S.; Liu, X.; Liu, G. Mesenchymal stem cell-derived exosomes ameliorate diabetic kidney disease through NOD2 signaling pathway. Ren. Fail. 2024, 46, 2381597. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, W.; Ji, C.; Zhu, Y.; Shan, Y.; Zhou, Z.; Chen, W.; Zhang, L.; Sun, Z.; Zhou, W.; et al. hucMSC-sEVs-Derived 14-3-3ζ Serves as a Bridge between YAP and Autophagy in Diabetic Kidney Disease. Oxidative Med. Cell. Longev. 2022, 2022, 3281896. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; Su, R.; Zhen, J.; Liu, X.; Liu, G. Small extracellular vesicles-shuttled miR-23a-3p from mesenchymal stem cells alleviate renal fibrosis and inflammation by inhibiting KLF3/STAT3 axis in diabetic kidney disease. Int. Immunopharmacol. 2024, 139, 112667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, S.; Wu, J.; He, J.; Ouyang, Y.; Ao, C.; Lang, R.; Jiang, Y.; Yang, Y.; Xiao, H.; et al. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate renal fibrosis in diabetic nephropathy by targeting Hedgehog/SMO signaling. FASEB J. 2024, 38, e23599. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Li, R.; Huang, M.; Yue, G.; Guan, L.; Deng, Y.; Cai, W.; Xu, J. Placental Mesenchymal Stem Cells Alleviate Podocyte Injury in Diabetic Kidney Disease by Modulating Mitophagy via the SIRT1-PGC-1alpha-TFAM Pathway. Int. J. Mol. Sci. 2023, 24, 4696. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Yue, G.; Deng, Y.; Cai, W.; Xu, J. Human placenta-derived mesenchymal stem cells ameliorate diabetic kidney disease by modulating the T helper 17 cell/ regulatory T-cell balance through the programmed death 1/programmed death-ligand 1 pathway. Diabetes Obes. Metab. 2024, 26, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Yue, G.; Xu, J. Placenta-derived mesenchymal stem cells protect against diabetic kidney disease by upregulating autophagy-mediated SIRT1/FOXO1 pathway. Ren. Fail. 2024, 46, 2303396. [Google Scholar] [CrossRef]
- Rao, N.; Wang, X.; Xie, J.; Li, J.; Zhai, Y.; Li, X.; Fang, T.; Wang, Y.; Zhao, Y.; Ge, L. Stem Cells from Human Exfoliated Deciduous Teeth Ameliorate Diabetic Nephropathy In Vivo and In Vitro by Inhibiting Advanced Glycation End Product-Activated Epithelial-Mesenchymal Transition. Stem Cells Int. 2019, 2019, 2751475. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Y.; Jiang, X.; Pu, T.; Zhang, L.; Li, S.; Hu, L.; Bai, B.; Hu, T.; Yu, L.; et al. Transplantation of Human Amniotic Mesenchymal Stem Cells Up-Regulates Angiogenic Factor Expression to Attenuate Diabetic Kidney Disease in Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 331–343. [Google Scholar] [CrossRef]
- Packham, D.K.; Fraser, I.R.; Kerr, P.G.; Segal, K.R. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 2016, 12, 263–269. [Google Scholar] [CrossRef]
- Perico, N.; Remuzzi, G.; Griffin, M.D.; Cockwell, P.; Maxwell, A.P.; Casiraghi, F.; Rubis, N.; Peracchi, T.; Villa, A.; Todeschini, M.; et al. Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). J. Am. Soc. Nephrol. 2023, 34, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Chi, Y.; Sun, T.; Gao, Y.; Dou, X.; Han, Z.; Xue, F.; Li, H.; Liu, W.; et al. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells in the treatment of refractory immune thrombocytopenia: A prospective, single arm, phase I trial. Signal Transduct. Target. Ther. 2024, 9, 102. [Google Scholar] [CrossRef]
- Salemkour, Y.; Yildiz, D.; Dionet, L.; t Hart, D.C.; Verheijden, K.A.T.; Saito, R.; Mahtal, N.; Delbet, J.-D.; Letavernier, E.; Rabant, M.; et al. Podocyte Injury in Diabetic Kidney Disease in Mouse Models Involves TRPC6-mediated Calpain Activation Impairing Autophagy. J. Am. Soc. Nephrol. 2023, 34, 1823–1842. [Google Scholar] [CrossRef]
- Lv, S.-S.; Liu, G.; Wang, J.-P.; Wang, W.-W.; Cheng, J.; Sun, A.-L.; Liu, H.-Y.; Nie, H.-B.; Su, M.-R.; Guan, G.-J. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int. Immunopharmacol. 2013, 17, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.F.; Williams, T.M.; Rudd, S.; Wells, C.A.; Kerr, P.G.; Ricardo, S.D. Human mesenchymal stem cells alter the gene profile of monocytes from patients with Type 2 diabetes and end-stage renal disease. Regen. Med. 2016, 11, 145–158. [Google Scholar] [CrossRef]
- Su, W.; Yin, Y.; Zhao, J.; Hu, R.; Zhang, H.; Hu, J.; Ren, R.; Zhang, Y.; Wang, A.; Lyu, Z.; et al. Exosomes derived from umbilical cord-derived mesenchymal stem cells exposed to diabetic microenvironment enhance M2 macrophage polarization and protect against diabetic nephropathy. FASEB J. 2024, 38, e23798. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Im, G.-B.; Luo, A.C.; Zhu, Y.; Hong, X.; Neumeyer, J.; Tang, H.-W.; Perrimon, N.; Melero-Martin, J.M. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 2024, 629, 660–668. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, L.; Li, L.; Liu, F.; Liu, J.; Chen, Y.; Cheng, J.; Lu, Y. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells 2021, 39, 913–928. [Google Scholar] [CrossRef]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 31. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, V.; Rehman, A.; Panda, S.K.; Torsiello, M.; Marigliano, M.; Nicoletti, M.M.; Ferraro, G.A.; De Falco, V.; Lappano, R.; Lieto, E.; et al. Mitochondrial transfer from Adipose stem cells to breast cancer cells drives multi-drug resistance. J. Exp. Clin. Cancer Res. 2024, 43, 166. [Google Scholar] [CrossRef] [PubMed]
- Simonson, M.S. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007, 71, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Liles, J.T.; Corkey, B.K.; Notte, G.T.; Budas, G.R.; Lansdon, E.B.; Hinojosa-Kirschenbaum, F.; Badal, S.S.; Lee, M.; Schultz, B.E.; Wise, S.; et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Investig. 2018, 128, 4485–4500. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Dai, C. Effects of Bone Marrow Mesenchymal Stem Cells on Plasminogen Activator Inhibitor-1 and Renal Fibrosis in Rats with Diabetic Nephropathy. Arch. Med. Res. 2016, 47, 71–77. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Wang, L.; Tao, Q.; Tu, C. Hippo/YAP signaling pathway: A new therapeutic target for diabetes mellitus and vascular complications. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231220134. [Google Scholar] [CrossRef]
- Yang, D.; Livingston, M.J.; Liu, Z.; Dong, G.; Zhang, M.; Chen, J.-K.; Dong, Z. Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell Mol. Life Sci. 2018, 75, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Ceccariglia, S.; Cargnoni, A.; Silini, A.R.; Parolini, O. Autophagy: A potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy 2020, 16, 28–37. [Google Scholar] [CrossRef]
- Regmi, S.; Raut, P.K.; Pathak, S.; Shrestha, P.; Park, P.-H.; Jeong, J.-H. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy 2021, 17, 2991–3010. [Google Scholar] [CrossRef]
- Verma, A.; Patel, A.B. Finerenone: A Non-steroidal Mineralocorticoid Receptor Blocker for Diabetic Kidney Disease. Trends Endocrinol. Metab. 2021, 32, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Heeba, G.H.; Khalifa, M.M.A. Effect of combined therapy of mesenchymal stem cells with GLP-1 receptor agonist, exenatide, on early-onset nephropathy induced in diabetic rats. Eur. J. Pharmacol. 2021, 892, 173721. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Chen, Y.L.; Sung, P.H.; Chiang, J.Y.; Chen, C.H.; Li, Y.C.; Yip, H.K. Repeated administration of adipose-derived mesenchymal stem cells added on beneficial effects of empagliflozin on protecting renal function in diabetic kidney disease rat. Biomed. J. 2024, 47, 100613. [Google Scholar] [CrossRef]
- Meng, J.; Gao, X.; Liu, X.; Zheng, W.; Wang, Y.; Wang, Y.; Sun, Z.; Yin, X.; Zhou, X. Effects of xenogeneic transplantation of umbilical cord-derived mesenchymal stem cells combined with irbesartan on renal podocyte damage in diabetic rats. Stem Cell Res. Ther. 2024, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lv, S.; Liu, J.; Liu, S.; Wang, Y.; Liu, G. Mesenchymal stem cells modified with angiotensin-converting enzyme 2 are superior for amelioration of glomerular fibrosis in diabetic nephropathy. Diabetes Res. Clin. Pract. 2020, 162, 108093. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Hickson, L.J.; Eirin, A.; Conley, S.M.; Taner, T.; Bian, X.; Saad, A.; Herrmann, S.M.; Mehta, R.A.; McKenzie, T.J.; Kellogg, T.A.; et al. Diabetic Kidney Disease Alters the Transcriptome and Function of Human Adipose-Derived Mesenchymal Stromal Cells but Maintains Immunomodulatory and Paracrine Activities Important for Renal Repair. Diabetes 2021, 70, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Yu, S.; Yin, Y.; Li, B.; Xue, J.; Wang, J.; Gu, Y.; Zhang, H.; Lyu, Z.; Mu, Y.; et al. Diabetic microenvironment preconditioning of adipose tissue-derived mesenchymal stem cells enhances their anti-diabetic, anti-long-term complications, and anti-inflammatory effects in type 2 diabetic rats. Stem Cell Res. Ther. 2022, 13, 422. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Saijo, Y.; Tsuchida, H.; Ishioka, S.; Nishikawa, A.; Saito, T.; et al. Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci. Rep. 2017, 7, 8484. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Hoang, D.M.; Nguyen, K.T.; Bui, D.M.; Nguyen, H.T.; Le, H.T.A.; Hoang, V.T.; Bui, H.T.H.; Dam, P.T.M.; Hoang, X.T.A.; et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl. Med. 2021, 10, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Wang, D.-A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011, 11, 189–197. [Google Scholar] [CrossRef]
- Wu, S.; Li, L.; Wang, G.; Shen, W.; Xu, Y.; Liu, Z.; Zhuo, Z.; Xia, H.; Gao, Y.; Tan, K. Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats. Int. J. Nanomed. 2014, 9, 5639–5651. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Wang, G.; Gao, Y.; Tan, K.; Zhuo, Z.; Liu, Z.; Xia, H.; Yang, D.; Li, P. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. BioMed Res. Int. 2013, 2013, 526367. [Google Scholar] [CrossRef]
- Wang, G.; Zhuo, Z.; Yang, B.; Wu, S.; Xu, Y.; Liu, Z.; Tan, K.; Xia, H.; Wang, X.; Zou, L.; et al. Enhanced Homing Ability and Retention of Bone Marrow Stromal Cells to Diabetic Nephropathy by Microbubble-Mediated Diagnostic Ultrasound Irradiation. Ultrasound Med. Biol. 2015, 41, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Zhang, Y.; Lv, H.; Yao, H.; Zhao, Y.; Li, J.; Li, X. Combined Placental Mesenchymal Stem Cells with Guided Nanoparticles Effective Against Diabetic Nephropathy in Mouse Model. Int. J. Nanomed. 2024, 19, 901–915. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, Y.; Chen, X.; Midgley, A.C.; Wang, Z.; Zhu, D.; Wu, J.; Chen, P.; Wu, L.; Wang, X.; et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 2020, 14, 12133–12147. [Google Scholar] [CrossRef]
- Peng, H.; Li, Y.; Ji, W.; Zhao, R.; Lu, Z.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Wang, J.; et al. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson’s Disease. ACS Nano 2022, 16, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Yu, C.; Bao, H.; Cheng, S.; Huang, J.; Zhang, Z. ROS-Responsive Janus Au/Mesoporous Silica Core/Shell Nanoparticles for Drug Delivery and Long-Term CT Imaging Tracking of MSCs in Pulmonary Fibrosis Treatment. ACS Nano 2023, 17, 6387–6399. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.C.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef]

| Type of MSCs | Subjects | Method of DKD Induction | Administration | Frequency and Dose | Effect | Reference |
|---|---|---|---|---|---|---|
| Mouse BM-MSCs | Male BALB/c mice | i.p. of 150 mg/kg STZ | Tail vein injection | Once every two weeks, three times after the onset 5 × 105 | MSCs reprogram Mφ into M2 via improvement of the lysosome–autophagy pathway and mitochondrial bioenergetics with transcription factor EB activation. | [24] |
| Male mice BTBR.Cg-Lepob/WiscJ | - | Intraperitoneal injection | 8th and 10th weeks of age 1 × 106 | MSC-treated animals exhibited lighter renal pathological impairment, upregulation of mitochondria-related survival genes, and a decrease in autophagy hyperactivation and apoptosis. | [25] | |
| Rat BM-MSCs | Male C57BL/6 mice male SD rats | i.p. of 150 mg/kg STZ (diabetic mice) tail vein injection of 55 mg/kg of STZ (diabetic rats) | Tail vein injection | 8 w and 10 w after the onset 1 × 104 | Mt transfer from BM-MSCs to damaged PTECs, injection of BM-MSC-derived isolated Mt in renal capsule share the same effect. | [26] |
| Male C57BL/6 mice | Not provided | Tail vein injection | Not provided | MSCs protect DKD kidneys by regulating M6 A methylation through Smad2/3/WTAP/ENO1. | [27] | |
| Male SD rats | i.p. of 55 mg/kg STZ | Tail vein injection | Once a week for 6 weeks 1 × 107 | Show immunosuppression of CD8 T-cell proliferation and activation mediated by CD103 DCs. | [28] | |
| Male SD rats | i.p. of 65 mg/kg STZ | Tail vein injection | 4 × 106 | Improve renal function and collagen accumulation; inhibit inflammatory and fibrotic cytokines by downregulating TLR-4/NF-κB expression. | [29] | |
| Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | Once a week for two continuous weeks 5 × 106 | MSCs suppress progression of diabetic nephropathy (DN) pathogenesis through LXA4 by targeting TGF-β/Smad signals and pro-inflammatory cytokines in DN. | [30] | |
| GECs | 30 mmol/L D-glucose | Co-culture | 5 × 105 | Rejuvenate damaged GEs via Mt transfer. | [31] | |
| BM-MSC-Exos | Male SD rats | i.p. of 35 mg/kg STZ | Tail vein injection | Once a week for 12 weeks 100 µg | Increase apoptosis; decrease GLU. Scr, BUN. | [32] |
| Rat BM-MSCs-derived exosomes | Male albino rats | i.p. of 60 mg/kg STZ | Tail vein injection | Two injections of exosomes 100 μg/kg/dose | Ameliorate diabetic nephropathy by autophagy induction through the mTOR signaling pathway. | [33] |
| Human BM-MSCs-derived extracellular vesicles (EVs) | Male NSG mice | i.p. of 37 mg/kg STZ for 4 days | Intravenous injection | Once a week for 4 weeks 1 × 1010 particles | Revert the progression of glomerular and interstitial fibrosis. | [34] |
| Rat ADSCs | Male SD rats | 8 weeks of high-fat diet (HFD) and a single dose of 25 mg/kg STZ | Tail vein injection | Once a week for 24 weeks 3 × 106 | Reduce blood glucose and insulin demand, reduce the expression of SLGT2 of PTEC and reduce kidney damage and inflammation. | [35] |
| Mouse ADSCs-EVs | C57BL/KsJ db/db | - | Tail vein injection | Once a week for 12 weeks - | miR-26a-5p delivered by ADSC-derived EVs suppress glomerular podocyte apoptosis and protect against DN by regulating TLR4. | [36] |
| Mouse ADSCs-Exos | C57BL/KsJ db/db | - | Tail vein injection | Once a week for 12 weeks - | Reduce proteinuria, Scr, blood urea nitrogen (BUN), and podocyte apoptosis; miR-486 of ADSCs-Exo promote autophagy flux. | [37] |
| Rat ADSCs-exos | Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | 50 μg exosomes t twice a week for 3 weeks | Decrease levels of blood glucose, serum creatinine (Scr), 24 h urinary protein, UACR, and kidney weight/body weight, and they suppressed mesangial hyperplasia and kidney fibrosis, which is related to miR-125a. | [38] |
| Human ADSCs-EVs | Mouse podocyte clone 5 (MPC5) | Normal glucose (NG, 5.5 mM), NG + mannitol (5.5 mM glucose +24.5 mM MA), HG (30 mM glucose) | - | 25 μg/mL | EV-derived miR-15b-5p could protect MPC5 cell apoptosis and inflammation via downregulation of the VEGF/PDK4 axis. | [39] |
| Rat ADSCs Sheet | Spontaneously Diabetic Torii (SDT) fatty rat | - | Femoral vein injection or renal capsule transplantation | 6 × 106 | Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney improves transplantation efficiency and suppresses inflammation and renal injury progression. | [40] |
| Human UC-MSCs | Male CD1 mice | i.p. of 80 mg/kg STZ | - | Thrice every 4 weeks after the onset 5 × 105 | Promote the expression of Arg1 in macrophages to promote M2 polarization and improve mitochondrial function of renal tubular epithelial cells. | [41] |
| Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | Once a week for two consecutive weeks 2 × 106 | Reduce urinary total protein, UACR, Scr, and BUN, improve renal pathological abnormalities, promote the expression of antiapoptotic protein Bcl-xl, and activate the apoptotic pathway. | [42] | |
| Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | 2 × 106 | Ameliorate functional parameters, improve renal pathological changes, reduce the levels of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) and profibrotic factor (TGF-β) in the kidney and blood. | [43] | |
| Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | Twice; group 1: weeks 7 and 8; group 2: weeks 9 and 10 2 × 106 | UC-MSC-derived miR-146a-5p restores renal function in DN rats by facilitating M2 macrophage polarization by targeting TRAF6. | [44] | |
| Male SD rats | HFD and i.p. of 35 mg/kg STZ | Tail vein injection | Three times every 10 days 2 × 106 | Attenuate renal oxidative damage and apoptosis, activate Nrf2. | [45] | |
| Male SD rats | 5/6 nephrectomy, followed by intraperitoneal administration of aminoguanidine (180 mg/kg) and streptozotocin (30 mg/kg) | Intrarenal arterial injection (IRA) | Day 21 after CKD induction 2.1 × 105 | IRA injection of xenogeneic MSCs was safe and effectively protected the residual renal function and architectural integrity. | [46] | |
| Male SD rats | i.p. of 50 mg/kg STZ | Intravenous injection | Once a week for 4 weeks Low-dose hucMSCs-treated group (MSC-L):5 × 106; high-dose hucMSCs-treated group (MSC-H):1 × 107 | Improve cell viability, wound healing, and senescence of the high glucose-damaged rat podocytes through a paracrine action mode; activate autophagy and attenuate senescence through the AMPK/mTOR pathway. | [47] | |
| Male SD rats | HFD for 4 w, followed by i.p. of 30 mg/kg STZ | Tail vein injection | Once per week for four consecutive weeks 1 × 107 | HUC-MSCs downregulated the expression of IGF1/IGF1R in the renal tissue of diabetic rats, inhibited the activity of the target genes CHK2 and p53, reduced apoptosis, and improved diabetic nephropathy. | [48] | |
| Male C57BL/6 mice | i.p. of 60 mg/kg STZ | Tail vein injection | 8 w or 16 w after the onset 5 × 105 | Reduce uACR and improve multiple glomerular and renal interstitial abnormalities; reduce circulating TGF-β1 and restore intrarenal autophagy; reduce early inflammation of the disease. | [49] | |
| Mouse UC-MSCs | Male BALB/C mice | i.p. of 150 mg/kg STZ | Tail vein injection | Weekly for 4 weeks 1 × 104 | Alleviate albuminuria, glomerulus injury, and fibrosis by inhibiting TGF-β1-triggered MFT and cell proliferation mediated by PI3K/Akt and MAPK signaling pathways and elevating the levels of MMP2 and MMP9. | [50] |
| Human UC-MSCs-exos | Male C57BL/KsJ-db/db | - | Tail vein injection | MSCs: every week for 6 weeks 1 × 106 MSC-exos: twice a week for 6 weeks 10 mg/kg bw | MSC-exos could inhibit high glucose-induced apoptosis and EMT through miR-424-5p targeting of YAP1. | [51] |
| Human UC-MSCs-exos | Male db/db mice | - | Tail vein injection | 3 times in the first week, and then twice a week for the next 3 weeks 100 μg | MSCs-Exos attenuated the expression of inflammatory factors in podocytes and DN mice in vivo and in vitro, inhibited the activation of the NLRP3 signaling pathway, and improved renal injury. MiR-22-3p may play a role in the anti-inflammatory effect of MSCs-Exo. | [52] |
| Human UC-MSCs-exos | Male C57BL/6J mice; male db/db mice | HFD for 6 weeks, followed by i.p. of 100 mg/kg STZ for three days | Not provided | 3 times in the first week, and then twice a week for the next 3 weeks 100 μg | Alleviate the inflammatory response; inhibit the activation of NOD2 signaling pathway; prevent apoptosis; increase cell viability in podocytes. | [53] |
| Human UC-MSCs-EVs | Male SD rats | HFD and i.p. of 35 mg/kg STZ | Tail vein injection | 10 mg/kg | Protein 14-3-3ζ in hucMSC-sEVs promotes YAP cytoplasmic retention instead of entering the nucleus, enhancing the level of autophagy in the cytoplasm to remove the excessive YAP protein. | [54] |
| Human UC-MSCs-EVs | Male C57BLKS/J db/db | - | Intravenous injection | Two times a week from 8 to 18 weeks old 100 ug | Attenuate renal fibrosis and inflammation; MiR-23a-3p and its target Krüppel-like factor 3 (KLF3) inhibit high glucose (HG)-induced STAT3 signaling pathway; miR-23a-3p is packaged into MSC sEV by RNA-binding motif protein X-linked (RBMX). | [55] |
| Human UC-MSCs/UC-MSCs-exos | Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | Twice UC-MSCs: 2 × 106 UC-MSC-exo: 100 μg/kg | Attenuate kidney damage; inhibit EMT and renal fibrosis; decrease SMO expression targeting Hedgehog/SMO pathway. | [56] |
| Human PMSCs | Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | 1 × 106 | Reverse podocyte injury and mitophagy. | [57] |
| Male SD rats | i.p. of 60 mg/kg STZ | Tail vein injection | Once a week for three weeks 1 × 106 | Regulate TH17/Treg through systemic immune regulation by upregulating PD-1; enhance the autophagy level of DKD rat kidney and podocyte by upregulating the expression of SIRT1 and FOXO1. | [58,59] | |
| SHED Human BM-MSCs | Male Goto-Kakizaki (GK) rats | HFD for 2–4 weeks | Tail vein injection | 4 × 106 | Attenuate hyperglycemia, hyperlipidemia, increased urinary albumin excretion, ECM accumulation, and a fractional mesangial area. | [60] |
| Human Amniotic MSCs | Male SD rats | i.p. of 55 mg/kg STZ | Penile vein injection | 2 × 106 | Increase the expression of ARF and decrease blood glucose, 24-h urinary protein, Scr, urea, kidney injury molecule-1 (KIM-1), and renal injury index. | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Zhang, C. Mesenchymal Stem Cell Therapy: Therapeutic Opportunities and Challenges for Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10540. https://doi.org/10.3390/ijms251910540
Cheng J, Zhang C. Mesenchymal Stem Cell Therapy: Therapeutic Opportunities and Challenges for Diabetic Kidney Disease. International Journal of Molecular Sciences. 2024; 25(19):10540. https://doi.org/10.3390/ijms251910540
Chicago/Turabian StyleCheng, Jia, and Chun Zhang. 2024. "Mesenchymal Stem Cell Therapy: Therapeutic Opportunities and Challenges for Diabetic Kidney Disease" International Journal of Molecular Sciences 25, no. 19: 10540. https://doi.org/10.3390/ijms251910540





