The Regulatory Role of miRNAs in Zebrafish Fin Regeneration
Abstract
:1. Introduction
2. Results
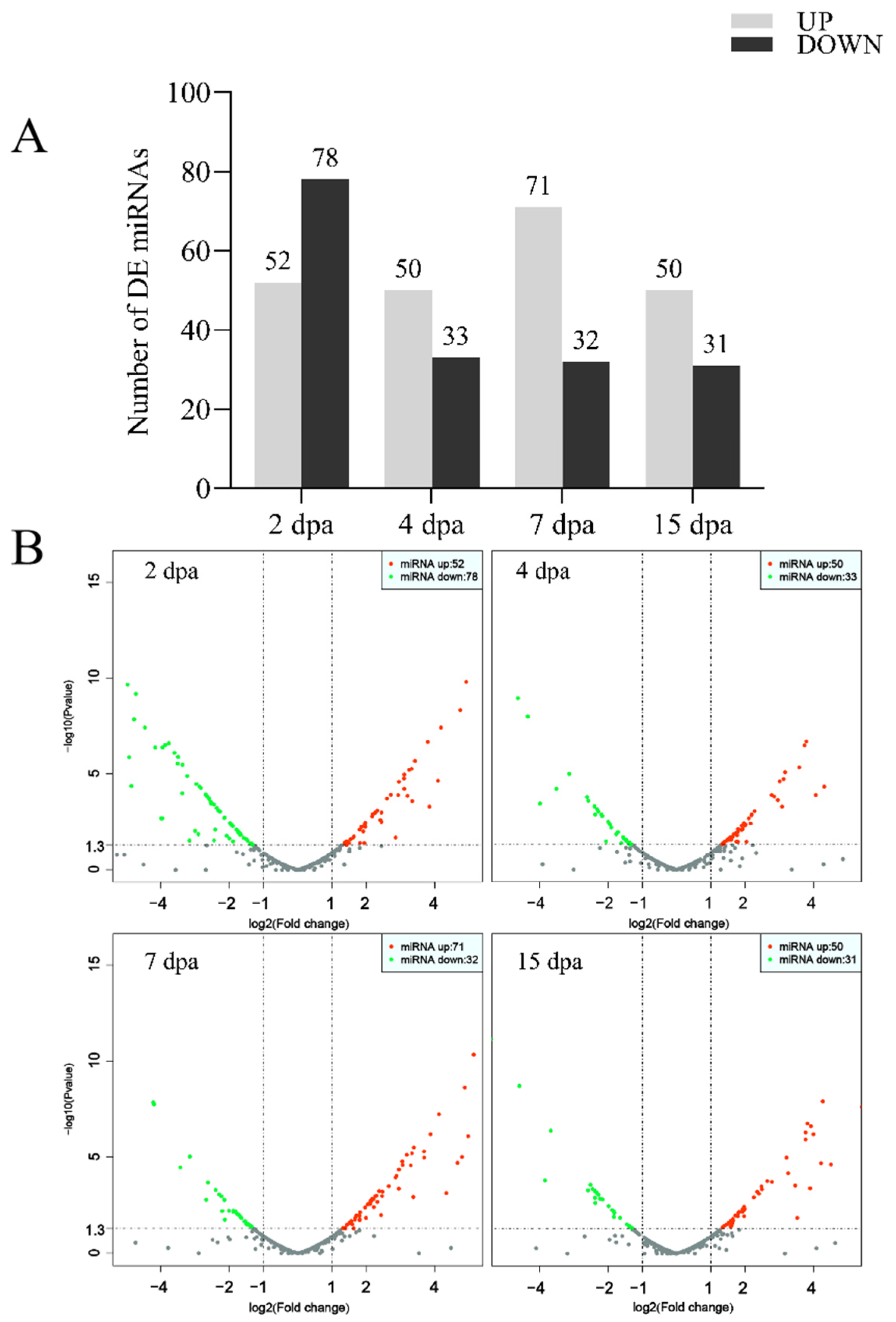
2.1. Analysis of miRNA Differential Expression
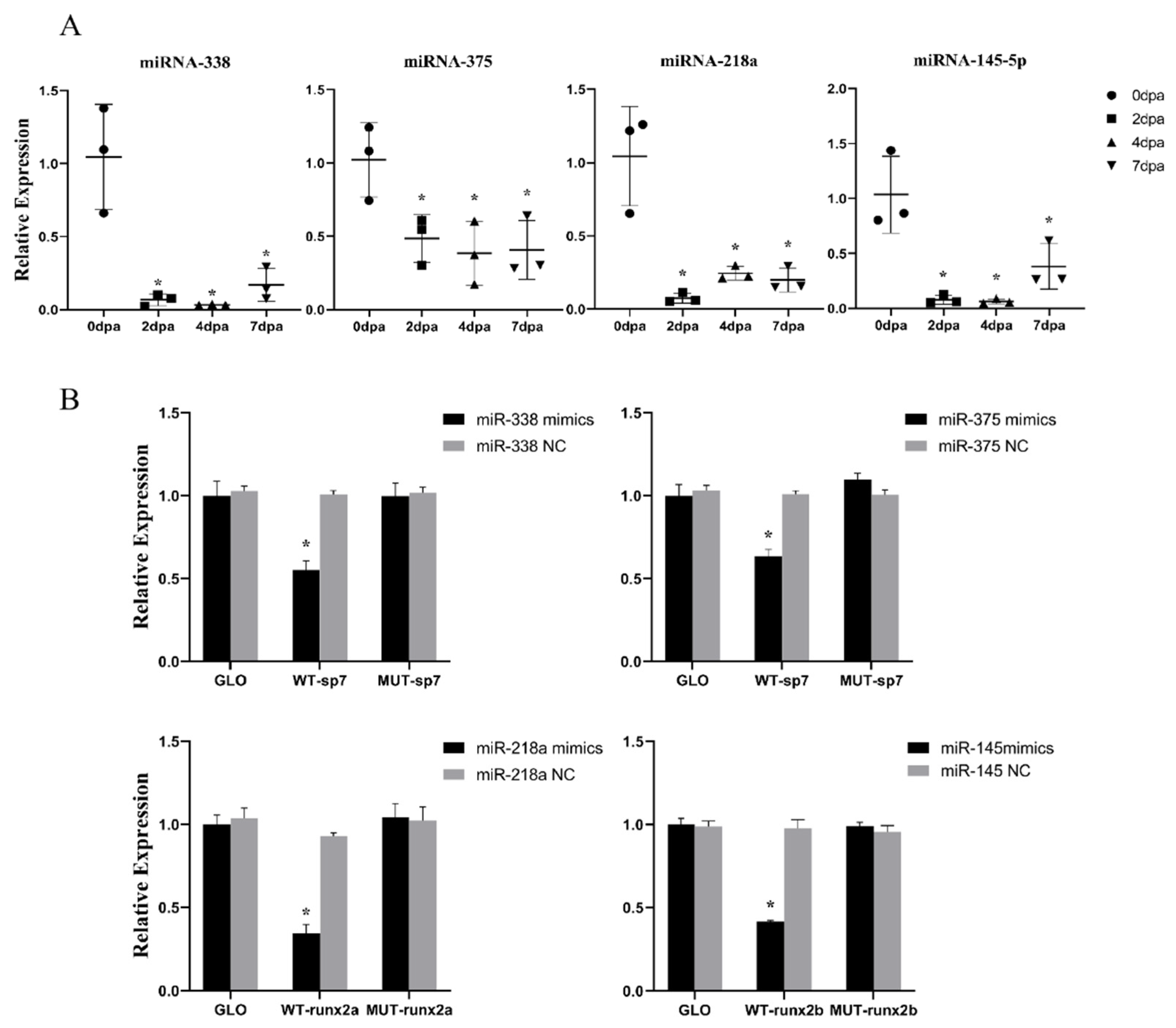
2.2. In Vitro Validation of the Targeting Relationship between miRNA and Predicted Target Genes
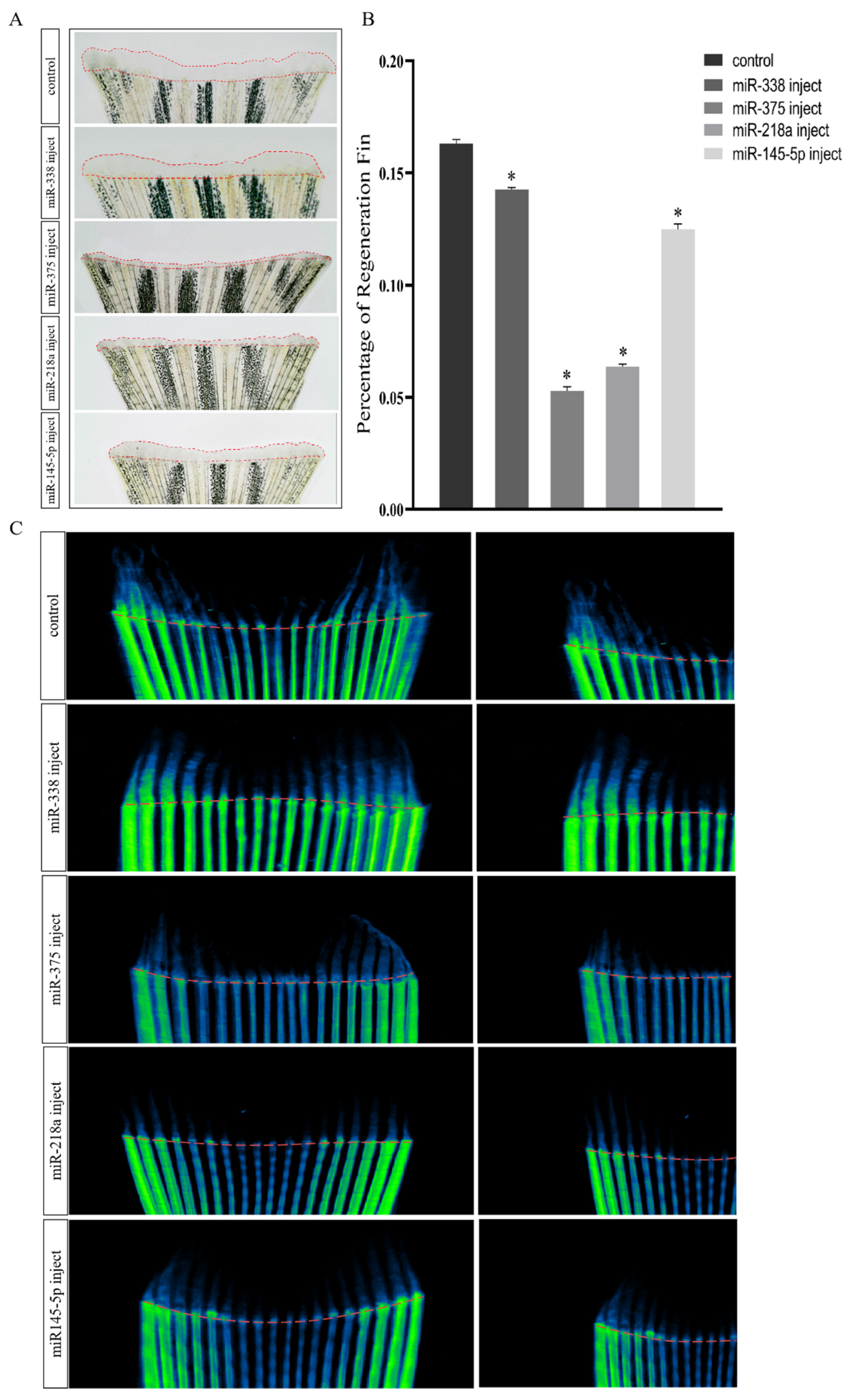
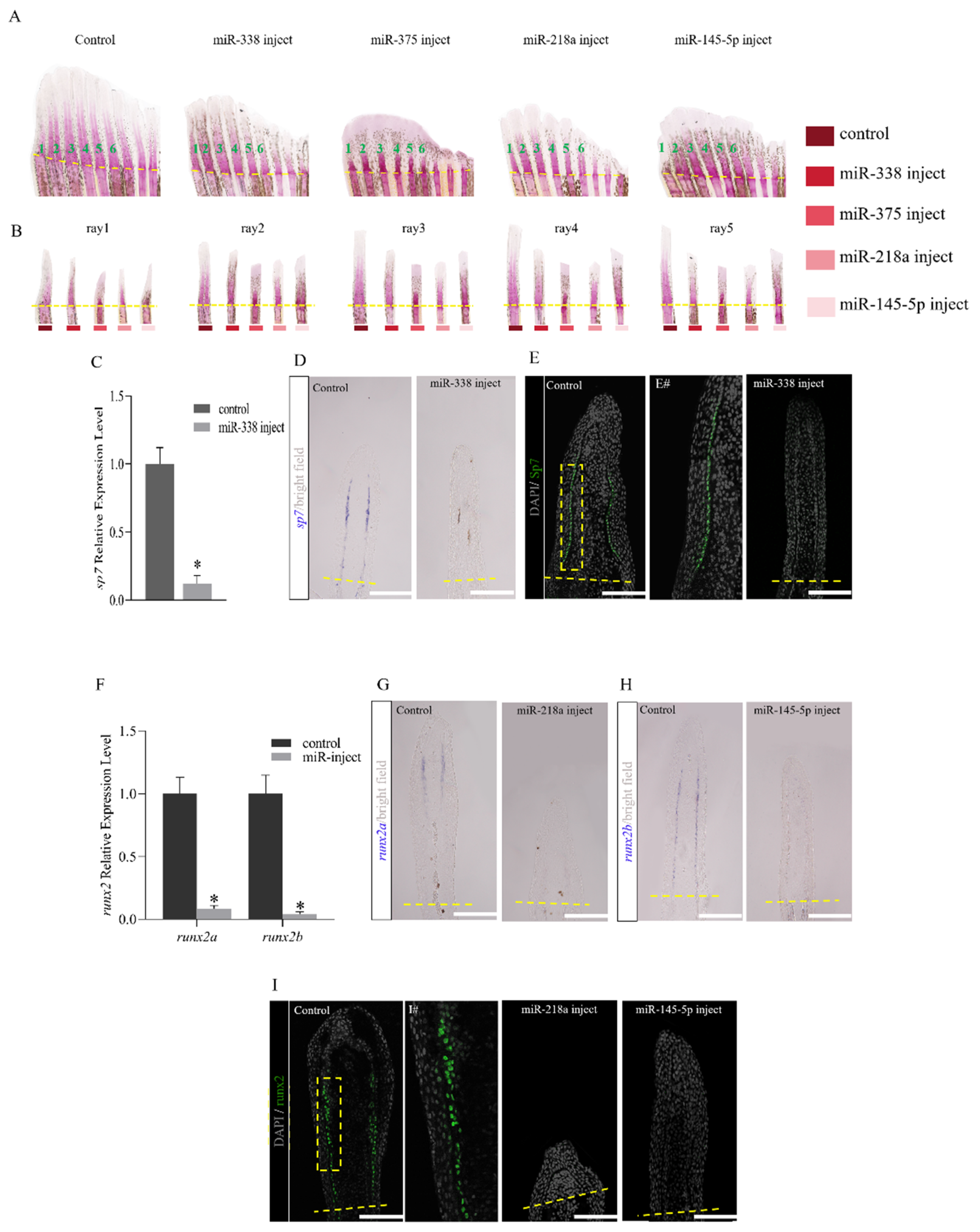
2.3. Overexpression of miRNAs Affects Regenerative Outgrowth
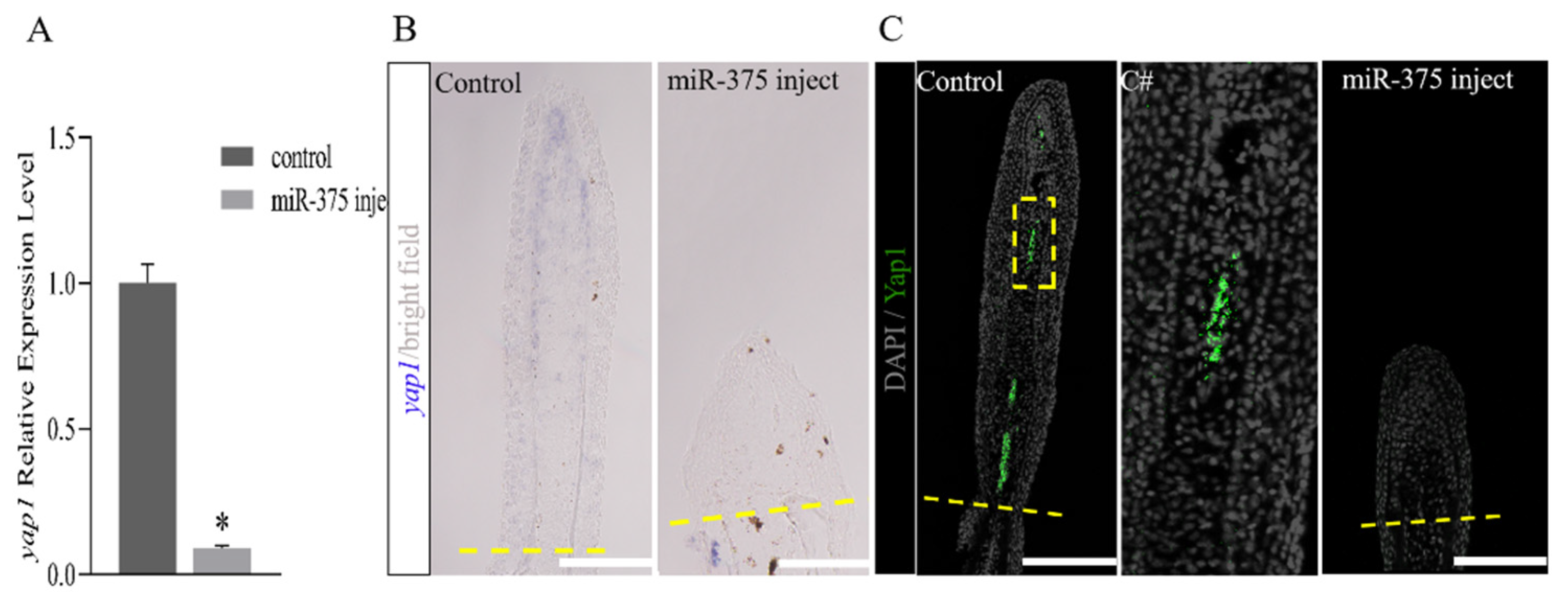
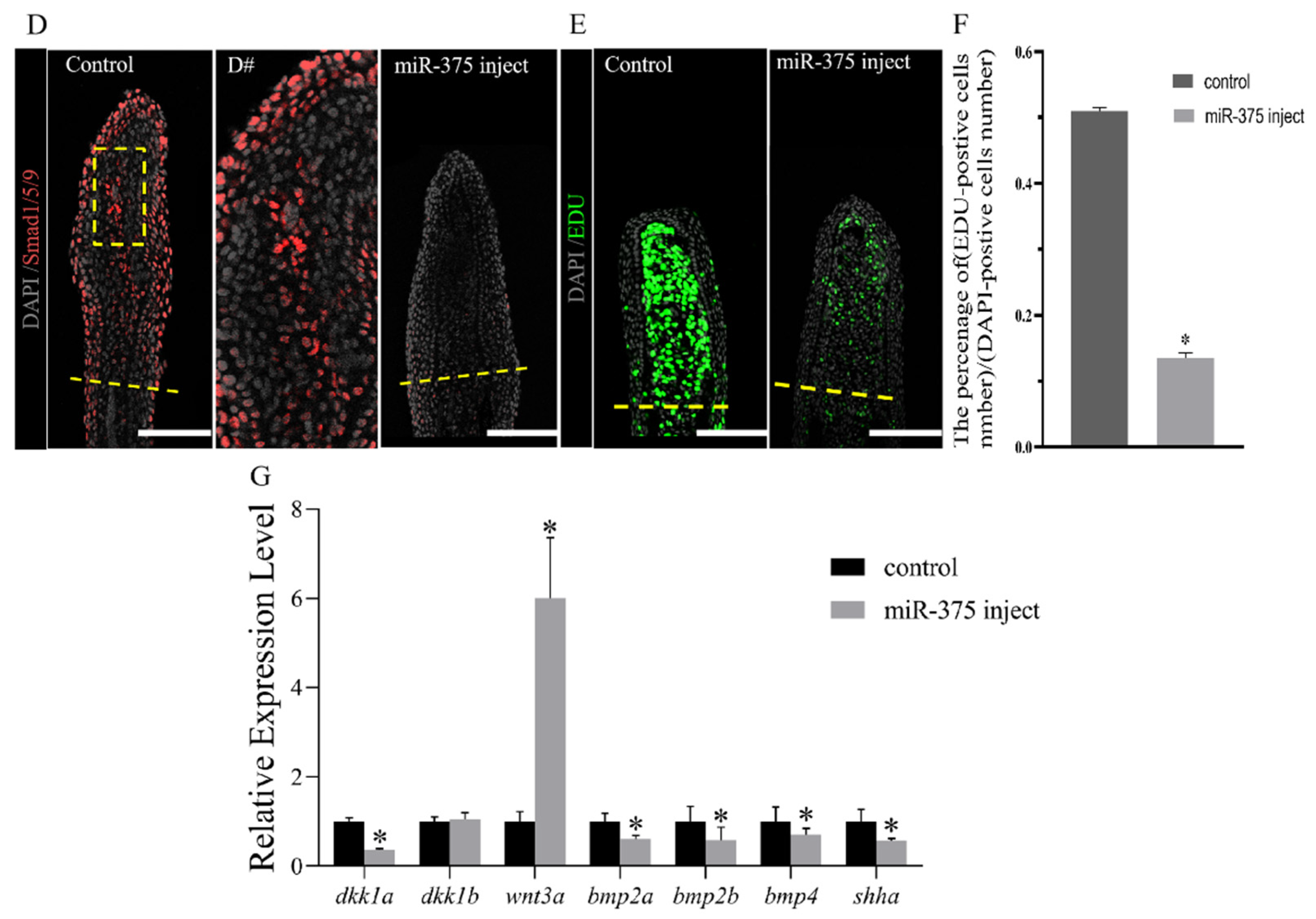
2.4. miRNAs Regulate Fish Fin Regeneration by Inhibiting Blastema Formation
2.5. miRNAs Regulate the Regeneration of Fish Fins by Delaying Bone Formation and Osteoblast Differentiation or Functionality
3. Discussion
4. Materials and Methods
4.1. Fish Care and Fin Amputations
4.2. Total RNA Isolation and miRNA-Seq
4.3. Quantitative Real-Time PCR
4.4. Dual-Luciferase Reporter Gene Assay
4.5. Drug Injections
4.6. EdU (5-Ethynyl-20–Deoxyuridine) Labeling
4.7. In Situ Hybridization
4.8. Immunostaining
4.9. Bone Staining and CT
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durante, L.D.S.; Hollmann, G.; Nazari, E.M. Impact of exposure to glyphosate-based herbicide on morphological and physiological parameters in embryonic and larval development of zebrafish. Environ. Toxicol. 2024, 39, 1822–1835. [Google Scholar] [CrossRef]
- Han, S.; Liu, X.; Xie, S.; Gao, M.; Liu, F.; Yu, S.; Sun, P.; Wang, C.; Archacki, S.; Lu, Z.; et al. Knockout of ush2a gene in zebrafish causes hearing impairment and late onset rod-cone dystrophy. Hum. Genet. 2018, 137, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Shi, W.; Fang, Z.; Li, L.; Luo, L. Using zebrafish as the model organism to understand organ regeneration. Sci. China. Life Sci. 2015, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Tanaka, K.; Chowdhury, G.; Nasu, K.; Kuroyanagi, Y.; Yamasu, K. Evolutionarily conserved roles of foxg1a in the developing subpallium of zebrafish embryos. Dev. Growth Differ. 2024, 66, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Cudak, N.; López-Delgado, A.C.; Keil, S.; Knopf, F. Fibroblast growth factor pathway component expression in the regenerating zebrafish fin. Gene Expr. Patterns GEP 2023, 48, 119307. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Chakravarty, A.; Panchal, A.; Patil, M.; Ghodadra, G.; Sudhakaran, J.; Nuesslein-Volhard, C. Zebrafish twist2/dermo1 regulates scale shape and scale organization during skin development and regeneration. Cells Dev. 2021, 166, 203684. [Google Scholar] [CrossRef]
- Veen, K.; Krylov, A.; Yu, S.; He, J.; Boyd, P.; Hyde, D.R.; Mantamadiotis, T.; Cheng, L.Y.; Jusuf, P.R. Her6 and Prox1a are novel regulators of photoreceptor regeneration in the zebrafish retina. PLoS Genet. 2023, 19, e1011010. [Google Scholar] [CrossRef]
- Weinberger, M.; Simões, F.C.; Gungoosingh, T.; Sauka-Spengler, T.; Riley, P.R. Distinct epicardial gene regulatory programs drive development and regeneration of the zebrafish heart. Dev. Cell 2024, 59, 351–367. [Google Scholar] [CrossRef]
- Akimenko, M.A.; Marí-Beffa, M.; Becerra, J.; Géraudie, J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 226, 190–201. [Google Scholar] [CrossRef]
- Poleo, G.; Brown, C.W.; Laforest, L.; Akimenko, M.A. Cell proliferation and movement during early fin regeneration in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 221, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Keating, M.T.; Nechiporuk, A. Tales of regeneration in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 226, 202–210. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef] [PubMed]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Ketting, R.F.; Fischer, S.E.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef]
- Hutvágner, G.; Zamore, P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tomari, Y. RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochim. Et Biophys. Acta 2016, 1859, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Behm-Ansmant, I.; Rehwinkel, J.; Izaurralde, E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Kobayashi, T.; Lu, J.; Cobb, B.S.; Rodda, S.J.; McMahon, A.P.; Schipani, E.; Merkenschlager, M.; Kronenberg, H.M. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 1949–1954. [Google Scholar] [CrossRef]
- Taipaleenmäki, H. Regulation of Bone Metabolism by microRNAs. Curr. Osteoporos. Rep. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.S.; Bensimon-Brito, A.; Lourenço, R.; Borbinha, J.; Soares, A.R.; Mateus, R.; Jacinto, A. Yap induces osteoblast differentiation by modulating Bmp signalling during zebrafish caudal fin regeneration. J. Cell Sci. 2019, 132, jcs231993. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.M.; Fekany-Lee, K.; Carmany-Rampey, A.; Erter, C.; Topczewski, J.; Wright, C.V.; Solnica-Krezel, L. Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes Dev. 2000, 14, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ziros, P.G.; Basdra, E.K.; Papavassiliou, A.G. Runx2: Of bone and stretch. Int. J. Biochem. Cell Biol. 2008, 40, 1659–1663. [Google Scholar] [CrossRef]
- Mizuno, Y.; Yagi, K.; Tokuzawa, Y.; Kanesaki-Yatsuka, Y.; Suda, T.; Katagiri, T.; Fukuda, T.; Maruyama, M.; Okuda, A.; Amemiya, T.; et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem. Biophys. Res. Commun. 2008, 368, 267–272. [Google Scholar] [CrossRef]
- Zhai, S.; Liu, C.; Vimalraj, S.; Subramanian, R.; Abullais, S.S.; Arora, S.; Saravanan, S. Glucagon-like peptide-1 receptor promotes osteoblast differentiation of dental pulp stem cells and bone formation in a zebrafish scale regeneration model. Peptides 2023, 163, 170974. [Google Scholar] [CrossRef]
- Riley, S.E.; Feng, Y.; Hansen, C.G. Hippo-Yap/Taz signalling in zebrafish regeneration. NPJ Regen. Med. 2022, 7, 9. [Google Scholar] [CrossRef]
- Zhong, D.; Xu, G.Z.; Wu, J.Z.; Liu, H.; Tang, J.Y.; Wang, C.G. Circ-ITCH sponges miR-214 to promote the osteogenic differentiation in osteoporosis via upregulating YAP1. Cell Death Dis. 2021, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Bay, S.; Öztürk, G.; Emekli, N.; Demircan, T. Downregulation of Yap1 during limb regeneration results in defective bone formation in axolotl. Dev. Biol. 2023, 500, 31–39. [Google Scholar] [CrossRef]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog. Neurobiol. 2013, 109, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Chendrimada, T.P.; Finn, K.J.; Ji, X.; Baillat, D.; Gregory, R.I.; Liebhaber, S.A.; Pasquinelli, A.E.; Shiekhattar, R. MicroRNA silencing through RISC recruitment of eIF6. Nature 2007, 447, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, D.T.; Westman, B.J.; Martin, D.I.; Preiss, T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. USA 2005, 102, 16961–16966. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, E.J.; Paydar, I.; Anderson, K.K.; Patton, J.G. Regulation of zebrafish fin regeneration by microRNAs. Proc. Natl. Acad. Sci. USA 2008, 105, 18384–18389. [Google Scholar] [CrossRef] [PubMed]
- Yin, V.P.; Thomson, J.M.; Thummel, R.; Hyde, D.R.; Hammond, S.M.; Poss, K.D. Fgf-dependent depletion of microRNA-133 promotes appendage regeneration in zebrafish. Genes Dev. 2008, 22, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Gomez, A.W.; Armstrong, B.E.; Henner, A.; Stankunas, K. Sequential and opposing activities of Wnt and BMP coordinate zebrafish bone regeneration. Cell Rep. 2014, 6, 482–498. [Google Scholar] [CrossRef] [PubMed]
- Inose, H.; Ochi, H.; Kimura, A.; Fujita, K.; Xu, R.; Sato, S.; Iwasaki, M.; Sunamura, S.; Takeuchi, Y.; Fukumoto, S.; et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 20794–20799. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hassan, M.Q.; Volinia, S.; van Wijnen, A.J.; Stein, J.L.; Croce, C.M.; Lian, J.B.; Stein, G.S. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc. Natl. Acad. Sci. USA 2008, 105, 13906–13911. [Google Scholar] [CrossRef]
- Yin, V.P.; Poss, K.D. New regulators of vertebrate appendage regeneration. Curr. Opin. Genet. Dev. 2008, 18, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.D. Integration of intercellular signaling through the Hippo pathway. Semin. Cell Dev. Biol. 2012, 23, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Irvine, K.D. The Hippo Signaling Network and Its Biological Functions. Annu. Rev. Genet. 2018, 52, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Reviews. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Quint, E.; Smith, A.; Avaron, F.; Laforest, L.; Miles, J.; Gaffield, W.; Akimenko, M.A. Bone patterning is altered in the regenerating zebrafish caudal fin after ectopic expression of sonic hedgehog and bmp2b or exposure to cyclopamine. Proc. Natl. Acad. Sci. USA 2002, 99, 8713–8718. [Google Scholar] [CrossRef]
- Renn, J.; Winkler, C. Osterix/Sp7 regulates biomineralization of otoliths and bone in medaka (Oryzias latipes). Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 34, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, Z.; Yang, J.; Huang, J.; Jiang, H. Sp7/osterix positively regulates dlx2b and bglap to affect tooth development and bone mineralization in zebrafish larvae. J. Biosci. 2019, 44, 127. [Google Scholar] [CrossRef]
- Ducy, P.; Starbuck, M.; Priemel, M.; Shen, J.; Pinero, G.; Geoffroy, V.; Amling, M.; Karsenty, G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999, 13, 1025–1036. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Holdway, J.E.; Poss, K.D. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev. Cell 2012, 22, 879–886. [Google Scholar] [CrossRef]
- McMillan, S.C.; Zhang, J.; Phan, H.E.; Jeradi, S.; Probst, L.; Hammerschmidt, M.; Akimenko, M.A. A regulatory pathway involving retinoic acid and calcineurin demarcates and maintains joint cells and osteoblasts in regenerating fin. Development 2018, 145, dev161158. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Felber, K.; Elks, P.; Croucher, P.; Roehl, H.H. Tracking gene expression during zebrafish osteoblast differentiation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 459–466. [Google Scholar] [CrossRef]
- Smith, A.; Zhang, J.; Guay, D.; Quint, E.; Johnson, A.; Akimenko, M.A. Gene expression analysis on sections of zebrafish regenerating fins reveals limitations in the whole-mount in situ hybridization method. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 417–425. [Google Scholar] [CrossRef]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2007, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Liu, X.; Duan, Z.; Zhao, H.; Chang, Z.; Li, L. The Regulatory Role of miRNAs in Zebrafish Fin Regeneration. Int. J. Mol. Sci. 2024, 25, 10542. https://doi.org/10.3390/ijms251910542
Fan J, Liu X, Duan Z, Zhao H, Chang Z, Li L. The Regulatory Role of miRNAs in Zebrafish Fin Regeneration. International Journal of Molecular Sciences. 2024; 25(19):10542. https://doi.org/10.3390/ijms251910542
Chicago/Turabian StyleFan, Jiaqi, Xinya Liu, Ziheng Duan, Hanya Zhao, Zhongjie Chang, and Li Li. 2024. "The Regulatory Role of miRNAs in Zebrafish Fin Regeneration" International Journal of Molecular Sciences 25, no. 19: 10542. https://doi.org/10.3390/ijms251910542



