miR-21 and miR-145 as Prognostic Biomarkers for Radiotherapy Responses in Cervical Cancer Patients: A Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Clinical Data
2.2. Correlation between Histopathology and Disease Staging towards Radiation Response
2.3. Relationship between miR-21 and miR-145 Level of Expression and Radiation Therapy Response
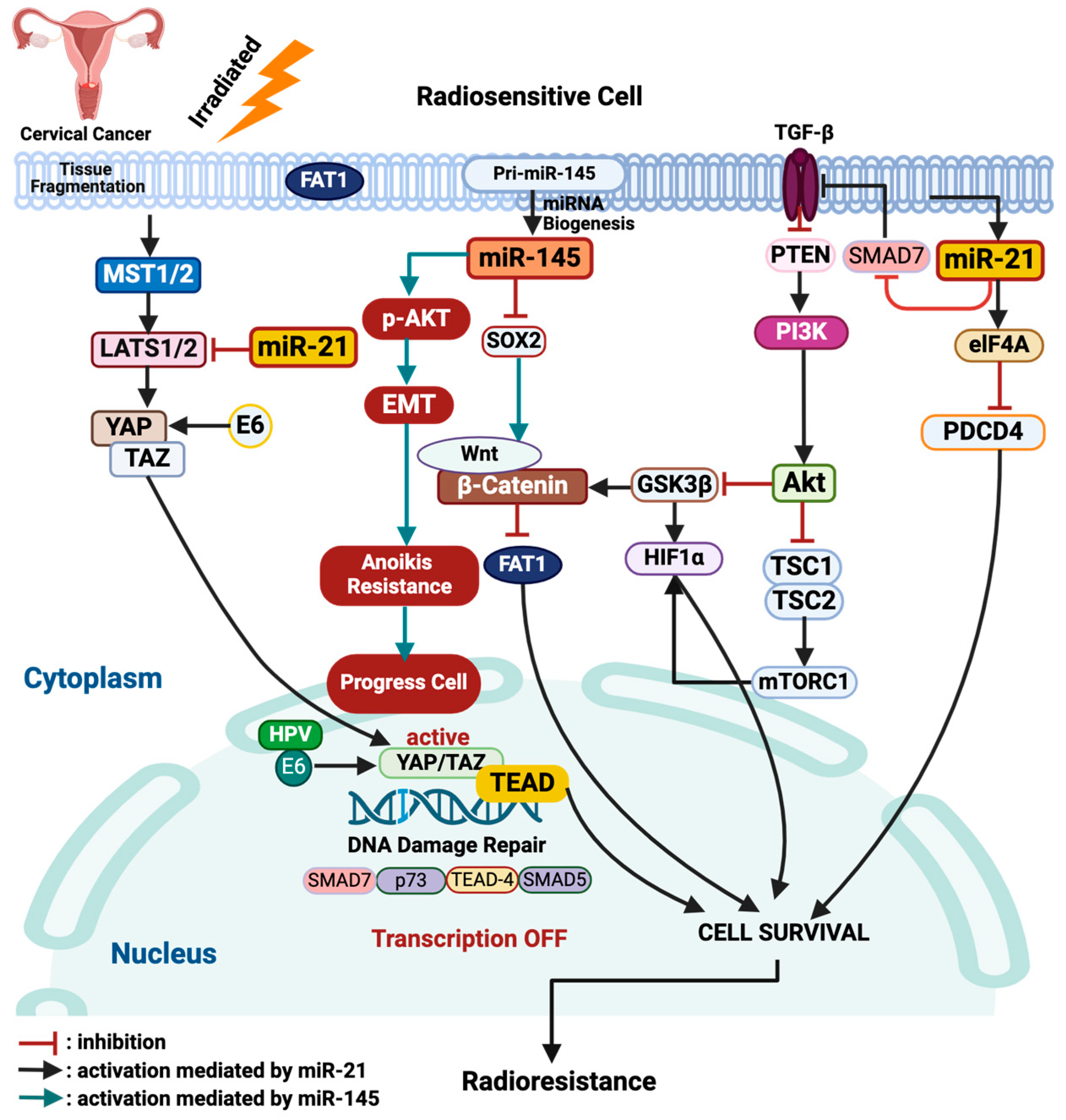
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Clearance
4.2. Radiation Response Evaluation Criteria
4.3. Measurement of miR-21 and miR-145 Expression Levels
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Cancer Observatory 2022: Incidence and Mortality by Country and by Region. 2022. Available online: https://gco.iarc.fr/today/en/dataviz/bars?types=0_1&mode=population (accessed on 2 April 2024).
- Nowakowski, A.; Cybulski, M.; Buda, I.; Janosz, I.; Olszak-Wąsik, K.; Bodzek, P.; Śliwczyński, A.; Teter, Z.; Olejek, A.; Baranowski, W. Cervical Cancer Histology, Staging and Survival before and after Implementation of Organised Cervical Screening Programme in Poland. PLoS ONE 2016, 11, e0155849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Chen, L.-J. The role of miRNAs in the invasion and metastasis of cervical cancer. Biosci. Rep. 2019, 39, BSR20181377. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, S.E.; Trianto, H.F.; Fatinah, N.N.; Ilmiawan, M.I.; Fitrianingrum, I.; Lestari, D. The Profile of Cervical Cancer Patients at Soedarso Hospital. Indones. J. Cancer 2022, 16, 33–38. [Google Scholar] [CrossRef]
- Nin, D.S.; Wujanto, C.; Tan, T.Z.; Lim, D.; Damen, J.M.A.; Wu, K.-Y.; Dai, Z.M.; Lee, Z.-W.; Idres, S.B.; Leong, Y.H.; et al. GAGE mediates radio resistance in cervical cancers via the regulation of chromatin accessibility. Cell Rep. 2021, 36, 109621. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Gorini, F.; Dondi, G.; Tesei, M.; De Crescenzo, E.; Morganti, A.G.; Hrelia, P.; De Iaco, P.; Angelini, S.; Perrone, A.M. Emerging Role of MicroRNAs in the Therapeutic Response in Cervical Cancer: A Systematic Review. Front. Oncol. 2022, 12, 847974. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, M.; Zehentmayr, F.; Niemöller, O.M.; Eigenbrod, S.; Kretzschmar, H.; Schulze-Osthoff, K.; Tonn, J.-C.; Atkinson, M.; Mörtl, S.; Belka, C. MiRNA expression patterns predict survival in glioblastoma. Radiat. Oncol. 2011, 6, 153. [Google Scholar] [CrossRef]
- Wang, X.-C.; Wang, W.; Zhang, Z.-B.; Zhao, J.; Tan, X.-G.; Luo, J.-C. Overexpression of miRNA-21 promotes radiation-resistance of non-small cell lung cancer. Radiat. Oncol. 2013, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. New progress and challenge in gynecological cancer. Ann. Transl. Med. 2022, 10, 119. [Google Scholar] [CrossRef]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int. J. Oncol. 2021, 59, 53. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Zheng, W.; Mo, X.; Liu, F.; Li, M.; Liu, Z.; Zhang, W.; Hu, X. Dysregulated microRNAs involved in the progression of cervical neoplasm. Arch. Gynecol. Obstet. 2015, 292, 905–913. [Google Scholar] [CrossRef]
- Du, G.; Cao, D.; Meng, L. miR-21 inhibitor suppresses cell proliferation and colony formation through regulating the PTEN/AKT pathway and improves paclitaxel sensitivity in cervical cancer cells. Mol. Med. Rep. 2017, 15, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- Zamani, S.; Sohrabi, A.; Hosseini, S.M.; Rahnamaye-Farzami, M.; Akbari, A. Deregulation of miR-21 and miR-29a in Cervical Cancer Related to HPV Infection. MicroRNA 2019, 8, 110–115. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Fan, K.; Jiang, C. MicroRNA-21 and its impact on signaling pathways in cervical cancer (Review). Oncol. Lett. 2019, 17, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Liolios, T.; Kastora, S.L.; Colombo, G. MicroRNAs in Female Malignancies. Cancer Inform. 2019, 18, 1176935119828746. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef]
- Ye, D.; Shen, Z.; Zhou, S. Function of microRNA-145 and mechanisms underlying its role in malignant tumor diagnosis and treatment. Cancer Manag. Res. 2019, 11, 969–979. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Wang, J.-J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.-H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Zhu, S.; Si, M.-L.; Wu, H.; Mo, Y.-Y. MicroRNA-21 Targets the Tumor Suppressor Gene Tropomyosin 1 (TPM1). J. Biol. Chem. 2007, 282, 14328–14336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Q.; Hu, Y.; Li, J.; Li, X.; Zhou, L.; Huang, Y. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol. Rep. 2012, 27, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, W.; Lv, Q.; Zhu, D. Overexpression of miR-21 promotes the proliferation and migration of cervical cancer cells via the inhibition of PTEN. Oncol. Rep. 2015, 33, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Wang, H.; Liu, J.; Wang, Z.-X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2012, 372, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-P.; He, C.-Y.; Zhu, Z.-T. Role of microRNA-21 in radiosensitivity in non-small cell lung cancer cells by targeting PDCD4 gene. Oncotarget 2017, 8, 23675–23689. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lu, X.; Liu, L.; Xu, J.; Feng, D.; Shu, Y. MiRNA-21. Cancer Biol. Ther. 2012, 13, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Martínez, S.Y.; Campos-Viguri, G.E.; Medina-García, S.E.; García-Flores, R.J.; Deas, J.; Gómez-Cerón, C.; Pedroza-Torres, A.; Bautista-Rodríguez, E.; Fernández-Tilapa, G.; Rodríguez-Dorantes, M.; et al. MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4086. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, S.; Wang, D. Overexpression of microRNA-21 decreased the sensitivity of advanced cervical cancer to chemoradiotherapy through SMAD7. Anti Cancer Drugs 2020, 31, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Mirdamadi, M.S.A.; Talebi, Y.; Khaniabad, N.; Banaei, G.; Daneii, P.; Gholami, S.; Ghorbani, A.; Tavakolpournegari, A.; Farsani, Z.M.; et al. Pre-clinical and clinical importance of miR-21 in human cancers: Tumorigenesis, therapy response, delivery approaches and targeting agents. Pharmacol. Res. 2023, 187, 106568. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shao, J.; Jin, Y.-J.; Kawase, H.; Ong, Y.T.; Troidl, K.; Quan, Q.; Wang, L.; Bonnavion, R.; Wietelmann, A.; et al. Endothelial FAT1 inhibits angiogenesis by controlling YAP/TAZ protein degradation via E3 ligase MIB2. Nat. Commun. 2023, 14, 1980. [Google Scholar] [CrossRef]
- Nozaki, M.; Yabuta, N.; Fukuzawa, M.; Mukai, S.; Okamoto, A. LATS1/2 kinases trigger self-renewal of cancer stem cells in aggressive oral cancer. Oncotarget 2019, 10, 1014–1030. [Google Scholar] [CrossRef]
- Liu, S.; Song, L.; Zhang, L.; Zeng, S.; Gao, F. miR-21 modulates resistance of HR-HPV positive cervical cancer cells to radiation through targeting LATS1. Biochem. Biophys. Res. Commun. 2015, 459, 679–685. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Mao, D.; Hua, G.; Lv, X.; Chen, X.; Angeletti, P.C.; Dong, J.; Remmenga, S.W.; Rodabaugh, K.J.; Zhou, J.; et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol. Med. 2015, 7, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, T.; He, D.; Hsieh, J.-T. MicroRNA-145 Modulates Tumor Sensitivity to Radiation in Prostate Cancer. Radiat. Res. 2015, 184, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, J.; Dong, W.; Xie, J.; Zhao, X. The diagnostic value of miR-145 and miR-205 in patients with cervical cancer. Am. J. Transl. Res. 2021, 13, 1825–1832. [Google Scholar] [PubMed]
- Cheng, X.; Shen, T.; Liu, P.; Fang, S.; Yang, Z.; Li, Y.; Dong, J. mir-145-5p is a suppressor of colorectal cancer at early stage, while promotes colorectal cancer metastasis at late stage through regulating AKT signaling evoked EMT-mediated anoikis. BMC Cancer 2022, 22, 1151. [Google Scholar] [CrossRef]
- Manvati, S.; Mangalhara, K.C.; Kalaiarasan, P.; Chopra, R.; Agarwal, G.; Kumar, R.; Saini, S.K.; Kaushik, M.; Arora, A.; Kumari, U.; et al. miR-145 supports cancer cell survival and shows association with DDR genes, methylation pattern, and epithelial to mesenchymal transition. Cancer Cell Int. 2019, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Martin, G.S.; Schulz, K.J.; Ronck, M.; Toussaint, L.G. Serial selection for invasiveness increases expression of miR-143/miR-145 in glioblastoma cell lines. BMC Cancer 2012, 12, 143. [Google Scholar] [CrossRef]
- Naito, Y.; Yasuno, K.; Tagawa, H.; Sakamoto, N.; Oue, N.; Yashiro, M.; Sentani, K.; Goto, K.; Shinmei, S.; Oo, H.Z.; et al. MicroRNA-145 is a potential prognostic factor of scirrhous type gastric cancer. Oncol. Rep. 2014, 32, 1720–1726. [Google Scholar] [CrossRef]
- Zhang, Q.; Gan, H.; Song, W.; Chai, D.; Wu, S. MicroRNA-145 promotes esophageal cancer cells proliferation and metastasis by targeting SMAD5. Scand. J. Gastroenterol. 2018, 53, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Salice, P.; Tescari, S.; Menna, E.; De Filippis, V.; Filippini, F. Enhanced neuronal cell differentiation combining biomimetic peptides and a carbon nanotube-polymer scaffold. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]

| Variable | Median (Q1–Q4) | n (%) |
|---|---|---|
| Age (years) | 52 (44–59) | |
| Marital status | ||
| No information | 2 (1.4) | |
| Not married | 4 (2.9) | |
| Married | 107 (76.4) | |
| Married > 1 time(s) | 27 (19,3) | |
| Parity | 3 (2–4) | |
| 0 | 8 (5.7) | |
| 1 | 14 (10) | |
| 2 | 40 (28.6) | |
| >2 | 78 (55.7) | |
| Abortion | 0 (0–0) | |
| 0 | 109 (77.9) | |
| 1 | 24 (17.1) | |
| 2 | 7 (5.0) |
| Variable | n (%) | Radiosensitive (CR/PR) n (%) | Radioresistant (SD/PD) n (%) | p-Value |
|---|---|---|---|---|
| Histopathology | ||||
| SCC | 119 (85) | 47 (39.5) | 72 (60.5) | 0.617 |
| AC | 11 (7.9) | 5 (45.5) | 6 (54.5) | |
| Others | 10 (7.1) | 3 (30) | 7 (70) | |
| FIGO Stage Classification | ||||
| IIIB | 102 (72.9) | 51 (50) | 51 (50) | <0.001 * |
| IVA | 38 (27.1) | 4 (10.5) | 34 (89.5) |
| Variable | Staging | Radiosensitive | Radioresistance | p-Value * |
|---|---|---|---|---|
| Median (Q1–Q4) | Median (Q1–Q4) | |||
| miR-21 nmol/(mg/mL) | Stage IIIB | 0.154 × 10−3 (0.00129 × 10−3–6.5512 × 10−3) | 2.737 × 10−3 (0.180 × 10−3–22.424 × 10−3) | 0.011 |
| Stage IVA | 0.029 × 10−3 (0.00031 × 10−3–0.096 × 10−3) | 3.582 × 10−3 (0.392 × 10−3–18.767 × 10−3) | 0.004 | |
| Total | 0.0801 × 10−3 (0.0013 × 10−3–6.31 × 10−3) | 2.9 ×10−3 (0.32 × 10−3–20 × 10−3) | <0.001 | |
| miR-145 nmol/(mg/mL) | Stage IIIB | 6.3 × 10−3 (0.2 × 10−3–122.4 × 10−3) | 25.1 × 10−3 (4.8 × 10−3–202.2 × 10−3) | 0.081 |
| Stage IVA | 5.0 × 10−3 (0.8 × 10−3–391.7 × 10−3) | 57.5 × 10−3 (4.2 × 10−3–369.6 × 10−3) | 0.407 | |
| Total | 6.4 × 10−3 (0.2 × 10−3–120 × 10−3) | 35.2 × 10−3 (4.8 × 10−3–220 × 10−3) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, A.D.; Andrijono; Winarto, H.; Prijanti, A.R.; Rachmadi, L.; Pakasi, T.A.; Gandamihardja, S.; Wirasugianto, J.; Amelia. miR-21 and miR-145 as Prognostic Biomarkers for Radiotherapy Responses in Cervical Cancer Patients: A Preliminary Study. Int. J. Mol. Sci. 2024, 25, 10545. https://doi.org/10.3390/ijms251910545
Putra AD, Andrijono, Winarto H, Prijanti AR, Rachmadi L, Pakasi TA, Gandamihardja S, Wirasugianto J, Amelia. miR-21 and miR-145 as Prognostic Biomarkers for Radiotherapy Responses in Cervical Cancer Patients: A Preliminary Study. International Journal of Molecular Sciences. 2024; 25(19):10545. https://doi.org/10.3390/ijms251910545
Chicago/Turabian StylePutra, Andi D., Andrijono, Hariyono Winarto, Ani R. Prijanti, Lisnawati Rachmadi, Trevino A. Pakasi, Supriadi Gandamihardja, Jourdan Wirasugianto, and Amelia. 2024. "miR-21 and miR-145 as Prognostic Biomarkers for Radiotherapy Responses in Cervical Cancer Patients: A Preliminary Study" International Journal of Molecular Sciences 25, no. 19: 10545. https://doi.org/10.3390/ijms251910545
APA StylePutra, A. D., Andrijono, Winarto, H., Prijanti, A. R., Rachmadi, L., Pakasi, T. A., Gandamihardja, S., Wirasugianto, J., & Amelia. (2024). miR-21 and miR-145 as Prognostic Biomarkers for Radiotherapy Responses in Cervical Cancer Patients: A Preliminary Study. International Journal of Molecular Sciences, 25(19), 10545. https://doi.org/10.3390/ijms251910545







