Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment
Abstract
1. Introduction
1.1. Influenza Viruses
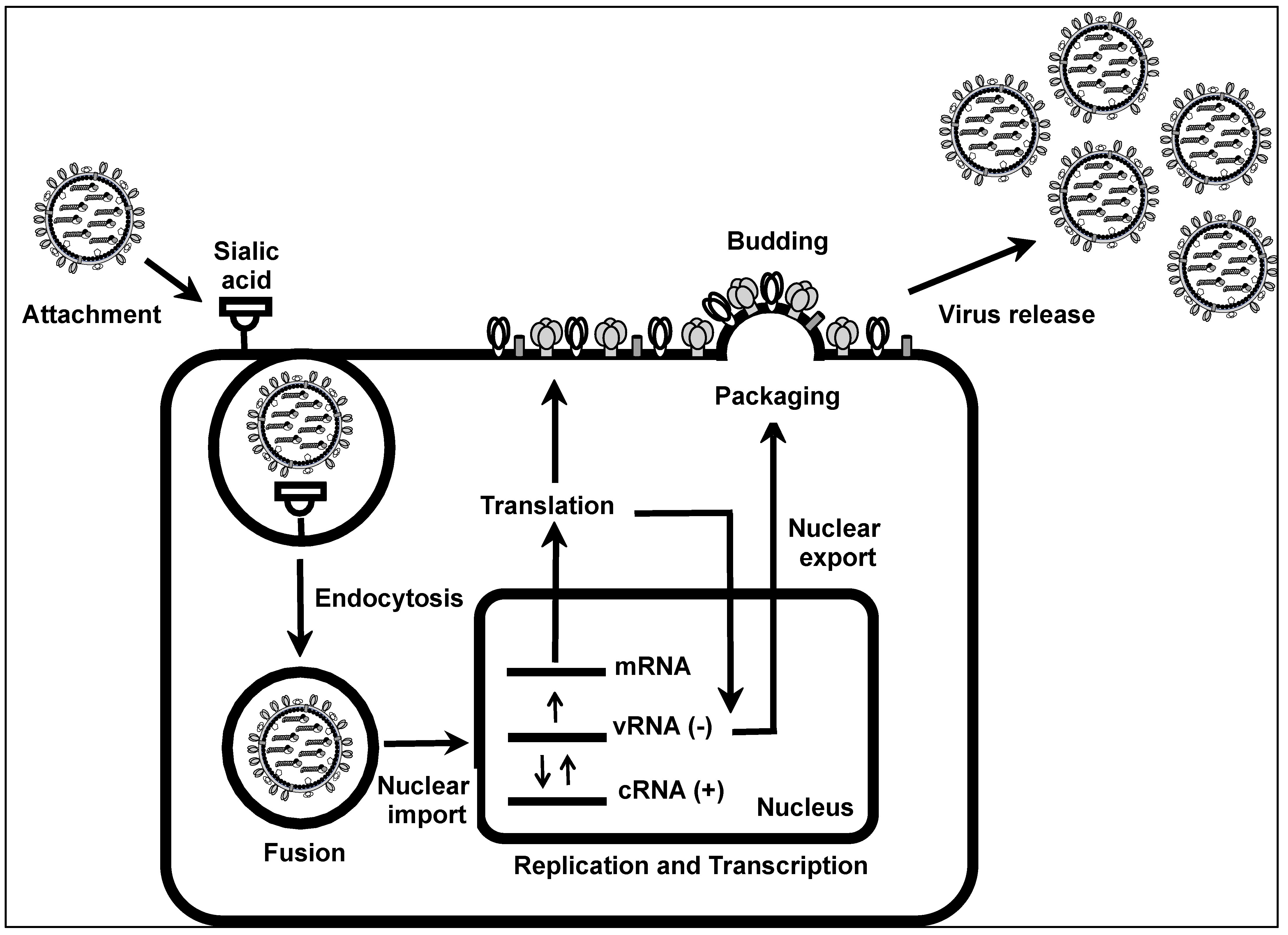
1.2. IAV Life Cycle
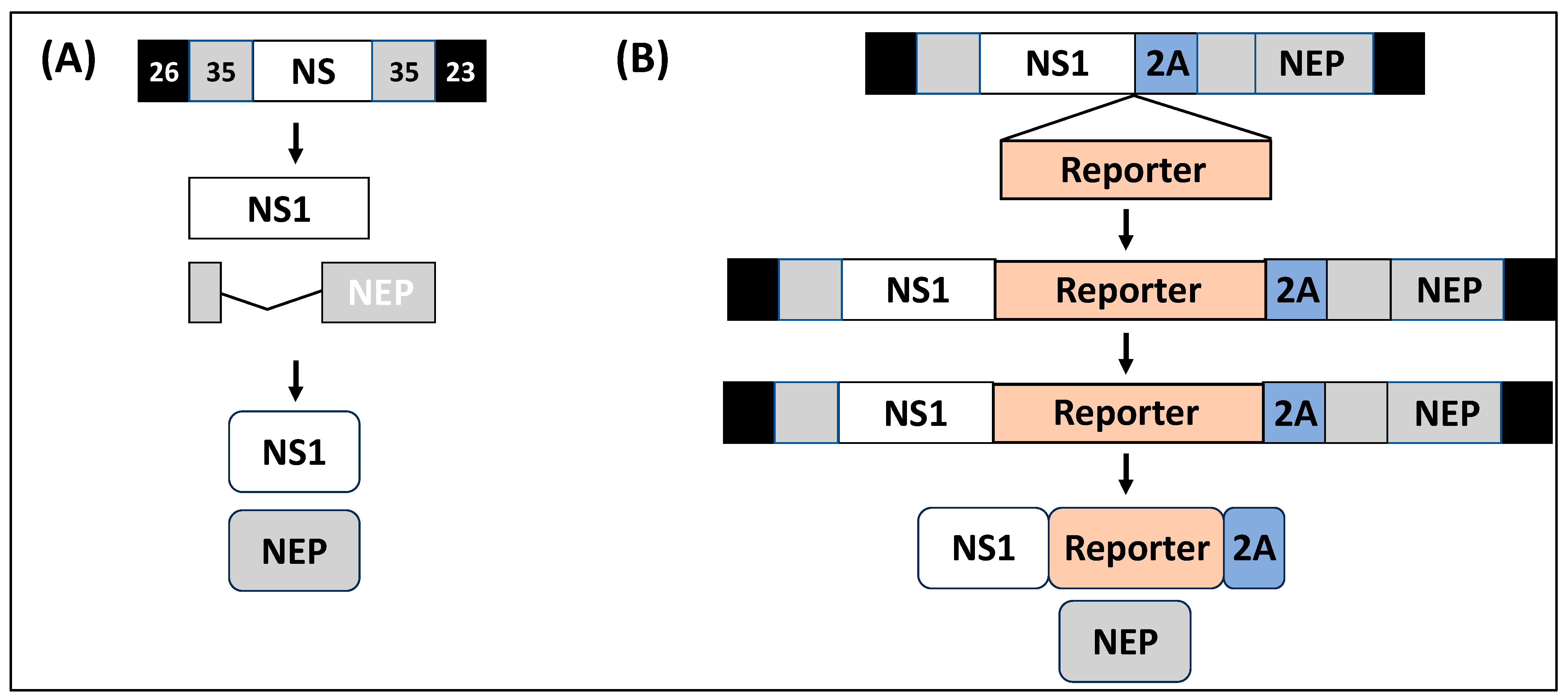
1.3. IAV NS Segment
1.4. Reverse Genetics of IAV
2. rIAV Expressing Reporter Genes from the NS Segment
2.1. rIAV Expressing Reporter Genes from a Modified NS Segment
2.2. rIAV Expressing Reporter Genes from Modified NS and HA Segments
2.3. rIAV Expressing Reporter Genes Instead of the Viral NS1 Protein
3. Conclusions and Future Directions: Recombinant
Author Contributions
Funding
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef] [PubMed]
- Rajao, D.S.; Perez, D.R. Universal Vaccines and Vaccine Platforms to Protect against Influenza Viruses in Humans and Agriculture. Front. Microbiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The 1918 Influenza Pandemic and Its Legacy. Cold Spring Harb. Perspect. Med. 2020, 10, a038695. [Google Scholar] [CrossRef]
- Blanco-Lobo, P.; Nogales, A.; Rodriguez, L.; Martinez-Sobrido, L. Novel Approaches for The Development of Live Attenuated Influenza Vaccines. Viruses 2019, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; DeDiego, M.L.; Martinez-Sobrido, L. Live attenuated influenza A virus vaccines with modified NS1 proteins for veterinary use. Front. Cell. Infect. Microbiol. 2022, 12, 954811. [Google Scholar] [CrossRef]
- Park, J.G.; Avila-Perez, G.; Nogales, A.; Blanco-Lobo, P.; de la Torre, J.C.; Martinez-Sobrido, L. Identification and Characterization of Novel Compounds with Broad-Spectrum Antiviral Activity against Influenza A and B Viruses. J. Virol. 2020, 94, 1110–1128. [Google Scholar] [CrossRef]
- Baker, S.F.; Nogales, A.; Santiago, F.W.; Topham, D.J.; Martinez-Sobrido, L. Competitive detection of influenza neutralizing antibodies using a novel bivalent fluorescence-based microneutralization assay (BiFMA). Vaccine 2015, 33, 3562–3570. [Google Scholar] [CrossRef]
- Chiem, K.; Nogales, A.; Martinez-Sobrido, L. Generation, Characterization, and Applications of Influenza A Reporter Viruses. Methods Mol. Biol. 2022, 2524, 249–268. [Google Scholar]
- Eckert, N.; Wrensch, F.; Gärtner, S.; Palanisamy, N.; Goedecke, U.; Jäger, N.; Pöhlmann, S.; Winkler, M. Influenza A virus encoding secreted Gaussia luciferase as useful tool to analyze viral replication and its inhibition by antiviral compounds and cellular proteins. PLoS ONE 2014, 9, e97695. [Google Scholar] [CrossRef]
- Nogales, A.; Avila-Perez, G.; Rangel-Moreno, J.; Chiem, K.; DeDiego, M.L.; Martinez-Sobrido, L. A Novel Fluorescent and Bioluminescent Bireporter Influenza A Virus To Evaluate Viral Infections. J. Virol. 2019, 93, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Chiem, K.; Breen, M.; DeDiego, M.L.; Parrish, C.R.; Martinez-Sobrido, L. Generation and Characterization of Single-Cycle Infectious Canine Influenza A Virus (sciCIV) and Its Use as Vaccine Platform. Methods Mol. Biol. 2022, 2465, 227–255. [Google Scholar] [PubMed]
- Hengrung, N.; El Omari, K.; Serna Martin, I.; Vreede, F.T.; Cusack, S.; Rambo, R.P.; Vonrhein, C.; Bricogne, G.; Stuart, D.I.; Grimes, J.M.; et al. Crystal structure of the RNA-dependent RNA polymerase from influenza C virus. Nature 2015, 527, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Fodor, E.; Te Velthuis, A.J.W. Structure and Function of the Influenza Virus Transcription and Replication Machinery. Cold Spring Harb. Perspect. Med. 2020, 10, a038398. [Google Scholar] [CrossRef]
- Zhu, Z.; Fodor, E.; Keown, J.R. A structural understanding of influenza virus genome replication. Trends Microbiol. 2023, 31, 308–319. [Google Scholar] [CrossRef]
- Carter, T.; Iqbal, M. The Influenza A Virus Replication Cycle: A Comprehensive Review. Viruses 2024, 16, 316. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Ostbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef]
- Rogers, G.N.; D’Souza, B.L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology 1989, 173, 317–322. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Srinivasan, A.; Raman, R.; Viswanathan, K.; Raguram, S.; Tumpey, T.M.; Sasisekharan, V.; Sasisekharan, R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat. Biotechnol. 2008, 26, 107–113. [Google Scholar] [CrossRef]
- Connor, R.J.; Kawaoka, Y.; Webster, R.G.; Paulson, J.C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 1994, 205, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Lakadamyali, M.; Zhang, F.; Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004, 11, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K. Architecture of a nascent viral fusion pore. EMBO J. 2010, 29, 1299–1311. [Google Scholar] [CrossRef]
- Skehel, J.J.; Bayley, P.M.; Brown, E.B.; Martin, S.R.; Waterfield, M.D.; White, J.M.; Wilson, I.A.; Wiley, D.C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc. Natl. Acad. Sci. USA 1982, 79, 968–972. [Google Scholar] [CrossRef]
- Chou, Y.Y.; Heaton, N.S.; Gao, Q.; Palese, P.; Singer, R.H.; Lionnet, T. Colocalization of different influenza viral RNA segments in the cytoplasm before viral budding as shown by single-molecule sensitivity FISH analysis. PLoS Pathog. 2013, 9, e1003358. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Chen, Q.; Wang, H.; Yao, Y.; Chen, J.; Chen, Z. A second CRM1-dependent nuclear export signal in the influenza A virus NS2 protein contributes to the nuclear export of viral ribonucleoproteins. J. Virol. 2013, 87, 767–778. [Google Scholar] [CrossRef]
- Amorim, M.J.; Bruce, E.A.; Read, E.K.; Foeglein, A.; Mahen, R.; Stuart, A.D.; Digard, P. A Rab11-and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J. Virol. 2011, 85, 4143–4156. [Google Scholar] [CrossRef] [PubMed]
- Lakdawala, S.S.; Wu, Y.; Wawrzusin, P.; Kabat, J.; Broadbent, A.J.; Lamirande, E.W.; Fodor, E.; Altan-Bonnet, N.; Shroff, H.; Subbarao, K. Influenza a virus assembly intermediates fuse in the cytoplasm. PLoS Pathog. 2014, 10, e1003971. [Google Scholar] [CrossRef]
- Li, X.; Gu, M.; Zheng, Q.; Gao, R.; Liu, X. Packaging signal of influenza A virus. Virol. J. 2021, 18, 36. [Google Scholar] [CrossRef]
- Goto, H.; Muramoto, Y.; Noda, T.; Kawaoka, Y. The genome-packaging signal of the influenza A virus genome comprises a genome incorporation signal and a genome-bundling signal. J. Virol. 2013, 87, 11316–11322. [Google Scholar] [CrossRef]
- Jakob, C.; Paul-Stansilaus, R.; Schwemmle, M.; Marquet, R.; Bolte, H. The influenza A virus genome packaging network—Complex, flexible and yet unsolved. Nucleic Acids Res. 2022, 50, 9023–9038. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Okura, T.; Kawahara, M.; Takizawa, N.; Momose, F.; Morikawa, Y. Apical Trafficking Pathways of Influenza A Virus HA and NA via Rab17- and Rab23-Positive Compartments. Front. Microbiol. 2019, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.P.; Lamb, R.A. Influenza virus assembly and budding at the viral budozone. Adv. Virus Res. 2005, 64, 383–416. [Google Scholar] [PubMed]
- Petrich, A.; Dunsing, V.; Bobone, S.; Chiantia, S. Influenza A M2 recruits M1 to the plasma membrane: A fluorescence fluctuation microscopy study. Biophys. J. 2021, 120, 5478–5490. [Google Scholar] [CrossRef]
- Calder, L.J.; Wasilewski, S.; Berriman, J.A.; Rosenthal, P.B. Structural organization of a filamentous influenza A virus. Proc. Natl. Acad. Sci. USA 2010, 107, 10685–10690. [Google Scholar] [CrossRef]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Balannik, V.; Pinto, L.H.; Lamb, R.A. Influenza virus m2 ion channel protein is necessary for filamentous virion formation. J. Virol. 2010, 84, 5078–5088. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Nogales, A.; Baker, S.F.; Martinez-Sobrido, L. Replication-competent influenza A viruses expressing a red fluorescent protein. Virology 2015, 476, 206–216. [Google Scholar] [CrossRef]
- Khalil, A.M.; Nogales, A.; Martinez-Sobrido, L.; Mostafa, A. Antiviral responses versus virus-induced cellular shutoff: A game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1. Front. Cell. Infect. Microbiol. 2024, 14, 1357866. [Google Scholar] [CrossRef]
- Evseev, D.; Magor, K.E. Molecular Evolution of the Influenza A Virus Non-structural Protein 1 in Interspecies Transmission and Adaptation. Front. Microbiol. 2021, 12, 693204. [Google Scholar] [CrossRef]
- Blake, M.E.; Kleinpeter, A.B.; Jureka, A.S.; Petit, C.M. Structural Investigations of Interactions between the Influenza a Virus NS1 and Host Cellular Proteins. Viruses 2023, 15, 2063. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Aydillo, T.; Ávila-Pérez, G.; Escalera, A.; Chiem, K.; Cadagan, R.; DeDiego, M.L.; Li, F.; García-Sastre, A.; Martínez-Sobrido, L. Functional Characterization and Direct Comparison of Influenza A, B, C, and D NS1 Proteins in vitro and in vivo. Front. Microbiol. 2019, 10, 2862. [Google Scholar] [CrossRef]
- Nogales, A.; Villamayor, L.; Utrilla-Trigo, S.; Ortego, J.; Martinez-Sobrido, L.; DeDiego, M.L. Natural Selection of H5N1 Avian Influenza A Viruses with Increased PA-X and NS1 Shutoff Activity. Viruses 2021, 13, 1760. [Google Scholar] [CrossRef]
- Villamayor, L.; Lopez-Garcia, D.; Rivero, V.; Martinez-Sobrido, L.; Nogales, A.; DeDiego, M.L. The IFN-stimulated gene IFI27 counteracts innate immune responses after viral infections by interfering with RIG-I signaling. Front. Microbiol. 2023, 14, 1176177. [Google Scholar] [CrossRef] [PubMed]
- Villamayor, L.; Rivero, V.; López-García, D.; Topham, D.J.; Martínez-Sobrido, L.; Nogales, A.; DeDiego, M.L. Interferon alpha inducible protein 6 is a negative regulator of innate immune responses by modulating RIG-I activation. Front. Immunol. 2023, 14, 1105309. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, P.L.; Muñoz-Moreno, R.; Fribourg, M.; Potla, U.; Mena, I.; Marjanovic, N.; Hartmann, B.M.; Sealfon, S.C.; García-Sastre, A.; Ramos, I.; et al. Differential Modulation of Innate Immune Responses in Human Primary Cells by Influenza A Viruses Carrying Human or Avian Nonstructural Protein 1. J. Virol. 2019, 94, 110–128. [Google Scholar] [CrossRef]
- Ramos, I.; Smith, G.; Ruf-Zamojski, F.; Martínez-Romero, C.; Fribourg, M.; Carbajal, E.A.; Hartmann, B.M.; Nair, V.D.; Marjanovic, N.; Monteagudo, P.L.; et al. Innate Immune Response to Influenza Virus at Single-Cell Resolution in Human Epithelial Cells Revealed Paracrine Induction of Interferon Lambda 1. J. Virol. 2019, 93, 110–128. [Google Scholar] [CrossRef]
- Golovko, A.O.; Koroleva, O.N.; Drutsa, V.L. Heterologous Expression and Isolation of Influenza A Virus Nuclear Export Protein NEP. Biochemistry 2017, 82, 1529–1537. [Google Scholar] [CrossRef]
- Gorai, T.; Goto, H.; Noda, T.; Watanabe, T.; Kozuka-Hata, H.; Oyama, M.; Takano, R.; Neumann, G.; Watanabe, S.; Kawaoka, Y. F1Fo-ATPase, F-type proton-translocating ATPase, at the plasma membrane is critical for efficient influenza virus budding. Proc. Natl. Acad. Sci. USA 2012, 109, 4615–4620. [Google Scholar] [CrossRef]
- Paterson, D.; Fodor, E. Emerging roles for the influenza A virus nuclear export protein (NEP). PLoS Pathog. 2012, 8, e1003019. [Google Scholar] [CrossRef]
- Martinez-Sobrido, L.; DeDiego, M.L.; Nogales, A. AGL2017-82570-RReverse genetics approaches for the development of new vaccines against influenza A virus infections. Curr. Opin. Virol. 2020, 44, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Yamada, S.; da Silva Lopes, T.J.; Dutta, J.; Khan, Z.; Kriti, D.; van Bakel, H.; Kawaoka, Y. Influenza Virus Polymerase Mutation Stabilizes a Foreign Gene Inserted into the Virus Genome by Enhancing the Transcription/Replication Efficiency of the Modified Segment. mBio 2019, 10, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Baker, S.F.; Domm, W.; Martinez-Sobrido, L. Development and applications of single-cycle infectious influenza A virus (sciIAV). Virus Res. 2016, 216, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Q.; Zhao, X.; Li, P.; Wang, Y.; Rong, L.; Du, R. Generation of a Reassortant Influenza A Subtype H3N2 Virus Expressing Gaussia Luciferase. Viruses 2019, 11, 665. [Google Scholar] [CrossRef]
- Breen, M.; Nogales, A.; Baker, S.F.; Martinez-Sobrido, L. Replication-Competent Influenza A Viruses Expressing Reporter Genes. Viruses 2016, 8, 179. [Google Scholar] [CrossRef]
- Fukuyama, S.; Katsura, H.; Zhao, D.; Ozawa, M.; Ando, T.; Shoemaker, J.E.; Ishikawa, I.; Yamada, S.; Neumann, G.; Watanabe, S.; et al. Multi-spectral fluorescent reporter influenza viruses (Color-flu) as powerful tools for in vivo studies. Nat. Commun. 2015, 6, 6600. [Google Scholar] [CrossRef]
- Reuther, P.; Gopfert, K.; Dudek, A.H.; Heiner, M.; Herold, S.; Schwemmle, M. Generation of a variety of stable Influenza A reporter viruses by genetic engineering of the NS gene segment. Sci. Rep. 2015, 5, 11346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, X.; Li, P.; Chen, Z.; Zhang, C.; Manicassamy, B.; Rong, L.; Cui, Q.; Du, R. Expanding the tolerance of segmented Influenza A Virus genome using a balance compensation strategy. PLoS Pathog. 2022, 18, e1010756. [Google Scholar] [CrossRef]
- Hoffmann, E.; Neumann, G.; Hobom, G.; Webster, R.G.; Kawaoka, Y. “Ambisense” approach for the generation of influenza A virus: vRNA and mRNA synthesis from one template. Virology 2000, 267, 310–317. [Google Scholar] [CrossRef]
- Hoffmann, E.; Neumann, G.; Kawaoka, Y.; Hobom, G.; Webster, R.G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 2000, 97, 6108–6113. [Google Scholar] [CrossRef]
- Nogales, A.; Piepenbrink, M.S.; Wang, J.; Ortega, S.; Basu, M.; Fucile, C.F.; Treanor, J.J.; Rosenberg, A.F.; Zand, M.S.; Keefer, M.C.; et al. A Highly Potent and Broadly Neutralizing H1 Influenza-Specific Human Monoclonal Antibody. Sci. Rep. 2018, 8, 4374. [Google Scholar] [CrossRef]
- Caceres, C.J.; Gay, L.C.; Faccin, F.C.; Perez, D.R. Use of Reverse Genetics for the Generation of Recombinant Influenza Viruses Carrying Nanoluciferase. Methods Mol. Biol. 2024, 2733, 47–74. [Google Scholar] [PubMed]
- Heaton, N.S.; Leyva-Grado, V.H.; Tan, G.S.; Eggink, D.; Hai, R.; Palese, P. In vivo bioluminescent imaging of influenza a virus infection and characterization of novel cross-protective monoclonal antibodies. J. Virol. 2013, 87, 8272–8281. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.A.; Meliopoulos, V.A.; Savage, C.; Livingston, B.; Mehle, A.; Schultz-Cherry, S. Visualizing real-time influenza virus infection, transmission and protection in ferrets. Nat. Commun. 2015, 6, 6378. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feng, L.; Pan, W.; Dong, Z.; Li, C.; Sun, C.; Chen, L. Generation of replication-competent recombinant influenza A viruses carrying a reporter gene harbored in the neuraminidase segment. J. Virol. 2010, 84, 12075–12081. [Google Scholar] [CrossRef]
- Munier, S.; Rolland, T.; Diot, C.; Jacob, Y.; Naffakh, N. Exploration of binary virus-host interactions using an infectious protein complementation assay. Mol. Cell. Proteom. 2013, 12, 2845–2855. [Google Scholar] [CrossRef]
- Pan, W.; Dong, Z.; Li, F.; Meng, W.; Feng, L.; Niu, X.; Li, C.; Luo, Q.; Li, Z.; Sun, C.; et al. Visualizing influenza virus infection in living mice. Nat. Commun. 2013, 4, 2369. [Google Scholar] [CrossRef]
- Spronken, M.I.; Short, K.R.; Herfst, S.; Bestebroer, T.M.; Vaes, V.P.; Van Der Hoeven, B.; Koster, A.J.; Kremers, G.J.; Scott, D.P.; Gultyaev, A.P.; et al. Optimisations and Challenges Involved in the Creation of Various Bioluminescent and Fluorescent Influenza A Virus Strains for In Vitro and In Vivo Applications. PLoS ONE 2015, 10, e0133888. [Google Scholar] [CrossRef]
- Tran, V.; Moser, L.A.; Poole, D.S.; Mehle, A. Highly sensitive real-time in vivo imaging of an influenza reporter virus reveals dynamics of replication and spread. J. Virol. 2013, 87, 13321–13329. [Google Scholar] [CrossRef]
- Yan, D.; Weisshaar, M.; Lamb, K.; Chung, H.K.; Lin, M.Z.; Plemper, R.K. Replication-Competent Influenza Virus and Respiratory Syncytial Virus Luciferase Reporter Strains Engineered for Co-Infections Identify Antiviral Compounds in Combination Screens. Biochemistry 2015, 54, 5589–5604. [Google Scholar] [CrossRef]
- Breen, M.; Nogales, A.; Baker, S.F.; Perez, D.R.; Martinez-Sobrido, L. Replication-Competent Influenza A and B Viruses Expressing a Fluorescent Dynamic Timer Protein for In Vitro and In Vivo Studies. PLoS ONE 2016, 11, e0147723. [Google Scholar] [CrossRef] [PubMed]
- Chiem, K.; Rangel-Moreno, J.; Nogales, A.; Martinez-Sobrido, L. A Luciferase-fluorescent Reporter Influenza Virus for Live Imaging and Quantification of Viral Infection. J. Vis. Exp. 2019, 150, e59890. [Google Scholar]
- De Baets, S.; Verhelst, J.; Van den Hoecke, S.; Smet, A.; Schotsaert, M.; Job, E.R.; Roose, K.; Schepens, B.; Fiers, W.; Saelens, X. A GFP expressing influenza A virus to report in vivo tropism and protection by a matrix protein 2 ectodomain-specific monoclonal antibody. PLoS ONE 2015, 10, e0121491. [Google Scholar] [CrossRef]
- Kittel, C.; Ferko, B.; Kurz, M.; Voglauer, R.; Sereinig, S.; Romanova, J.; Stiegler, G.; Katinger, H.; Egorov, A. Generation of an influenza A virus vector expressing biologically active human interleukin-2 from the NS gene segment. J. Virol. 2005, 79, 10672–10677. [Google Scholar] [CrossRef]
- Kittel, C.; Sereinig, S.; Ferko, B.; Stasakova, J.; Romanova, J.; Wolkerstorfer, A.; Katinger, H.; Egorov, A. Rescue of influenza virus expressing GFP from the NS1 reading frame. Virology 2004, 324, 67–73. [Google Scholar] [CrossRef] [PubMed]
- DiPiazza, A.; Nogales, A.; Poulton, N.; Wilson, P.C.; Martinez-Sobrido, L.; Sant, A.J. Pandemic 2009 H1N1 Influenza Venus reporter virus reveals broad diversity of MHC class II-positive antigen-bearing cells following infection in vivo. Sci. Rep. 2017, 7, 10857. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, B.; Manicassamy, S.; Belicha-Villanueva, A.; Pisanelli, G.; Pulendran, B.; Garcia-Sastre, A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc. Natl. Acad. Sci. USA 2010, 107, 11531–11536. [Google Scholar] [CrossRef]
- Gabor, K.A.; Goody, M.F.; Mowel, W.K.; Breitbach, M.E.; Gratacap, R.L.; Witten, P.E.; Kim, C.H. Influenza A virus infection in zebrafish recapitulates mammalian infection and sensitivity to anti-influenza drug treatment. Dis. Model Mech. 2014, 7, 1227–1237. [Google Scholar] [CrossRef]
- Pang, I.K.; Pillai, P.S.; Iwasaki, A. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. Proc. Natl. Acad. Sci. USA 2013, 110, 13910–13915. [Google Scholar] [CrossRef]
- Resa-Infante, P.; Thieme, R.; Ernst, T.; Arck, P.C.; Ittrich, H.; Reimer, R.; Gabriel, G. Importin-alpha7 is required for enhanced influenza A virus replication in the alveolar epithelium and severe lung damage in mice. J. Virol. 2014, 88, 8166–8179. [Google Scholar] [CrossRef]
- Roberts, K.L.; Manicassamy, B.; Lamb, R.A. Influenza A virus uses intercellular connections to spread to neighboring cells. J. Virol. 2015, 89, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, M.; De, A. Bioluminescence based in vivo screening technologies. Curr. Opin. Pharmacol. 2012, 12, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Poole, D.S.; Jeffery, J.J.; Sheahan, T.P.; Creech, D.; Yevtodiyenko, A.; Peat, A.J.; Francis, K.P.; You, S.; Mehle, A. Multi-Modal Imaging with a Toolbox of Influenza A Reporter Viruses. Viruses 2015, 7, 5319–5327. [Google Scholar] [CrossRef]
- Zhao, H.; Doyle, T.C.; Coquoz, O.; Kalish, F.; Rice, B.W.; Contag, C.H. Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J. Biomed. Opt. 2005, 10, 41210. [Google Scholar] [CrossRef] [PubMed]
- Lao, G.; Ma, K.; Qiu, Z.; Qi, W.; Liao, M.; Li, H. Real-Time Visualization of the Infection and Replication of a Mouse-Lethal Recombinant H9N2 Avian Influenza Virus. Front. Vet. Sci. 2022, 9, 849178. [Google Scholar] [CrossRef]
- Nogales, A.; Schotsaert, M.; Rathnasinghe, R.; DeDiego, M.L.; Garcia-Sastre, A.; Martinez-Sobrido, L. Replication-Competent DeltaNS1 Influenza A Viruses Expressing Reporter Genes. Viruses 2021, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.V.; Moisy, D.; Munier, S.; Schraidt, O.; Naffakh, N.; Cusack, S. Replication-competent influenza A virus that encodes a split-green fluorescent protein-tagged PB2 polymerase subunit allows live-cell imaging of the virus life cycle. J. Virol. 2012, 86, 1433–1448. [Google Scholar] [CrossRef]
- Domm, W.; Yee, M.; Misra, R.S.; Gelein, R.; Nogales, A.; Martinez-Sobrido, L.; O’reilly, M.A.; Lignelli, E.; Palumbo, F.; Myti, D.; et al. Oxygen-dependent changes in lung development do not affect epithelial infection with influenza A virus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L940–L949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Sastre, A.; Egorov, A.; Matassova, D.; Brandtbc, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Musterbc, T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef]
- Nogales, A.; Rodriguez-Sanchez, I.; Monte, K.; Lenschow, D.J.; Perez, D.R.; Martinez-Sobrido, L. Replication-competent fluorescent-expressing influenza B virus. Virus Res. 2016, 213, 69–81. [Google Scholar] [CrossRef]


| Gene | Virus Backbone (1) | Transgene (2) | Insertion Mechanism | Application | Reference |
|---|---|---|---|---|---|
| NS | pH1N1 | Venus | 2A site | Virus pathogenesis | [76] |
| NS | PR8 | maxGFP | 2A site | Virus pathogenesis | [77,78,79,80,81] |
| NS | PR8 | maxGFP, turboRFP, Gluc | 2A site | Antiviral and virus–host interaction | [10] |
| NS | PR8 pH1N1 | mCherry | 2A site | Antivirals, neutralizing antibodies, virus pathogenesis | [38] |
| NS | pH1N1 | Timer | 2A site | Virus propagation | [71] |
| NS | PR8 VN1203 | Venus, eGFP, eCFP, mCherry | 2A site | Virus–host interaction and virus pathogenesis | [56] |
| NS | PR8 | Nluc, mCherry | ΔNS1 and 2A site | Virus–host interaction | [82] |
| NS | Guan2008 | Nluc | 2A site | Virus pathogenesis | [81] |
| NS | PR8 WSN | GFP, mCherry | 2× 2A site | Virus pathogenesis and virus–host interaction | [44,45,57,73] |
| Gene | Virus Backbone (1) | Transgene (2) | Insertion Mechanism | Application | Reference |
|---|---|---|---|---|---|
| NS | B/Brisbane | maxGFP, mCherry, Timer | 2A site | Antivirals, neutralizing antibodies, virus pathogenesis | [71,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Sobrido, L.; Nogales, A. Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment. Int. J. Mol. Sci. 2024, 25, 10584. https://doi.org/10.3390/ijms251910584
Martinez-Sobrido L, Nogales A. Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment. International Journal of Molecular Sciences. 2024; 25(19):10584. https://doi.org/10.3390/ijms251910584
Chicago/Turabian StyleMartinez-Sobrido, Luis, and Aitor Nogales. 2024. "Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment" International Journal of Molecular Sciences 25, no. 19: 10584. https://doi.org/10.3390/ijms251910584
APA StyleMartinez-Sobrido, L., & Nogales, A. (2024). Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment. International Journal of Molecular Sciences, 25(19), 10584. https://doi.org/10.3390/ijms251910584







