Abstract
Chlortetracycline hydrochloride (CTC) is a broad-spectrum tetracycline antibiotic with a wide range of antibacterial activities. Due to low solubility, poor stability, and low bioavailability, clinical preparation development is limited. We sought to improve these solubility and dissolution rates by preparing solid dispersions. A hydrophilic polymer was selected as the carrier, and a solid dispersion was prepared using a medium grinding method, with samples characterized by scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), powder X-ray diffraction (PXRD), Fourier-transform infrared spectroscopy (FT-IR), and particle size distribution (PSD). To maximize CTC solubility and stability, different polymer types and optimal drug-to-polymer ratios were screened. The solubility of optimized povidone K30 (PVPK30) (1/0.75, w/w)-, hydroxypropyl-β-cyclodextrin (HP-β-CD) (1/2, w/w)-, and gelatin (1/1, w/w)-based solid dispersions was 6.25-, 7.7-, and 3.75-fold higher than that of pure CTC powder, respectively. Additionally, in vitro dissolution studies showed that the gelatin-based solid dispersion had a higher initial dissolution rate. SEM and PS analyses confirmed that this dispersion had smaller and more uniform particles than PVPK30 and HP-β-CD dispersions. Therefore, successful solid polymer dispersion preparations improved the CTC solubility, dissolution rates, and stability, which may have potential as drug delivery systems.
1. Introduction
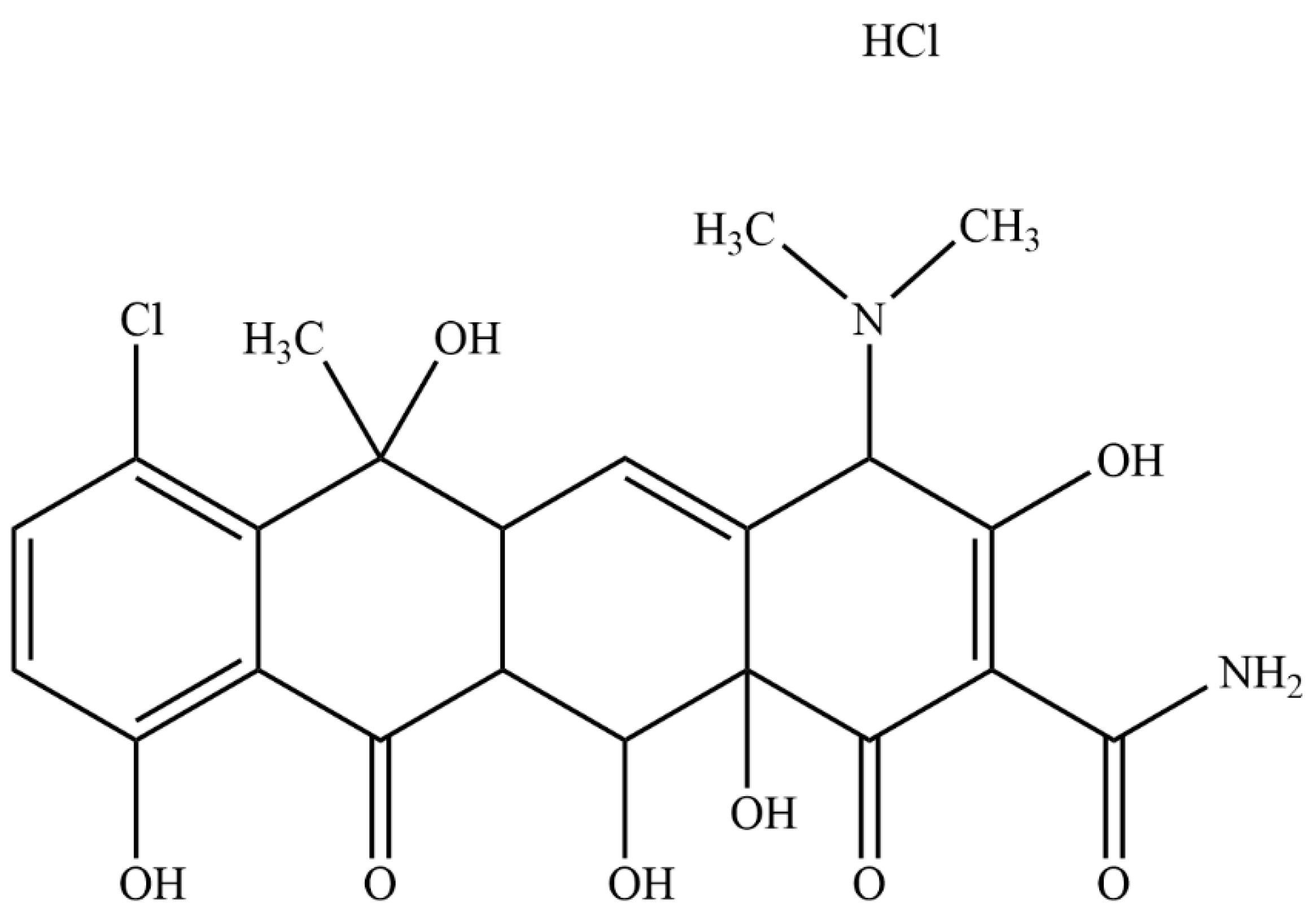
Chlortetracycline hydrochloride (CTC, Figure 1) (6-methyl-4-(dimethylamino)-3,6,10,12,12α-pentahydroxy-1,1-dioxo-7-chloro-1,4,4α,5,5α,6,11,12α-octahydro-2-tetrabenamide hydrochloride) is a broad-spectrum antibiotic of the tetracycline family [1], produced by the genus Actinomyces. As CTC acts by inhibiting protein synthesis in sensitive microorganisms, it has wide antibacterial activity ranges against both Gram-positive and Gram-negative bacteria and is widely used to treat animal and human infectious diseases [2,3,4]. CTC is a class II drug (Biopharmaceutical Classification System), and its low aqueous solubility (approximately 4 mg/mL) is associated with low oral bioavailability [5], which limits clinical applications. Therefore, an effective and appropriate CTC drug delivery system is required to address these shortcomings.
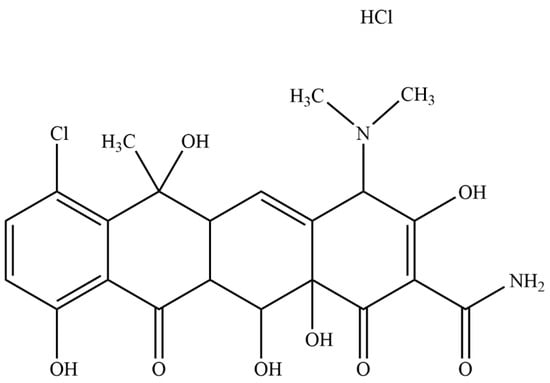
Figure 1.
Chemical structure of chlortetracycline hydrochloride (CTC).
Many different solubilization technologies have been studied, including physical and chemical modification methods, such as cyclodextrin inclusion complexes [6], nanosuspensions [7], and salt/eutectic [8] and solid [9,10,11] dispersions. Of the various technologies, amorphous solid dispersion [12] effectively improves drug solubility and bioavailability by reducing the drug particle size (PS) and improving wettability, and it has higher drug loading and stability. This technology is widely used to convert drugs from crystalline to amorphous forms [13]. When compared with crystalline forms, amorphous forms significantly improve solubility and dissolution rates, as evidenced by the literature [14]. However, as amorphous forms are in a physically unstable high-energy state, this greatly affects drug stability [15]. To overcome such issues, polymers have shown promise as crystallization inhibitors, with some studies proposing that polymers generate long-term supersaturation by reducing drug molecule mobility [16]. As polymers with different molecular weights have different solubilization effects on insoluble drugs and also on the crystallization inhibition degree, the correct polymers must be selected for insoluble drugs.
Povidone K30 (PVPK30) is a common solid-dispersion carrier; its carbonyl oxygen interacts with the hydrogen-bond forming molecules in a drug, which inhibits amorphous drug crystallization. PVPK30’s effects on drug stability have been widely reported [17,18]. Similarly, both hydroxypropyl-β-cyclodextrin (HP-β-CD) and gelatin have also been widely used in drug research due to their water solubility, non-toxicity, and excellent biocompatibility traits [19,20,21].
The aim of this study is to prepare a new solid drug–polymer dispersion using medium ball milling to improve CTC solubility and stability. Based on solubility studies, the optimal polymer type and drug/polymer ratio are selected. Finally, the solid dispersion’s physical and chemical properties are analyzed.
2. Results and Discussion
2.1. Effects of Different Types of Polymers on the Solubility of CTC
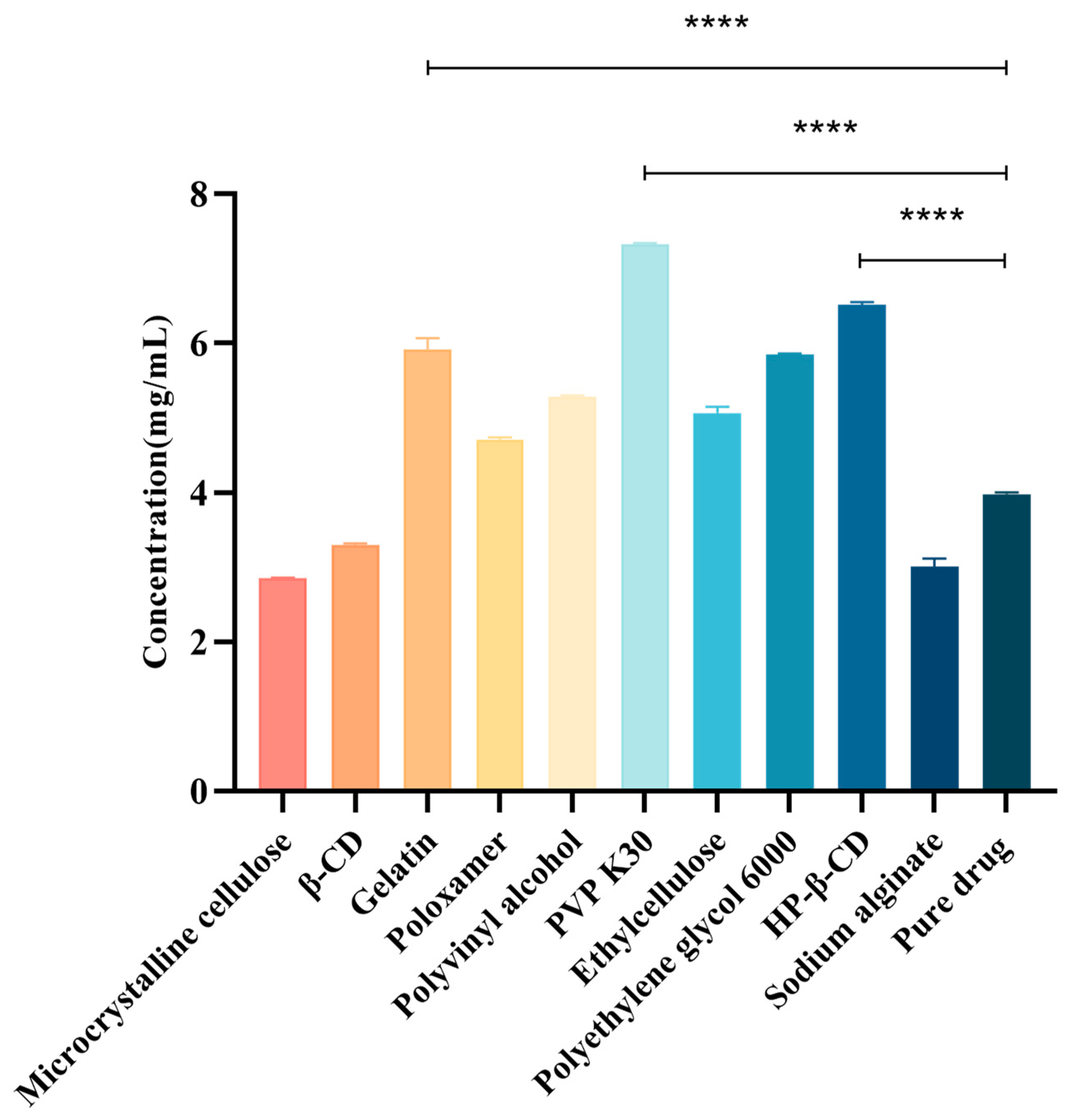
The choice of the polymer carrier is crucial when determining insoluble drug solubility and how it directly affects drug stability and bioavailability [22]. Therefore, to select a suitable hydrophilic polymer as the CTC solid-dispersion carrier, we evaluated the effects of ten polymers on the CTC solubility. As shown (Figure 2), the polymers and CTC were determined at a 1:1 mass ratio. We observed that drug solubility increased with polymer addition. Of these polymers, PVPK30 significantly improved the CTC solubility; the solubility at 7.35 mg/mL was 1.84-fold higher than that of pure CTC (4 mg/mL), suggesting that interactions between PVPK30 and CTC disrupted the drug’s interactions with itself and thereby improved solubility. Also, HP-β-CD’s solubility effects toward CTC (6.55 mg/mL) were increased 1.64-fold, while gelatin’s solubility effects toward CTC (6.05 mg/mL) were increased approximately 1.51-fold. In contrast, microcrystalline cellulose, β-CD, and sodium alginate had no solubilizing effects toward CTC. Thus, polymer choice is a critical factor when improving insoluble CTC’s solubility. Therefore, the PVPK30, HP-β-CD, and gelatin carriers were selected for further research.
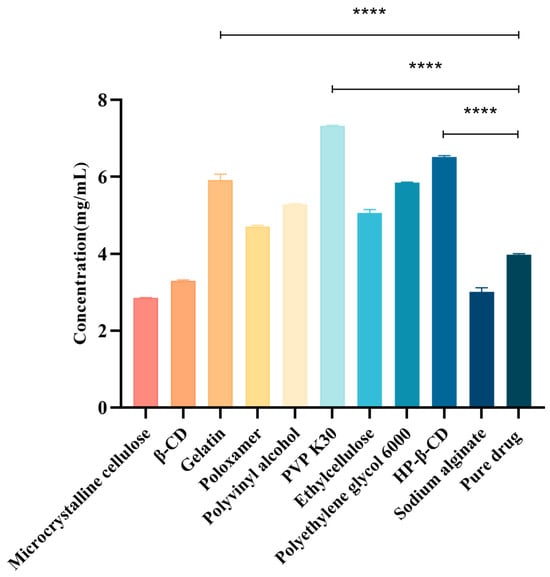
Figure 2.
Different compounds’ effects on CTC at 1:1 mass ratios. **** p-Value < 0.0001.
2.2. Polymer Concentration Effects on Solid-Dispersion Solubility in CTC
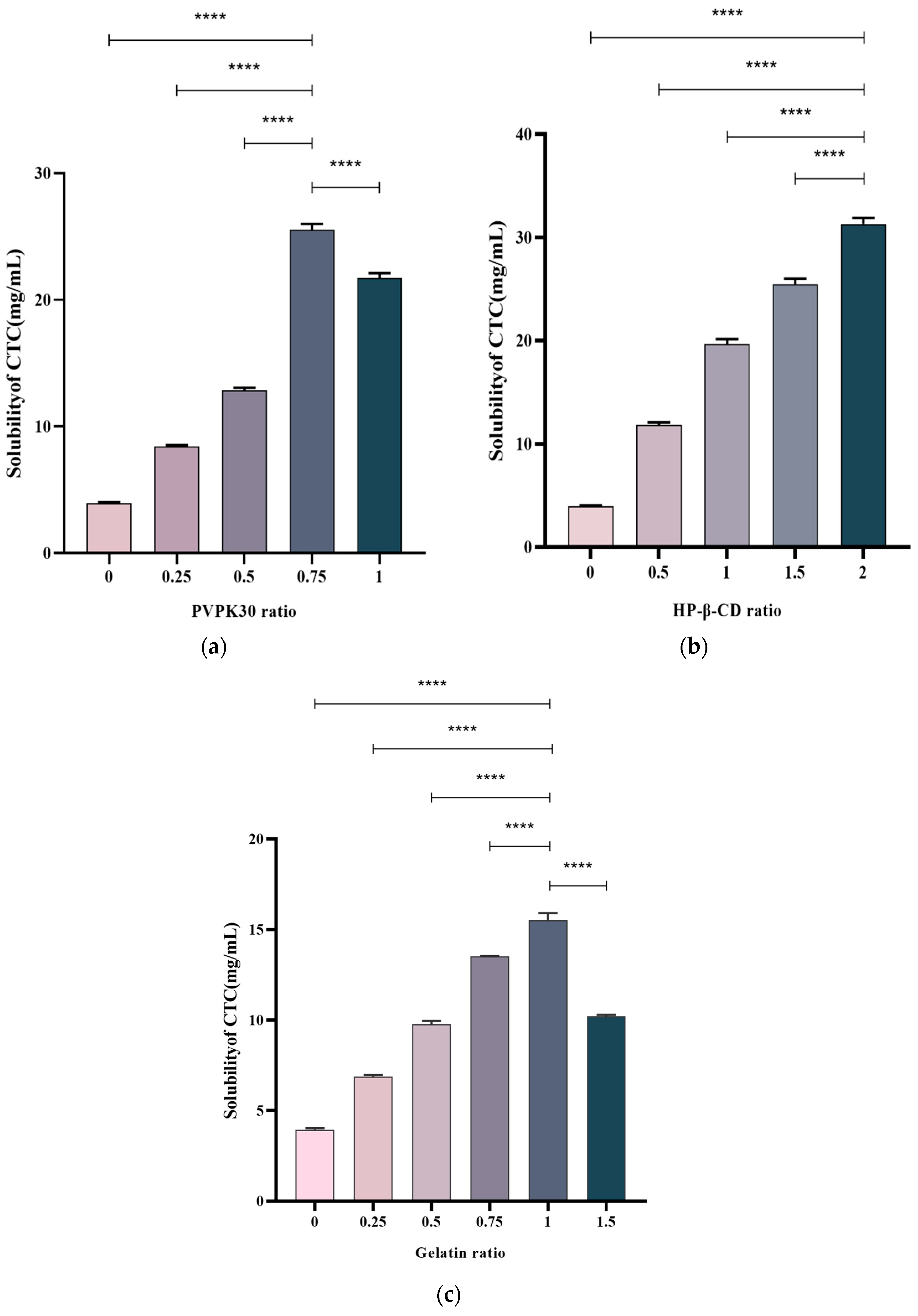
The drug/polymer effects at different ratios on the CTC solubility were examined. Multiple ratios were selected to prepare solid CTC dispersions. PVPK30-based solid dispersions, with CTC-to-PVPK30 weight ratios of 1/0, 1/0.25, 1/0.5, and 1/1, were prepared using a medium grinding method. As observed (Figure 3a), with a gradual increase in the PVPK30 dose, the CTC solubility in solid dispersions significantly increased. In general, insoluble drugs’ solubility increases with an increase in water-soluble polymers [23]. However, when the CTC/PVPK30 ratio reached 1/0.75, the CTC solubility decreased as the PVPK30 quantity increased. This phenomenon also occurred in gelatin-based solid dispersions (Figure 3c). When the CTC-to-gelatin ratio reached 1/1, the CTC solubility decreased with an increased gelatin dose. These results show that the drug-to-polymer ratio was critical for improving drug solubility. Therefore, the PVPK30- (weight ratio = 1/0.75) and gelatin-based solid dispersions (weight ratio = 1/1) were selected for further study. Subsequently, HP-β-CD-based solid dispersions with 1/0, 1/0.5, 1/1, 1/1.5, and 1/2 CTC and HP-β-CD weight ratios were prepared using the medium grinding method. The CTC solubility (Figure 3b) increased with increasing HP-β-CD quantity. This observation was consistent with most solid drug solubility dispersions [24]. Therefore, we also selected HP-β-CD-based solid dispersions (weight ratio = 1/2) for further research. According to the above results, PVPK30-, HP-β-CD-, and gelatin-based solid dispersions improved the CTC solubility by approximately 6.25-, 7.69-, and 3.77-fold, respectively, when compared with pure CTC powder, indicating that optimal drug-to-polymer ratios, based on polymer type, were critical for maximizing the solid-dispersion systems.
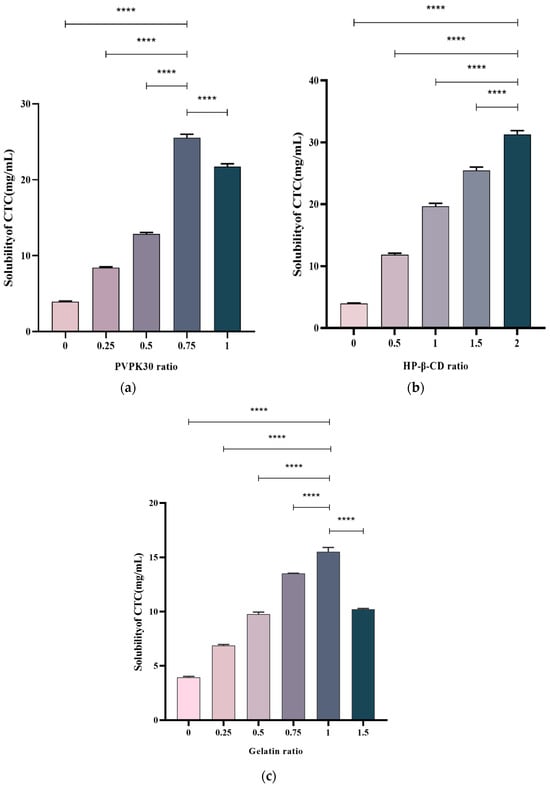
Figure 3.
Polymer concentration effects on CTC-loaded solid-dispersion solubility. (a) PVPK30; (b) HP-β-CD; and (c) gelatin. **** p-Value < 0.0001.
2.3. In Vitro Dissolution Studies
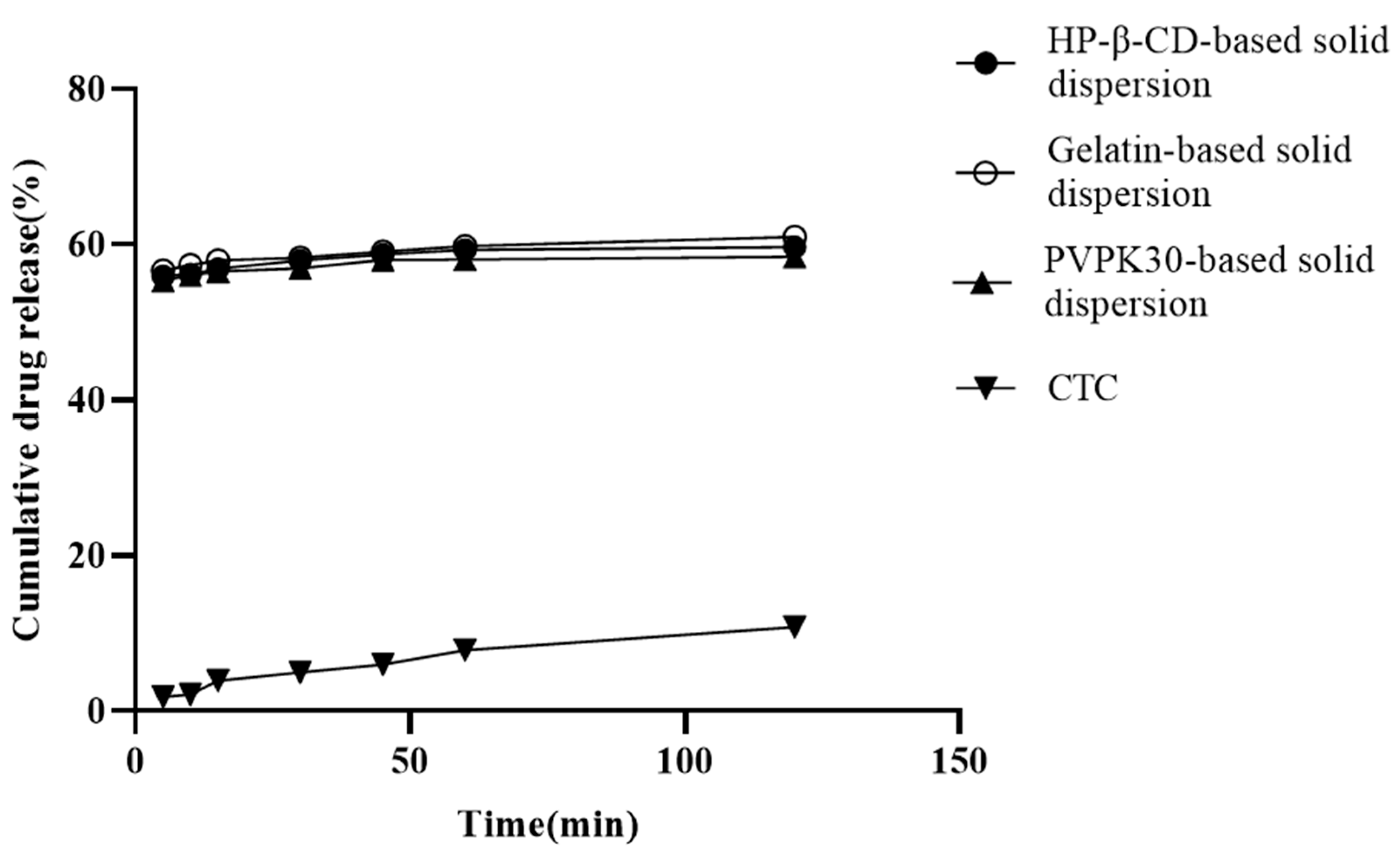
In vitro release is a key factor affecting insoluble drug bioavailability during oral absorption [25]. Solid dispersions based on hydrophilic carries help improve the insoluble drugs’ wettability and reduce interfacial tension between the carrier and the dissolved medium [26]. The three solid dispersions prepared in this study are shown in Figure 4. At 120 min, the PVPK30-, HP-β-CD-, and gelatin-based solid dispersions’ dissolution rates were approximately 57.78%, 59.95%, and 60.89%, respectively. When compared with pure CTC (10.65%) and CTC premix [27], these dissolution rates were significantly higher, implying that the hydrophilic nature of pentaerythritol and the polymer may form a tight association with the CTC drug particles, causing partial amorphism and thus increasing drug dissolution. This results in increased wettability and dispersion of the drug. Additionally, the initial dissolution rates are also used to evaluate CTC’s dissolution in polymers. We observed that the gelatin-based solid dispersions’ dissolution rate in the first 30 min was significantly higher than that for both PVPK30- and HP-β-CD-based solid dispersions, indicating a higher initial wettability, which was possibly due to the smaller PS in gelatin-based solid dispersions. This result was consistent with the characterization results (Figure 5d). After 60 min, it was observed that the dissolution rates of all three solid dispersions were relatively slow, suggesting a potential final dissolution rate when compared to long-term saturation solubility results. Similarly, the release data were also used to study the release kinetics, and it was found that the release of CTC in the three solid dispersing regime agents followed the Peppas–Korsmeyer model, in which drug release followed the diffusion process.
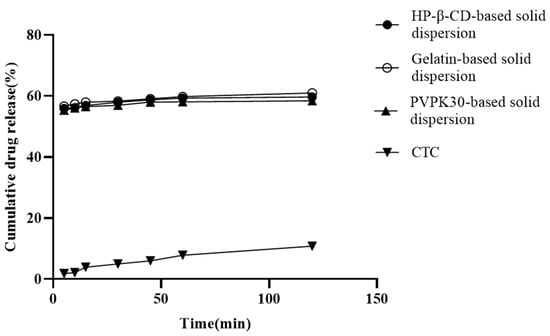
Figure 4.
In vitro CTC-loaded solid-dispersion dissolution profiles.
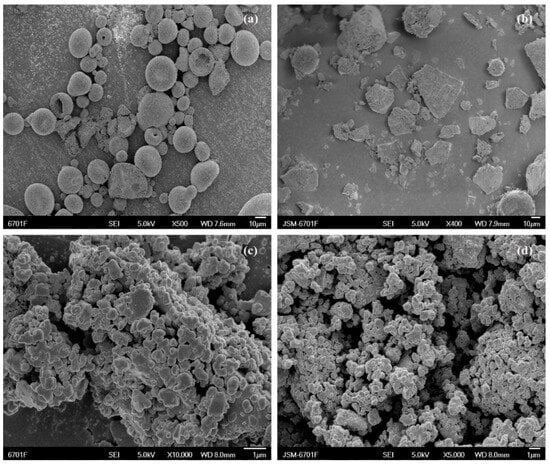
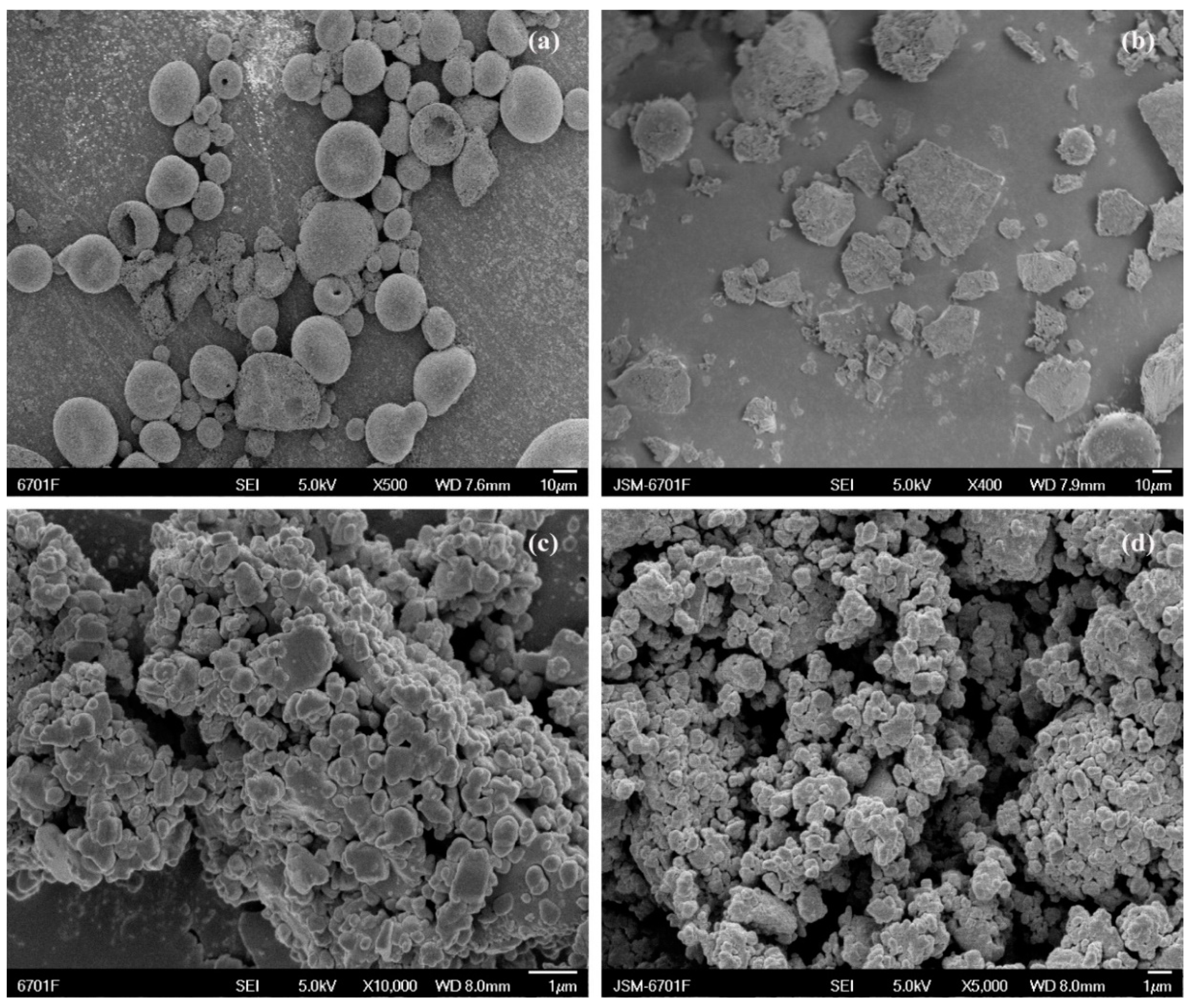
Figure 5.
Scanning electron micrographs showing (a) CTC powder; (b) PVPK30-based solid dispersion; (c) HP-β-CD-based solid dispersion; and (d) gelatin-based solid dispersion.
2.4. Stability of CTC-Loaded Solid Dispersion
The results of the influencing-factor experiments on the solid dispersions including high temperature, high humidity, and strong light are shown in Table 1. The appearance of the three solid dispersions was yellow and did not change during the whole period of the influencing-factor experiments. In strong light, there was no significant difference in the content of PVPK30-based solid dispersions at 5 days and 10 days, which markedly enhanced the stability of CTC under illumination. Only 3.47% of the HP-β-CD-based solid dispersion was degraded after 10 days. The gelatin-based solid dispersion exhibited slight sensitivity, with degradation rates of 7.5% and 8.27% at 5 days and 10 days, respectively. No differences were noted in the content of the PVPK30- and HP-β-CD-based solid dispersions when subjected to high-temperature and high-humidity conditions. The gelatin-based solid dispersion showed mild sensitivity at 60 °C/75% ± 1%, resulting in degradation rates of 5.22% and 6.73% on days five and ten, respectively. Following an assessment of the influencing factors, all three types of solid dispersions complied with the standards set forth by the Chinese Veterinary Pharmacopoeia (labeled amount: 90–110%) [28] and demonstrated good stability.

Table 1.
The influence factor test of CTC-loaded solid dispersion (mean ± SD, n = 3).
2.5. Characterization
2.5.1. Scanning Electron Microscopy (SEM)
SEM facilitates surface morphological observations and element composition analyses of different solid materials (e.g., PVPK30). SEM was used to examine the surface morphology of pure CTC and its complex (Figure 5 and Figure S1). Pure CTC had spherical structures (Figure 5a), while PVPK30 was composed of smooth surfaces and amorphous spherical particles (Figure S1e), while HP-β-CD showed spherical cavity structures (Figure S1f), and gelatin showed massive, irregular structures (Figure S1c). These surface morphologies were consistent with previous reports [29,30,31]. The physical mixture, simple M-SD prepared by fully mixing with CTC, showed a common CTC and polymer morphology (Figure S1c,e,f) without changing host and guest molecule morphologies during the process. In contrast, solid dispersions prepared by medium ball milling underwent drastic changes; amorphous forms were observed with disappearing spherical drug structures, suggesting that CTC was possibly amorphously distributed in the carrier. Additionally, gelatin-based solid dispersions exhibited smaller, more uniform particles that combined to form aggregates, indicating improved initial wettability and higher initial dissolution rates than the other solid dispersions.
2.5.2. Particle Size Distributions (PSDs)
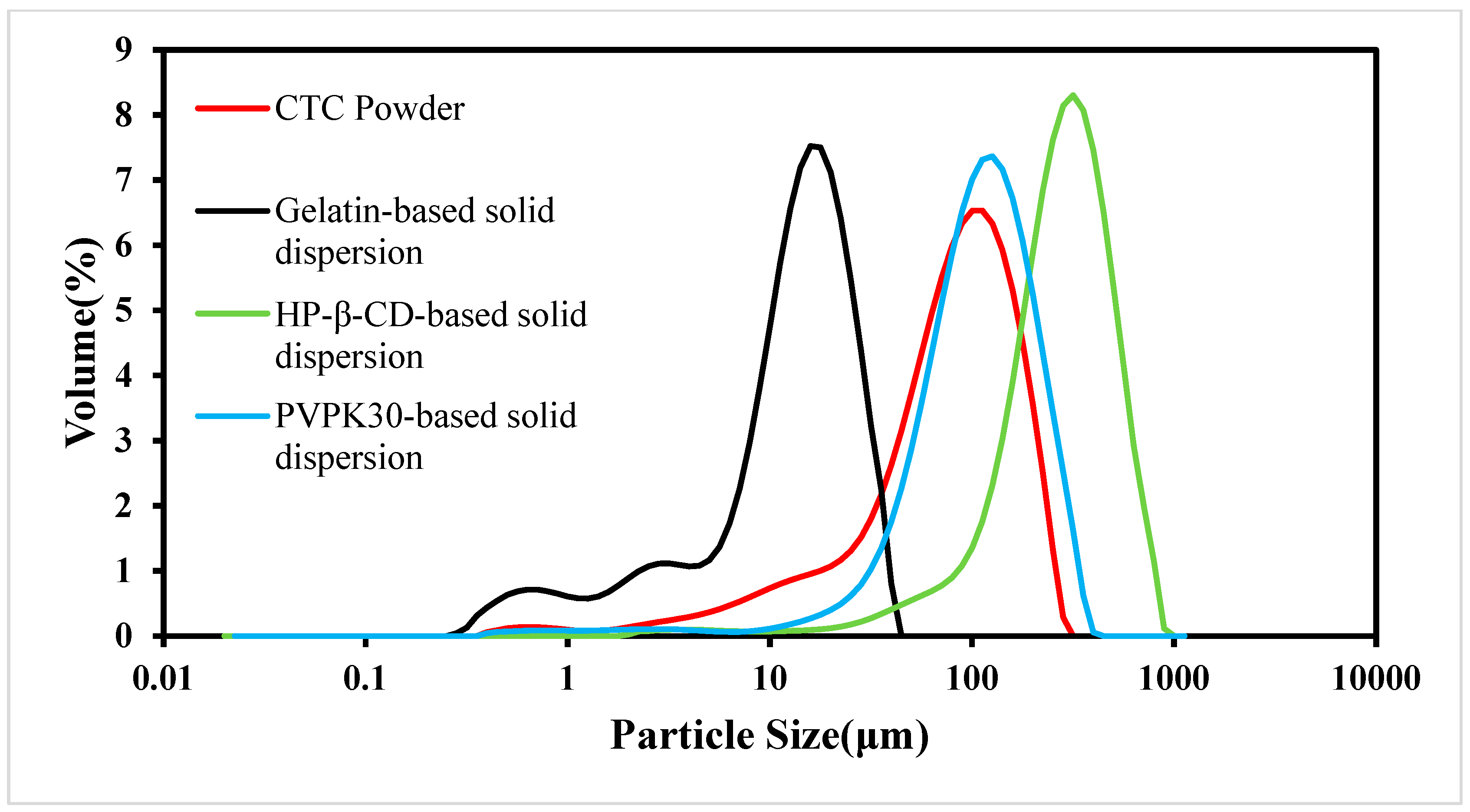
As the PS is an important factor affecting drug dissolution rates, solid dispersions must have uniform PSs and shapes [32]. In this study, we examined CTC PS distributions in the three solid dispersions (Figure 6). When compared with CTC powder, PS in the gelatin-based solid dispersions was significantly reduced. However, PS in the HP-β-CD- and PVPK30-based solid dispersions did not decrease when compared with that of the CTC powder, especially the PS in PVPK30-based solid dispersions, which increased significantly. This was because the PVPK30-based solid dispersion particles showed irregular bulk structures, resulting in wide PS distributions. In addition, the wettability of solid dispersions is also related to the improvement of the solubility of insoluble drugs [33]. The data show [34] that there is a direct correlation between the wettability of solid dispersions and the dissolution rate. Therefore, with an increase in the wettability of gelatin-based solid dispersions, the initial dissolution rate is higher than that of the HP-β-CD and PVPK30-based solid dispersions. These results were consistent with the SEM trends.
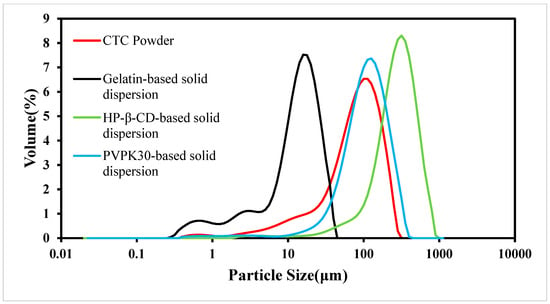
Figure 6.
CTC and CTC-loaded solid-dispersion particle size distributions.
2.5.3. Differential Scanning Calorimetry (DSC)
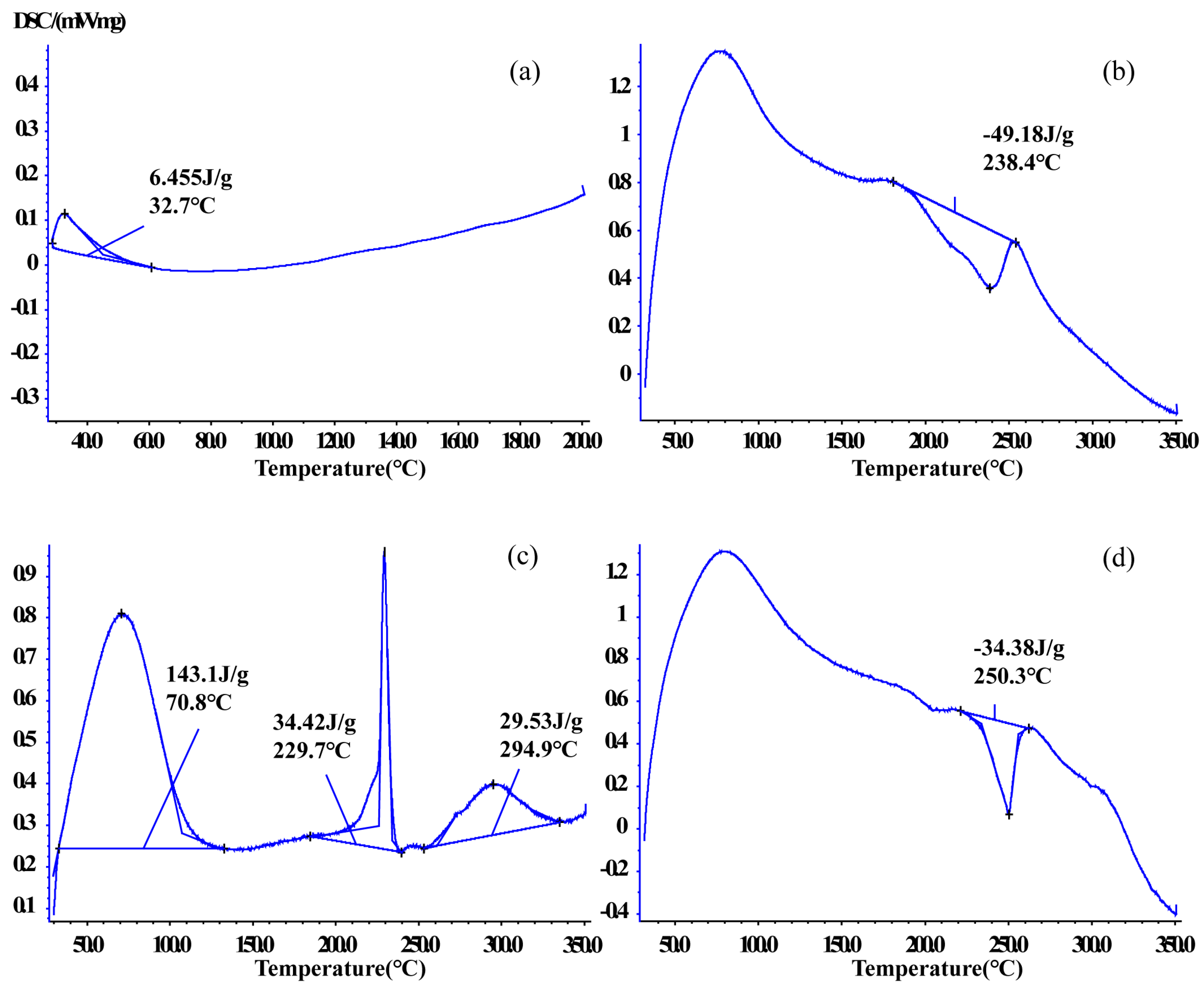
DSC is used to monitor interactions between host and guest molecules and shows melting point changes in drugs [35]. DSC was used to verify thermal behaviors in CTC and solid dispersions in this study (Figure 7 and Figure S2). The CTC powder DSC curve showed an endothermic peak at 32.7 °C. PVPK30 showed two broad endothermic peaks at 78.4 °C and 431.9 °C. The first spike was attributed to water molecule release and the second spike to PVPK30 decomposition. However, the physical mixture curve was dissimilar to that for PVPK30 and had a sharp exothermic peak at 249.8 °C (ΔH = −20.17 J/g), indicating a crystalline form.
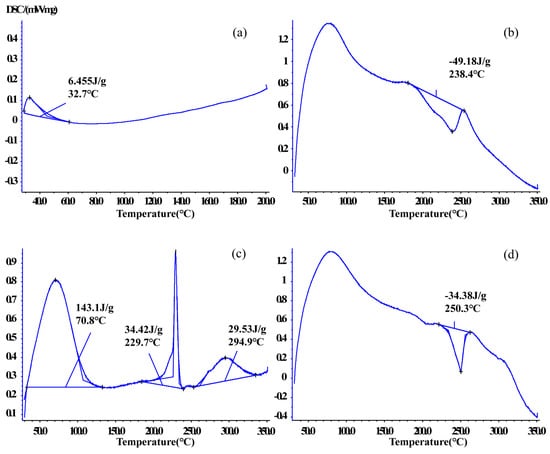
Figure 7.
Comparing differential scanning calorimetric thermograms: (a) CTC powder; (b) PVPK30-based solid dispersion; (c) HP-β-CD-based solid dispersion; and (d) gelatin-based solid dispersion.
The same phenomenon was observed for gelatin, which had two endothermic peaks at 221.9 °C and 268.5 °C and an exothermic peak at 244.1 °C. But the sample weight did not change. The physical mixture curve had a sharp exothermic peak at 250.2 °C (ΔH = −44.52 J/g).
The HP-β-CD physical mixture was different to the other mixtures; an endothermic peak was recorded at 70.4 °C, indicating water loss due to dehydration, consistent with previous reports [36]. The physical mixture also showed endothermic peaks at 72.5 °C, 238.3 °C, and 290.3 °C and an exothermic peak at 335.9 °C, indicating sample melting. In medium ball milling-prepared PVPK30- and gelatin-based solid dispersions (Figure 7b,d), the characteristic peaks relative to the drug disappeared, with exothermic peaks at 238.4 °C and 250.3 °C, respectively. The ΔH value (−34.38 J/g) of the gelatin-based solid dispersion was lower than that of the physical mixture (ΔH = −44.52 J/g), which indicated that CTC had partially replaced water binding in gelatin, resulting in physical interactions. The HP-β-CD solid dispersion contained three endothermic peaks at 70.8 °C, 229.7 °C, and 294.9 °C, with corresponding ΔH (34.42 J/g) and ΔH (29.53 J/g) values lower than those in the physical mixture. Additionally, characteristic peaks related to the CTC boiling point disappeared, further confirming enhanced CTC thermal stability.
2.5.4. Thermogravimetric Analysis (TGA)
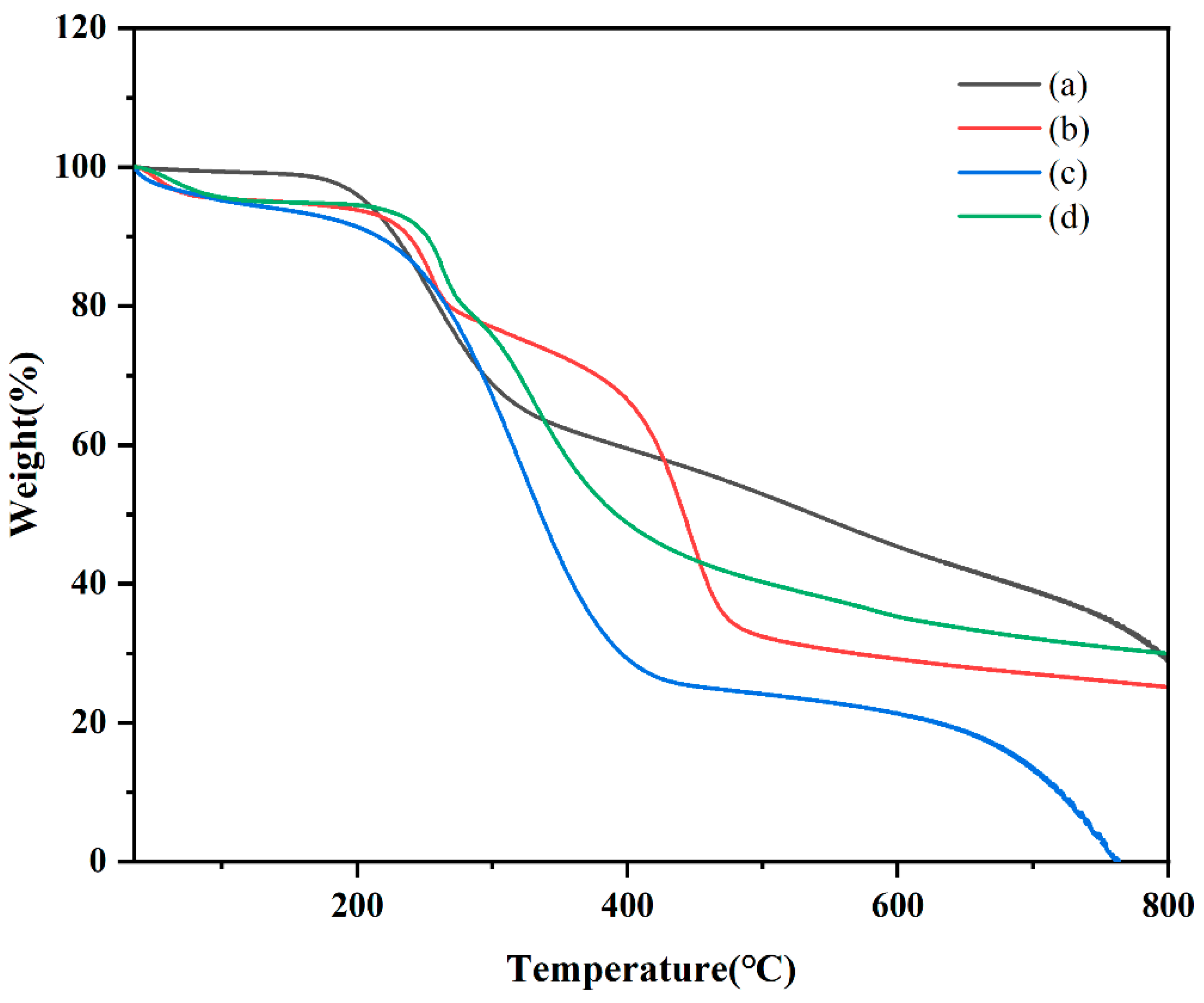
TGA is used to evaluate physical and chemical property adjustments in different materials [37]. In this study, TGA was used to examine CTC, PVPK30, HP-β-CD, gelatin, physical mixtures, and solid dispersions to determine mass loss due to temperature increases. The TGA data are shown in Figure 8 and Figure S3. Although thermograms show the sum of pure component curves, substantial differences can be observed in complex curves [38]. At 400 °C, PVPK30 showed more stability than HP-β-CD and gelatin, with a residual weight of 85.35%. For CTC, decomposition was divided into two periods: the first at 200 °C and the second at 781 °C, with residual weight reduced from 98.639 wt% to 33.806 wt%. In considering unstable CTC physicochemical properties, the TGA curve of the physical mixture showed drug and polymer superposition, with the first decomposition corresponding to drug decomposition and the second corresponding to the polymer. According to the TGA of solid dispersions prepared by medium ball milling, The CTC thermal stability was improved after complexing with polymers, with the polymers decomposing at higher temperatures than the free CTC. It should be noted that the CTC solid dispersion’s thermal stability was less than that for the pure components (PVPK30, HP-β-CD, and gelatin), but the CTC solid dispersion still retained reasonable thermal stability.
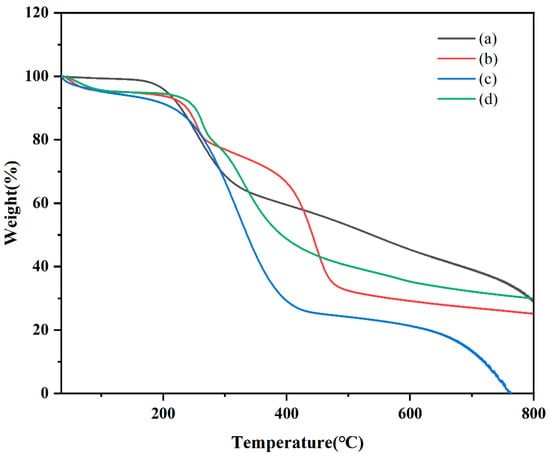
Figure 8.
Comparing thermal gravimetric analyzer thermograms: (a) CTC powder; (b) PVPK30-based solid dispersion; (c) HP-β-CD-based solid dispersion; and (d) gelatin-based solid dispersion.
2.5.5. Powder X-ray Diffraction (PXRD)
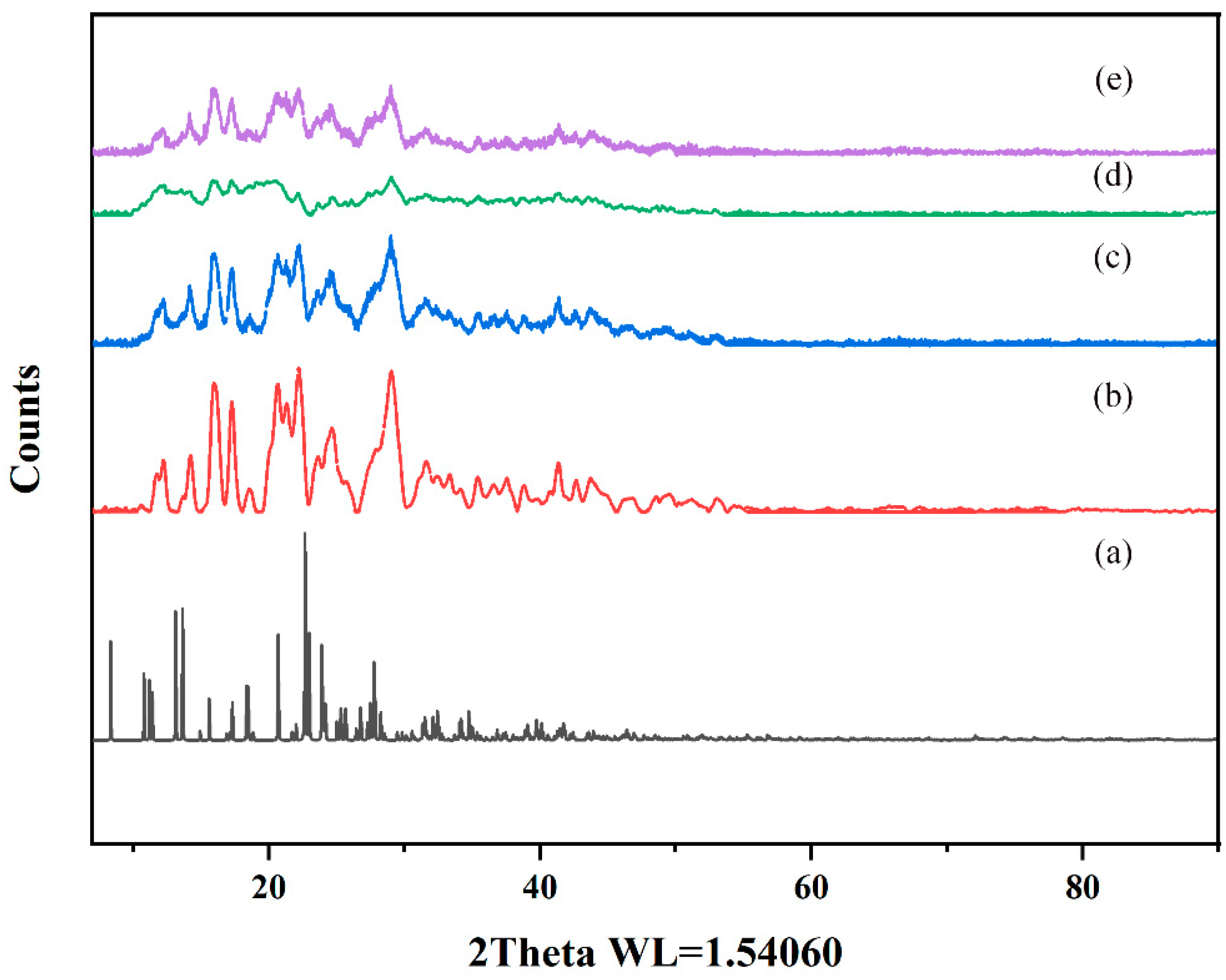
PXRD effectively characterizes interactions between host and guest molecules and verifies crystal structure properties in mixtures [39]. In this study, the CTC and the solid dispersions were evaluated by PXRD. As shown (Figure 9 and Figure S4), pure CTC powder exhibited obvious characteristic diffraction peaks, indicating a crystal form. The characteristic peaks that appeared coincided highly with the simulated XRD pattern, but the relative intensities differed. This could be due to the preferred orientation of the sample used for diffractogram measurement. PVPK30, HP-β-CD, and gelatin all showed wide peak characteristics indicative of amorphous forms, consistent with previous studies [40,41]. It is worth noting that the PVPK30 and gelatin physical mixtures showed reduced characteristic peak strength for CTC, bearing in mind that both mixtures were composed of CTC/PVPK30 and CTC/gelatin, respectively, with 1:1 and 1:075 weight ratios. Therefore, when compared with pure CTC powder, the CTC peak strength was reduced and weakened. The disappearance of the HP-β-CD physical mixture drug crystals was possibly related to the amorphous properties of cyclodextrin. However, the diffraction patterns of PVPK30- and gelatin-based solid dispersions, prepared by medium ball milling, showed that the characteristic peak intensity was significantly lower than that for the pure CTC powder and the physical mixtures, indicating the presence of amorphous CTC in the solid dispersion. The HP-β-CD-based solid dispersions showed wide peak characteristics, indicating an amorphous CTC form.
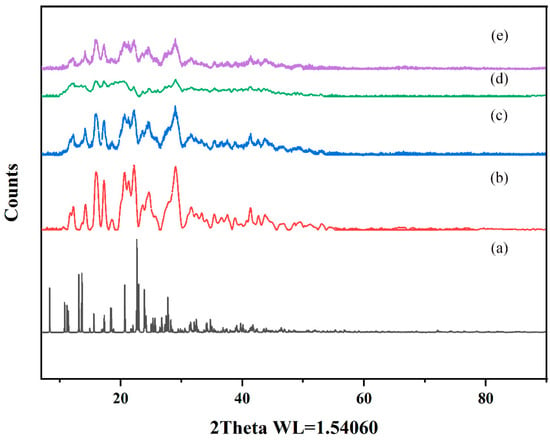
Figure 9.
Comparing powder X-ray diffraction patterns: (a) CTC simulated; (b) CTC powder; (c) PVPK30-based solid dispersion; (d) HP-β-CD-based solid dispersion; (e) and gelatin-based solid dispersion.
2.5.6. Fourier-Transform Infrared Spectroscopy (FT-IR)
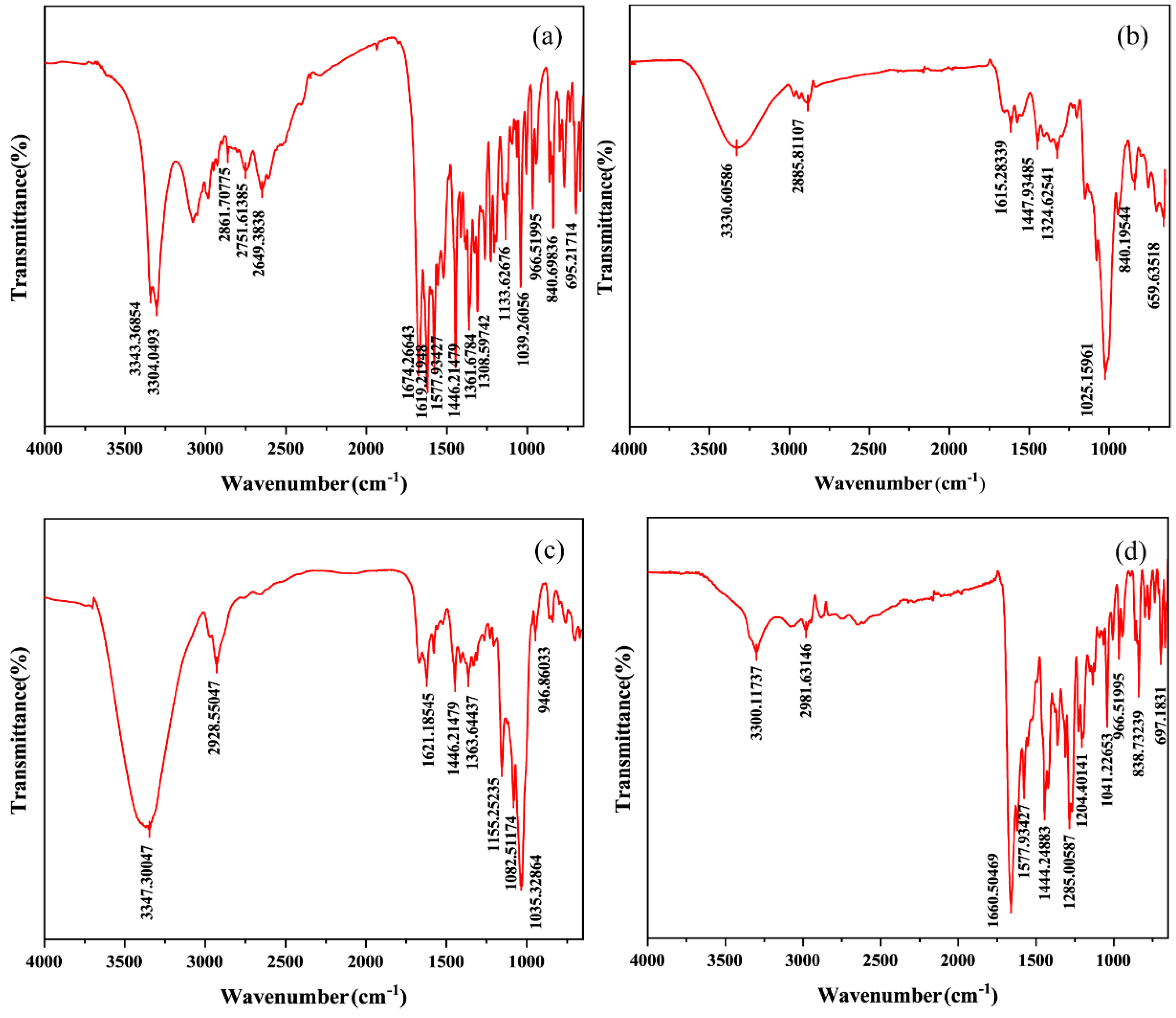
FT-IR is used to study the nature and extent of interactions between drugs and carriers and to identify interaction differences between drugs and carriers in physical and media grinding mixtures [39]. Our FT-IR spectra are shown in Figure 10 and Figure S5. The pure CTC spectra showed (Table 2) N-H stretching vibration signals at 3343 and 3304 cm−1; C=O double signals at 1674 cm−1; aliphatic C-H stretching vibrations at 1446, 1361, and 1308 cm−1; and bending vibrations outside the C-H aromatic ring at 840 and 695 cm−1. The physical mixtures’ spectral patterns (Figure S5d,e,f) reflected the sum of the FT-IR spectral band positions of PVPK30, HP-β-CD, gelatin, and CTC, with accumulation effects, thus indicating little or no interactions between the drug and polymer in the physical mixtures. However, the spectral patterns for the solid dispersions prepared by medium ball grinding (Figure 10) were significantly different to those for the physical mixtures. In the three solid-dispersion formulations, partial peak displacement occurred, but no new absorption peaks appeared, indicating that CTC had not formed new chemical bonds and that intermolecular interactions occurred between the drug and polymers, leading to hydrogen bond formation.
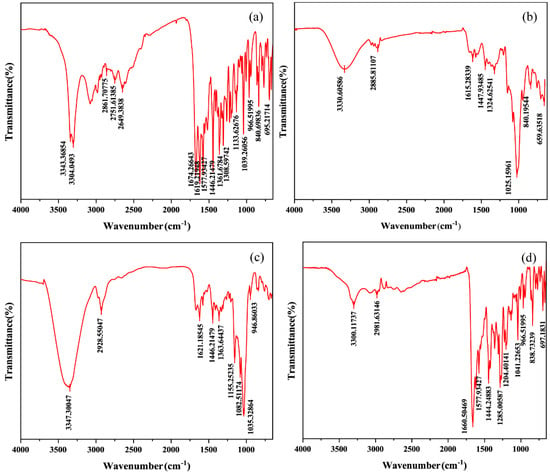
Figure 10.
Comparing Fourier infrared spectroscopy spectra: (a) CTC powder; (b) PVPK30-based solid dispersion; (c) HP-β-CD-based solid dispersion; and (d) gelatin-based solid dispersion.

Table 2.
Infrared absorption peak characteristics of functional compounds of infrared spectrometer.
3. Materials and Methods
3.1. Materials
The CTC (purity > 85%) and poloxamer were purchased from Beijing Solarbio Technology Co., Ltd. (Beijing, China). The β-CD, HP-β-CD, gelatin, and PVPK30 were obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Polyethylene glycol was purchased from Shanghai Guangnuo Chemical Technology Co., Ltd. (Shanghai, China). Microcrystalline cellulose was purchased from IMCD Co., Ltd. (Shanghai, China). Sodium alginate, polyvinyl alcohol, and ethyl cellulose were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). All the other materials were of analytical reagent grade and used as received. All experiments were conducted using de-ionized water.
3.2. Preparing Solid Dispersions by Grinding with CTC-Loaded Medium
The CTC solid dispersions were prepared using the medium grinding method, and the best carrier was determined. At room temperature, CTC was mixed with different PVPK30, gelatin, and HP-β-CD quantities in a small, laboratory-scale medium grinder (Chishun technology development Co., Ltd., Nanjing, China). We used two zirconia grinding tanks to balance the grinding (45 mL); 16 built-in porcelain balls of different sizes, four grinding cycles, 530 revolutions, a grinding time = 30 min, and a combination of forward and reverse rotation were used.
3.3. Polymer Effects on CTC Solubility
To select suitable polymers for the CTC solid-dispersion preparations, the CTC solubility was screened using different polymers (gelatin, sodium alginate, HP-β-CD, β-CD, polyvinyl alcohol, PVPK30, poloxamer188, microcrystalline cellulose, ethyl cellulose, and polyethylene glycol). Equal CTC and polymer proportions were added to a 20 mL aqueous solution for 2 h with stirring and then ultrasonicated for 5 min, oscillated at 25 °C (in a water bath) for 7 days, and centrifuged at 12,000 rpm for 15 min, and the supernatants were filtered through 0.22 μm filters. Finally, the CTC concentrations were determined using high-performance liquid chromatography (HPLC).
3.4. Polymer Dose Effects on CTC Solubility in Solid Dispersions
PVPK30-based solid dispersions, using CTC-to-PVPK30 weight ratios of 1/0, 1/0.25, 1/0.5, and 1/1, were prepared using the medium grinding method. The HP-β-CD-based solid dispersions were prepared using CTC-and-HP-β-CD weight ratios of 1/0, 1/0.5, 1/1, 1/1.5 and 1/2; and the gelatin-based solid dispersions were prepared using CTC-to-gelatin weight ratios of 1/0, 1/0.25, 1/0.5, 1/0.75, 1/1, and 1/1.5. The ratios were then screened to determine the optimal solid-dispersion proportions. Finally, the CTC concentrations were determined using HPLC.
3.5. Stability Study
The stability of the solid dispersions was evaluated by influencing-factor experiments including high temperature, high humidity, and strong light [42]. Precision-weighed amounts of 2.0 g solid-dispersion samples were placed in a plate and placed in a stable oven at 60 °C/75% ± 1%, 40 °C/75% ± 1% (high-temperature, high-humidity test) and 4500 ± 500 l× (light test) for 10 days, respectively. The samples were taken on the fifth and the tenth day to assess changes in the appearance and drug content.
3.6. Solubility and Dissolution Studies on CTC-Loaded Solid Dispersions
The dissolution rates of the PVPK30-, HP-β-CD- and gelatin-based solid dispersions (each containing 100 mg CTC) were measured by a dissolution tester at 37 °C and 900 mL distilled water with a rotational speed of 150 rpm/min. Then, 5 mL samples were taken at 5, 10, 15, 30, 45, 60, and 120 min, and the same volume of distilled water added [43]. The samples were then filtered through 0.22 µm microporous filter membranes, and the drug concentrations were determined by HPLC.
3.7. HPLC
HPLC was performed using a Waters2489 system (Milford, Massachusetts, USA). The sample solubility and dissolution were analyzed on a C18 column (250 × 4.6 mm, inner diameter = 5 μm; Milford, MA, USA) in a mobile phases of 0.01 M oxalic acid, acetonitrile, and methanol (7:2:1). The chromatographic conditions were flow rate = 1.0 mL/min, temperature = 35 °C, and sample volume = 20 μL. A standard curve was used to calculate the CTC concentrations: Y = 4.9526x − 0.3447, R2 = 1. All the experiments were conducted in triplicate.
3.8. CTC-Loaded Solid-Dispersion Physicochemical Properties
3.8.1. SEM
The sample powders were analyzed using a JSM-15 SEM (JEOL, Tokyo, Japan) to characterize their morphology. The samples were fixed to a brass column using conductive double-sided tape and kept in a vacuum, using the JFC 40 (JEOL, Tokyo, Japan) ion sputtering coating machine gold plating to improve the electrical conductivity. The samples were then placed into the sample tank of the scanning analyzer, where they were observed and photographed at an accelerated voltage = 10 kV [44].
3.8.2. DSC
Thermal analysis was performed using the MDSC Q999 (T-zero™ DSC Technology, TA Instruments, New Castle, DE, USA) differential scanning calorimeter. The samples were placed in a sealed aluminum pan and heated in a dynamic nitrogen atmosphere at 50 mL/min at a heating rate of 10 °C/min. Among them, the gelatin physical mixture and solid dispersion were heated in the temperature range of 40–350 °C [45], CTC in the temperature range of 28–200, and PVPK30 in the temperature range of 130–600.
3.8.3. TGA
A TGA (Q5000, TA Corporation, New Castle, DE, USA) was used to determine thermal stability. A sample was placed in a platinum sample tray and, in a N2 environment (40 mL/min), the heating rates were increased from 40 °C to 900 °C at 10 °C/min intervals. A TGA curve was generated as the sample mass changed with the increasing temperature [36].
3.8.4. PXRD
PXRD patterns were generated by evaluating the physical state of a sample using a Bruker D40 Advance diffractometer (Bruker, Germany). The samples were analyzed using Cu-Kα radiation (λ = 8.1 A) in the diffraction angle (2θ) range from 7 to 90°. The running data were as follows: scanning step = 0.02° and scanning speed = 1°/s [29]. In addition, the simulated XRD pattern was generated by the vesta3.5.8 software.
3.8.5. FT-IR
The FT-IR spectra were obtained in a Bruker Tensor 27 FT-IR spectrometer (Bruker, Germany). The samples were thoroughly mixed and ground in KBr, after which the powders were compressed to prepare KBr disks, and finally 32 scans were accumulated at 2 cm−1 resolution in the 4000–400 cm−1 range [46].
3.8.6. PS Distribution Analyses
The average PS and distribution were characterized using laser diffraction. The samples were diluted 100-fold in deionized water and added to a colorimetric dish. The dispersant and refractive indices were set to ensure that the diluted samples were in clear and transparent solutions. Ultimately, the measured sample PS distribution categorized by D90, D50, and D10 is given [47].
3.9. Statistical Analyses
The data were expressed as the mean ± SD. The results were analyzed using one-way analysis of variance and Duncan’s multiple range tests in SPSS 20.0 software. The significance level was set at p < 0.05.
4. Conclusions
In summary, this study successfully prepared a polymer-based solid dispersion using the medium ball milling method, which included hydrophilic polymers such as PVPK30, HP-β-CD, and gelatin to improve the solubility of CTC. And characterization was carried out using the SEM, DSC, TGA, PXRD, FT-IR, and particle size distribution methods. It was found that the physical properties and particle size of the insoluble drug CTC were affected by the type and proportion of the polymer, causing CTC to transition from a crystalline state to an amorphous state, thereby improving the solubility and dissolution rate of the drug. The solubility studies showed that the solubility values for the 1/0.75 PVPK30- (approximately 25 mg/mL), 1/2 HP-β-CD- (approximately 30.8 mg/mL), and 1/1 gelatin-based solid dispersions (approximately 15 mg/mL) were significantly improved when compared with those of pure CTC powder (4 mg/mL). Also, the PVPK30 (57.78%), HP-β-CD (59.95%), and gelatin (60.89%) solid dispersions’ dissolution rates in vitro were significantly higher than those for other CTC preparations. Additionally, the initial dissolution rate of the gelatin-based solid dispersion was significantly higher than that of the PVPK30- and HP-β-CD-based solid dispersions, as the gelatin-based solid dispersion consisted of smaller, more uniform particles bound together to form aggregates. Therefore, the results of this study indicate that the selection of polymer types and ratios is an important and promising strategy for improving the solubility of poorly soluble drugs and ultimately enhancing their bioavailability.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms251910591/s1: Figure S1: Scanning electron micrographs; Figure S2: Comparing differential scanning calorimetric thermograms; Figure S3: Comparing thermal gravimetric analyzer thermograms; Figure S4: Comparing powder X-ray diffraction patterns; Figure S5: Comparing Fourier-transform infrared spectroscopy spectral patterns.
Author Contributions
Conceptualization, J.Z. and Y.Z.; methodology, C.Z.; software, C.Z.; validation, C.Z., Y.L. and Y.B.; formal analysis, C.Z.; investigation, B.L.; resources, J.Z.; data curation, C.Z.; writing—original draft preparation, C.Z.; writing—review and editing, B.L.; visualization, J.Z.; supervision, B.L.; project administration, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grants from the Science and Technology Plan Project of Gansu Province (Grant Nos. 24JRRA028), the National Beef Cattle and Yak Industry Technology System (CARS-37), the Central Public-interest Scientific Institution Basal Research Fund (1610322024016), and the Chinese Academy of Agricultural Sciences Innovation Project (Grant 25-LZIHPS-05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors would like to thank the Key Laboratory of Veterinary Medicine Creation, Chinese Academy of Agricultural Sciences for providing key reagents and equipment for this study. We would also like to express our sincere gratitude to the editor and anonymous reviewers for their valuable comments, which have greatly improved this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, N.; Liu, K.; Yan, B.; Zhu, L.; Zhao, C.; Gao, J.; Ruan, J.; Zhang, W.; Qiu, R. Chlortetracycline hydrochloride removal by different biochar/Fe composites: A comparative study. J. Hazard. Mater. 2021, 403, 123889. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, W.; Liao, Y.; Jiang, X.; Cheng, Z. Alliance between doping Ag and dual ligands-enhanced fluorescent gold nanoclusters for the assays of vitamin B12 and chlortetracycline hydrochloride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120194. [Google Scholar] [CrossRef]
- He, B.; Yang, Y.; Liu, B.; Zhao, Z.; Shang, J.; Cheng, X. Degradation of chlortetracycline hydrochloride by peroxymonosulfate activation on natural manganese sand through response surface methodology. Environ. Sci. Pollut. Res. Int. 2022, 29, 82584–82599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Q.; Yang, G.; Li, X.; Du, W.; Leong, Y.K.; Chang, J.-S. Enhanced chlortetracycline removal by iron oxide modified spent coffee grounds biochar and persulfate system. Chemosphere 2022, 301, 134654. [Google Scholar] [CrossRef]
- Liu, D.; Chen, P.; Yang, X.; Wang, J. Synthesis and evaluation of bisulfate/mesylate-conjugated chlortetracycline with high solubility and bioavailability. Acta Pharm. 2020, 70, 483–498. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef]
- Lipert, M.P.; Rodríguez-Hornedo, N. Cocrystal Transition Points: Role of Cocrystal Solubility, Drug Solubility, and Solubilizing Agents. Mol. Pharm. 2015, 12, 3535–3546. [Google Scholar] [CrossRef]
- Shen, J.; Hu, A.; Yang, Y.; Nie, T.; Huang, S.; Cheng, Z.; Liu, W. Ternary solid dispersions of lacidipine: Enhancing dissolution and supersaturation maintenance through strategic formulation optimization. Int. J. Pharm. 2024, 654, 123989. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, F. Modelling drug degradation of amorphous solid dispersion during twin-screw extrusion. Eur. J. Pharm. Biopharm. 2023, 190, 197–205. [Google Scholar] [CrossRef]
- Mondal, S.; Sirvi, A.; Jadhav, K.; Sangamwar, A.T. Supersaturating lipid-based solid dispersion of atazanavir provides enhanced solubilization and supersaturation in the digestive aqueous phase. Int. J. Pharm. 2023, 638, 122919. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, L.; Su, M.; Heng, W.; Wei, Y.; Gao, Y.; Qian, S. Deaggregation and Crystallization Inhibition by Small Amount of Polymer Addition for a Co-Amorphous Curcumin-Magnolol System. Pharmaceutics 2021, 13, 1725. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, S.; Hao, Y.; Fan, K.; Wang, L.; Zhao, X.; He, X. Amorphous solid dispersion preparation via coprecipitation improves the dissolution, oral bioavailability, and intestinal health enhancement properties of magnolol. Poult. Sci. 2023, 102, 102676. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, H.; Wang, Y.; Wang, R.; Xu, J.; Zhang, C. Amorphous Solid Dispersions: Role of the Polymer and Its Importance in Physical Stability and In Vitro Performance. Pharmaceutics 2022, 14, 1747. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef] [PubMed]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. The role of polymer concentration on the molecular mobility and physical stability of nifedipine solid dispersions. Mol. Pharm. 2015, 12, 1477–1484. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. The role of drug-polymer hydrogen bonding interactions on the molecular mobility and physical stability of nifedipine solid dispersions. Mol. Pharm. 2015, 12, 162–170. [Google Scholar] [CrossRef]
- Sarpal, K.; Delaney, S.; Zhang, G.G.Z.; Munson, E.J. Phase Behavior of Amorphous Solid Dispersions of Felodipine: Homogeneity and Drug-Polymer Interactions. Mol. Pharm. 2019, 16, 4836–4851. [Google Scholar] [CrossRef]
- Xia, N.; Wan, W.; Zhu, S.; Liu, Q. Preparation of crystalline nanocellulose/hydroxypropyl β cyclodextrin/carboxymethyl cellulose polyelectrolyte complexes and their controlled release of neohesperidin-copper (II) in vitro. Int. J. Biol. Macromol. 2020, 163, 1518–1528. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef]
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural biomacromolecule based composite scaffolds from silk fibroin, gelatin and chitosan toward tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef] [PubMed]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tong, M.; Li, S.; Yu, X.; Hu, Z.; Zhang, Q.; Xu, R.; Wang, J. Surfactant-free amorphous solid dispersion with high dissolution for bioavailability enhancement of hydrophobic drugs: A case of quercetin. Drug Dev. Ind. Pharm. 2021, 47, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Luanda, A.; Manohar, M.; Charyulu, R.N.; Badalamoole, V. Evaluation of drug release efficiency and antibacterial property of a pH-responsive dextran-based silver nanocomposite hydrogel. Int. J. Biol. Macromol. 2024, 268, 131783. [Google Scholar] [CrossRef]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Hu, L. Study on the in Vitro Release of Streptomycin Microcapsules and Their Impact on Rumen Bacterial Diversity in Sheep. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Pilet, C.; Lanneluc, B. Pharmacopoeia and veterinary medicine. Ann. Dell’Istituto Super. Sanità 1975, 11, 245–253. [Google Scholar]
- Yang, M.; He, S.; Fan, Y.; Wang, Y.; Ge, Z.; Shan, L.; Gong, W.; Huang, X.; Tong, Y.; Gao, C. Microenvironmental pH-modified solid dispersions to enhance the dissolution and bioavailability of poorly water-soluble weakly basic GT0918, a developing anti-prostate cancer drug: Preparation, characterization and evaluation in vivo. Int. J. Pharm. 2014, 475, 97–109. [Google Scholar] [CrossRef]
- Tang, P.; Ma, X.; Wu, D.; Li, S.; Xu, K.; Tang, B.; Li, H. Posaconazole/hydroxypropyl-β-cyclodextrin host–guest system: Improving dissolution while maintaining antifungal activity. Carbohydr. Polym. 2016, 142, 16–23. [Google Scholar] [CrossRef]
- Baybaş, D.; Serdaroğlu, G.; Semerci, B. The composite microbeads of alginate, carrageenan, gelatin, and poly(lactic-co-glycolic acid): Synthesis, characterization and Density Functional Theory calculations. Int. J. Biol. Macromol. 2021, 181, 322–338. [Google Scholar] [CrossRef]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and Improved Dissolution of Glycyrrhetinic Acid Solid Dispersion by Alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.J.; Zia, H.; Sandhu, H.K.; Shah, N.H.; Malick, W.A. Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution—Pros and cons. Drug Deliv. 2007, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, N.; Lian, R.; Qi, J.; Wu, W. Understanding the relationship between wettability and dissolution of solid dispersion. Int. J. Pharm. 2014, 465, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Petkar, B.; Pore, Y.; Kulkarni, A.; Burade, K. Physicochemical and molecular modeling studies of cefixime–l-arginine–cyclodextrin ternary inclusion compounds. Carbohydr. Polym. 2013, 98, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Marcolino, A.I.P.; Macedo, L.B.; Nogueira-Librelotto, D.R.; Fernandes, J.R.; Bender, C.R.; Wust, K.M.; Frizzo, C.P.; Mitjans, M.; Vinardell, M.P.; Rolim, C.M.B. Preparation, characterization and in vitro cytotoxicity study of dronedarone hydrochloride inclusion complexes. Mater. Sci. Eng. C 2019, 100, 48–61. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, P.; Sun, P.; Kang, Y.; Niu, Y.; She, Y.; Zhao, D. Inclusion complexes of β-cyclodextrin with isomeric ester aroma compounds: Preparation, characterization, mechanism study, and controlled release. Carbohydr. Polym. 2024, 333, 121977. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review. Bioresour. Technol. 2017, 246, 88–100. [Google Scholar] [CrossRef]
- Slámová, M.; Prausová, K.; Epikaridisová, J.; Brokešová, J.; Kuentz, M.; Patera, J.; Zámostný, P. Effect of co-milling on dissolution rate of poorly soluble drugs. Int. J. Pharm. 2021, 597, 120312. [Google Scholar] [CrossRef]
- Yousaf, A.M.; Malik, U.R.; Shahzad, Y.; Mahmood, T.; Hussain, T. Silymarin-laden PVP-PEG polymeric composite for enhanced aqueous solubility and dissolution rate: Preparation and in vitro characterization. J. Pharm. Anal. 2019, 9, 34–39. [Google Scholar] [CrossRef]
- Ma, S.X.; Chen, W.; Yang, X.D.; Zhang, N.; Wang, S.J.; Liu, L.; Yang, L.J. Alpinetin/hydroxypropyl-β-cyclodextrin host-guest system: Preparation, characterization, inclusion mode, solubilization and stability. J. Pharm. Biomed. Anal. 2012, 67–68, 193–200. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Chen, D.; Xu, W.; Tao, Y.; Pan, Y.; Meng, K.; Shabbir, M.A.B.; Liu, Q.; Huang, L.; et al. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int. J. Nanomed. 2019, 14, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Woo, M.R.; Choi, H.G.; Jin, S.G. Effects of Polymers on the Drug Solubility and Dissolution Enhancement of Poorly Water-Soluble Rivaroxaban. Int. J. Mol. Sci. 2022, 23, 9491. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.O.K.; Tan, Y.T.F.; Peh, K.K. Effect of HPMC concentration on β-cyclodextrin solubilization of norfloxacin. Carbohydr. Polym. 2014, 101, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Pan, L.; Ning, K.; Fang, Y.; Muhitdinov, B.; Liu, E.; Huang, Y. Asiatic acid cyclodextrin inclusion micro-cocrystal for insoluble drug delivery and acute lung injury therapy enhancement. J. Nanobiotechnol. 2024, 22, 119. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, C.; Tian, F.; Xiao, Z.; Sun, Z.; Lu, L.; Dai, W.; Zhang, Q.; Mei, X. Improving the Dissolution Rate and Bioavailability of Curcumin via Co-Crystallization. Pharmaceuticals 2024, 17, 489. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Xu, D. Encapsulation of calcium carbonate with a ternary mixture of sodium caseinate/gelatin/xanthan gum to enhance the dispersion stability of solid/oil/water emulsions. Front. Nutr. 2022, 9, 1090827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).