Polyamines in Dysbiotic Oral Conditions of Older Adults: A Scoping Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
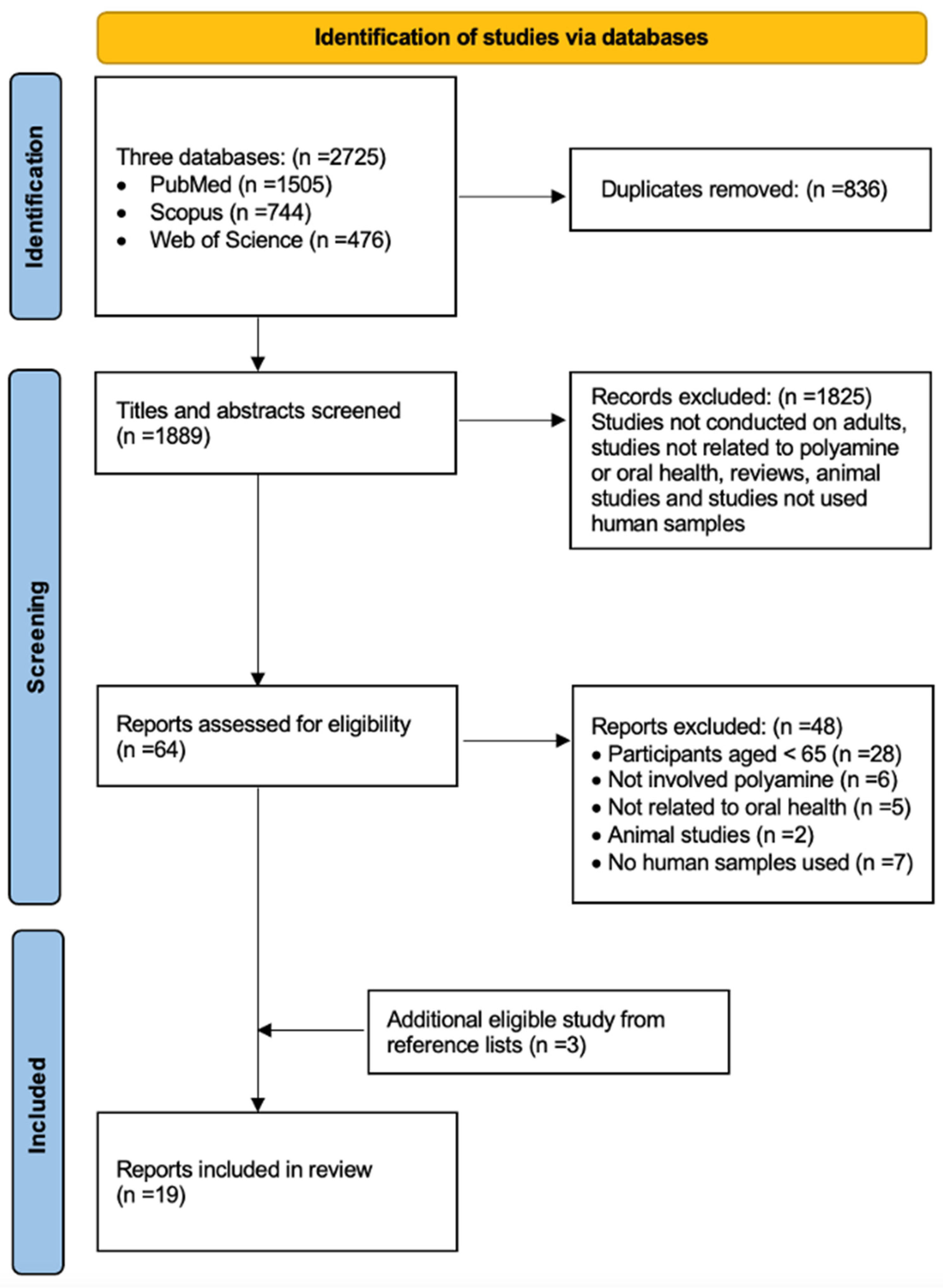
2.2. Study Selection
2.3. Data Extraction and Synthesis
3. Results
3.1. Oral Cancer
3.2. Periodontal Infection
3.3. Halitosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Uemura, T.; Matsunaga, M.; Yokota, Y.; Takao, K.; Furuchi, T. Inhibition of polyamine catabolism reduces cellular senescence. Int. J. Mol. Sci. 2023, 24, 13397. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Gutierrez, G.E.; Borbolla Jiménez, F.V.; Muñoz, L.G.; Tapia Guerrero, Y.S.; Murillo Melo, N.M.; Cristóbal-Luna, J.M.; eGarcia, N.L.; Cordero-Martínez, J.; Magaña, J.J. The molecular role of polyamines in age-related diseases: An update. Int. J. Mol. Sci. 2023, 24, 16469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, E.; Radaic, A.; Yu, X.; Yang, S.; Yu, C.; Xiao, S.; Ye, C. Diagnostic potential and future directions of matrix metalloproteinases as biomarkers in gingival crevicular fluid of oral and systemic diseases. Int. J. Biol. Macromol. 2021, 188, 180–196. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. Common medical and dental problems of older adults: A narrative review. Geriatrics 2021, 6, 76. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A systematic review on caries status of older adults. Int. J. Environ. Res. Public. Health 2021, 18, 10662. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Paudel, G.; Acharya, R.; George, A.; Borgnakke, W.S.; Rawal, L.B. Oral health and healthy ageing: A scoping review. BMC Geriatr. 2024, 24, 33. [Google Scholar] [CrossRef]
- Kurihara, S. Polyamine metabolism and transport in gut microbes. Biosci. Biotechnol. Biochem. 2022, 86, 957–966. [Google Scholar] [CrossRef]
- Ramani, D.; De Bandt, J.P.; Cynober, L. Aliphatic polyamines in physiology and diseases. Clin. Nutr. 2014, 33, 14–22. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-promoting effects of dietary polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Wawrzyniak, M.; Groeger, D.; Frei, R.; Ferstl, R.; Wawrzyniak, P.; Krawczyk, K.; Pugin, B.; Barcik, W.; Westermann, P.; Dreher, A.; et al. Spermidine and spermine exert protective effects within the lung. Pharmacol. Res. Perspect. 2021, 9, e00837. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A., Jr.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer. 2022, 22, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Carriche, G.M.; Almeida, L.; Stüve, P.; Velasquez, L.; Dhillon-LaBrooy, A.; Roy, U.; Lindenberg, M.; Strowig, T.; Plaza-Sirvent, C.; Schmitz, I.; et al. Regulating T-cell differentiation through the polyamine spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348.e11. [Google Scholar] [CrossRef] [PubMed]
- Maier, T. Oral microbiome in health and disease: Maintaining a healthy, balanced ecosystem and reversing dysbiosis. Microorganisms 2023, 11, 1453. [Google Scholar] [CrossRef]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory networks linking oral microbiome with systemic health and disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef]
- Suárez, L.J.; Garzón, H.; Arboleda, S.; Rodríguez, A. Oral dysbiosis and autoimmunity: From local periodontal responses to an imbalanced systemic immunity: A review. Front. Immunol. 2020, 11, 591255. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Narayanan, R.; Shankar, V.; Ethiraj, S.; Wang, X.; Duan, N.; Ni, Y.-H.; Hu, Q.; Zare, R.N. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc. Natl. Acad. Sci. USA 2020, 117, 16167–16173. [Google Scholar] [CrossRef]
- Michailidou, E.; Tzimagiorgis, G.; Chatzopoulou, F.; Vahtsevanos, K.; Antoniadis, K.; Kouidou, S.; Markopoulos, A.; Antoniades, D. Salivary mRNA markers having the potential to detect oral squamous cell carcinoma segregated from oral leukoplakia with dysplasia. Cancer Epidemiol. 2016, 43, 112–118. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Edamatsu, K.; Sugano, A.; Kitabatake, K.; Iino, M. Discrimination of oral squamous cell carcinoma from oral lichen planus by salivary metabolomics. Oral. Dis. 2020, 26, 35–42. [Google Scholar] [CrossRef]
- Cheng, Y.S.L.; Jordan, L.; Rees, T.; Chen, H.S.; Oxford, L.; Brinkmann, O.; Wong, D. Levels of potential oral cancer salivary mRNA biomarkers in oral cancer patients in remission and oral lichen planus patients. Clin. Oral. Investig. 2014, 18, 985–993. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Chen, Y.T.; Hsieh, Y.J.; Chang, K.P.; Hsueh, P.C.; Chen, T.W.; Yu, J.-S.; Chang, Y.-S.; Li, L.; Wu, C.-C. Integrated analyses utilizing metabolomics and transcriptomics reveal perturbation of the polyamine pathway in oral cavity squamous cell carcinoma. Anal. Chim. Acta. 2019, 1050, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Sugahara, K.; Kasahara, K.; Katakura, A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017, 37, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- de Sá Alves, M.; de Sá Rodrigues, N.; Bandeira, C.M.; Chagas, J.F.S.; Pascoal, M.B.N.; Nepomuceno, G.L.J.T.; Martinho, H.d.S.; Alves, M.G.O.; Mendes, M.A.; Dias, M.; et al. Identification of possible salivary metabolic biomarkers and altered metabolic pathways in south american patients diagnosed with oral squamous cell carcinoma. Metabolites 2021, 11, 650. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Jordan, L.; Chen, H.S.; Kang, D.; Oxford, L.; Plemons, J.; Parks, H.; Rees, T. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J. Periodontal Res. 2017, 52, 428–437. [Google Scholar] [CrossRef]
- Rodrigues, W.F.; Miguel, C.B.; Agostinho, F.; da Silva, G.V.; Lazo-Chica, J.E.; Naressi Scapin, S.M.; Napimoga, M.H.; Trindade-Da-Silva, C.A.; Krieger, J.E.; Pereira, A.d.C.; et al. Metabolomic evaluation of chronic periodontal disease in older adults. Mediat. Inflamm. 2021, 2021, 1796204. [Google Scholar] [CrossRef]
- Ozeki, M.; Nozaki, T.; Aoki, J.; Bamba, T.; Jensen, K.R.; Murakami, S.; Toyoda, M. Metabolomic Analysis of gingival crevicular fluid using gas chromatography/mass spectrometry. Mass Spectrom 2016, 5, A0047. [Google Scholar] [CrossRef]
- Andörfer, L.; Holtfreter, B.; Weiss, S.; Matthes, R.; Pitchika, V.; Schmidt, C.O.; Samietz, S.; Kastenmüller, G.; Nauck, M.; Völker, U.; et al. Salivary metabolites associated with a 5-year tooth loss identified in a population-based setting. BMC Med. 2021, 19, 161. [Google Scholar] [CrossRef]
- Montis, N.; Cotti, E.; Noto, A.; Fattuoni, C.; Barberini, L. Salivary metabolomics fingerprint of chronic apical abscess with sinus tract: A pilot study. Sci. World J. 2019, 2019, 3162063. [Google Scholar] [CrossRef]
- Levine, M.; Lohinai, Z.; Teles, R.P. Low biofilm lysine content in refractory chronic periodontitis. J. Periodontol. 2017, 88, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lo, K.L.; Liman, A.N.; Feng, X.P.; Ye, W. Tongue-coating microbial and metabolic characteristics in halitosis. J. Dent. Res. 2024, 103, 484–493. [Google Scholar] [CrossRef]
- Jo, J.K.; Seo, S.H.; Park, S.E.; Kim, H.W.; Kim, E.J.; Na, C.S.; Cho, K.-M.; Kwon, S.-J.; Moon, Y.-H.; Son, H.-S. Identification of salivary microorganisms and metabolites associated with halitosis. Metabolites 2021, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Dadamio, J.; Van Tornout, M.; Vancauwenberghe, F.; Federico, R.; Dekeyser, C.; Quirynen, M. Clinical utility of a novel colorimetric chair side test for oral malodour. J. Clin. Periodontol. 2012, 39, 645–650. [Google Scholar] [CrossRef]
- Pan, C.; Rizvi, Z. Oral Cancer: What the general surgeon should know. Surg. Clin. North. Am. 2022, 102, 309–324. [Google Scholar] [CrossRef] [PubMed]
- International WCRF. Mouth and Oral Cancer Statistics 2022. Available online: https://www.wcrf.org/cancer-trends/mouth-and-oral-cancer-statistics/ (accessed on 30 September 2024).
- Yang, J.; Guo, K.; Zhang, A.; Zhu, Y.; Li, W.; Yu, J.; Wang, P. Survival analysis of age-related oral squamous cell carcinoma: A population study based on SEER. Eur. J. Med. Res. 2023, 28, 413. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Panta, P.; Venna, V.R. Salivary RNA signatures in oral cancer detection. Anal. Cell Pathol. 2014, 2014, 450629. [Google Scholar] [CrossRef]
- Li, Y.; St John, M.A.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.H.; et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef]
- Brinkmann, O.; Kastratovic, D.A.; Dimitrijevic, M.V.; Konstantinovic, V.S.; Jelovac, D.B.; Antic, J.; Nesic, V.S.; Markovic, S.Z.; Martinovic, Z.R.; Akin, D. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral. Oncol. 2011, 47, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Elashoff, D.; Zhou, H.; Reiss, J.; Wang, J.; Xiao, H.; Henson, B.; Hu, S.; Arellano, M.; Sinha, U.; Le, A.; et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomark. Prev. 2012, 21, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Pink, C.; Holtfreter, B.; Völzke, H.; Nauck, M.; Dörr, M.; Kocher, T. Periodontitis and systemic inflammation as independent and interacting risk factors for mortality: Evidence from a prospective cohort study. BMC Med. 2023, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.Y.; Chu, C.H.; Ogawa, H.; Lai, E.H. Improving oral health of older adults for healthy ageing. J. Dent. Sci. 2024, 19, 1–7. [Google Scholar] [CrossRef]
- Rashid, M.E.; Alam, M.K.; Akhter, K.; Abdelghani, A.; Babkair, H.A.; Sghaireen, M.G. Assessing the impact of smoking cessation interventions on periodontal health and gingival inflammation in smokers with periodontitis. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 1), S742–S744. [Google Scholar] [CrossRef]
- Baima, G.; Corana, M.; Iaderosa, G.; Romano, F.; Citterio, F.; Meoni, G.; Tenori, L.; Aimetti, M. Metabolomics of gingival crevicular fluid to identify biomarkers for periodontitis: A systematic review with meta-analysis. J. Periodontal Res. 2021, 56, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Basic, A.; Dahlén, G. Microbial metabolites in the pathogenesis of periodontal diseases: A narrative review. Front. Oral. Health 2023, 4, 1210200. [Google Scholar] [CrossRef]
- Barnes, V.M.; Teles, R.; Trivedi, H.M.; Devizio, W.; Xu, T.; Lee, D.P.; Mitchell, M.W.; Wulff, J.E.; Milburn, M.V.; Guo, L. Assessment of the effects of dentifrice on periodontal disease biomarkers in gingival crevicular fluid. J. Periodontol. 2010, 81, 1273–1279. [Google Scholar] [CrossRef]
- Maita, E.; Horiuchi, H. Polyamine analysis of infected root canal contents related to clinical symptoms. Endod. Dent. Traumatol. 1990, 6, 213–217. [Google Scholar]
- Matos-Sousa, J.M.; Chemelo, V.S.; Frazão, D.R.; Bittencourt, L.O.; de Moura, J.D.M.; Mesquita, C.M.; Marañón-Vásquez, G.; Fagundes, N.C.F.; Paranhos, L.R.; Maia, L.C.; et al. Is there any association between the presence of biomarkers and apical periodontitis? A systematic review. Front. Immunol. 2024, 15, 1366954. [Google Scholar] [CrossRef]
- Wu, J.; Cannon, R.D.; Ji, P.; Farella, M.; Mei, L. Halitosis: Prevalence, risk factors, sources, measurement and treatmen—A review of the literature. Aust. Dent. J. 2020, 65, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Schertel Cassiano, L.; Abdullahi, F.; Leite, F.R.M.; López, R.; Peres, M.A.; Nascimento, G.G. The association between halitosis and oral-health-related quality of life: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.H.; Balan, P.; Pang, L.M.; Laine, M.L.; Seneviratne, C.J. Role of the oral microbiome, metabolic pathways, and novel diagnostic tools in intra-oral halitosis: A comprehensive update. Crit. Rev. Microbiol. 2021, 47, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.; Karpiński, T.M. The role of oral microbiota in intra-oral halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Study types | Clinical studies and laboratory studies using human samples | Studies not on human samples, case reports, commentaries, letters and reviews |
| Participants | Included any participants aged ≥ 65 | No participants aged ≥ 65 |
| Agents | Reporting polyamines and related molecules | No reporting polyamines and related molecules |
| Outcomes | Reporting dysbiotic oral conditions | No reporting dysbiotic oral conditions |
| Dysbiotic Oral Conditions | |||
|---|---|---|---|
| Oral Cancer | Periodontal Infection | Halitosis | |
| Polyamines | |||
| Cadaverine | + | + | + |
| Putrescine | + | + | + |
| Spermidine | − | ||
| Spermine | |||
| Polyamine Derivatives | |||
| N-acetylputrescine | + | ||
| N-acetylspermidine | + | ||
| Butyrylputrescine | + | + | |
| N-acetylcadaverine | + | + | |
| N-acetylspermine | + | ||
| Polyamine-related genes | |||
| OAZ1 | + | + | |
| SAT1 | + | + | |
| ODC1 | + | ||
| SMOX | + | ||
| SMS | + | ||
| SRM | + | ||
| Cadaverine-associated functional gene | + | ||
| Polyamine Precursors | |||
| Arginine | + | ||
| Lysine | + | +/− | |
| Ornithine | +/− | + | |
| Metabolites | |||
| S-adenosylmethionine | + | ||
| 5-aminovaleric acid | + | ||
| N-acetylornithine | + | ||
| Source | Polyamine, or Related Molecule | Type of Study | Methodology | Potential Application | Country [Reference] |
|---|---|---|---|---|---|
| Precancerous Conditions | |||||
| Saliva | Putrescine, Cadaverine, Spermidine, Spermine | In vitro | CPSI-MS with ML | Detection | China [17] |
| Saliva | Polyamine related mRNA | In vitro | Sequence-specific primers and RT-qPCR | Detection | Greece [18] |
| Saliva | Putrescine | In vitro | CE-TOF-MS | Detection | Japan [19] |
| Saliva | Polyamine related mRNA | In vitro | Pre-amplification RT-qPCR with nested gene-specific primers | Detection | USA [20] |
| Oral Cancer | |||||
| Saliva, Cancerous tissue | Putrescine, Cadaverine, Spermidine | In vitro | CE-TOF-MS | Detection | Japan [21] |
| Saliva | Putrescine, Cadaverine | In vitro | CE-TOF-MS | Detection | USA [22] |
| Cancerous tissue | Putrescine, Spermidine | In vitro | LC-MS | Detection | China [23] |
| Saliva | Cadaverine | In vitro | CE-MS | Detection | Japan [24] |
| Saliva | Spermidine | In vitro | GC-MS | Detection | Brazil [25] |
| Saliva | Polyamine related mRNA | In vitro | Pre-amplification RT-qPCR with nested gene-specific primers | Detection | USA [26] |
| Periodontal infection | |||||
| Gingival crevicular fluid | Putrescine | In vitro | GC-MS | Detection | Brazil [27] |
| Gingival crevicular fluid | Putrescine | In vitro | GC-MS | Detection | Japan [28] |
| Gingival crevicular fluid | Putrescine, Cadaverine | In vitro | GC-MS, UH-LCTMS | Detection Monitor of therapy | USA [26] |
| Saliva | Putrescine, Cadaverine | In vitro | CE-TOF-MS | Detection | USA [22] |
| Saliva | Cadaverine | In vitro | UMS, UH-LCTMS | Detection | Germany [29] |
| Saliva | Polyamine related mRNA | In vitro | Pre-amplification RT-qPCR with nested gene-specific primers | Detection | USA [26] |
| Saliva | Putrescine | In vitro | GC-MS | Detection | Italy [30] |
| Biofilm from gingival crevice | Cadaverine | In vitro | LC | Detection Monitor of therapy | USA [31] |
| Halitosis | |||||
| Biofilm from tongue | Polyamine- associated functional gene | In vitro | LC-MS | Detection | China [32] |
| Saliva | Putrescine | In vitro | GC-MS | Detection | Korea [33] |
| Saliva | Putrescine, Cadaverine | In vitro | Chairside colorimetric test GC-MS | Detection | Belgium [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.; Chan, A.K.Y.; Chu, C.H. Polyamines in Dysbiotic Oral Conditions of Older Adults: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 10596. https://doi.org/10.3390/ijms251910596
Chu S, Chan AKY, Chu CH. Polyamines in Dysbiotic Oral Conditions of Older Adults: A Scoping Review. International Journal of Molecular Sciences. 2024; 25(19):10596. https://doi.org/10.3390/ijms251910596
Chicago/Turabian StyleChu, Stephanie, Alice Kit Ying Chan, and Chun Hung Chu. 2024. "Polyamines in Dysbiotic Oral Conditions of Older Adults: A Scoping Review" International Journal of Molecular Sciences 25, no. 19: 10596. https://doi.org/10.3390/ijms251910596






