Regulatory Role of IL6 in Immune-Related Adverse Events during Checkpoint Inhibitor Treatment in Melanoma
Abstract
:1. Introduction
2. Results and Discussion
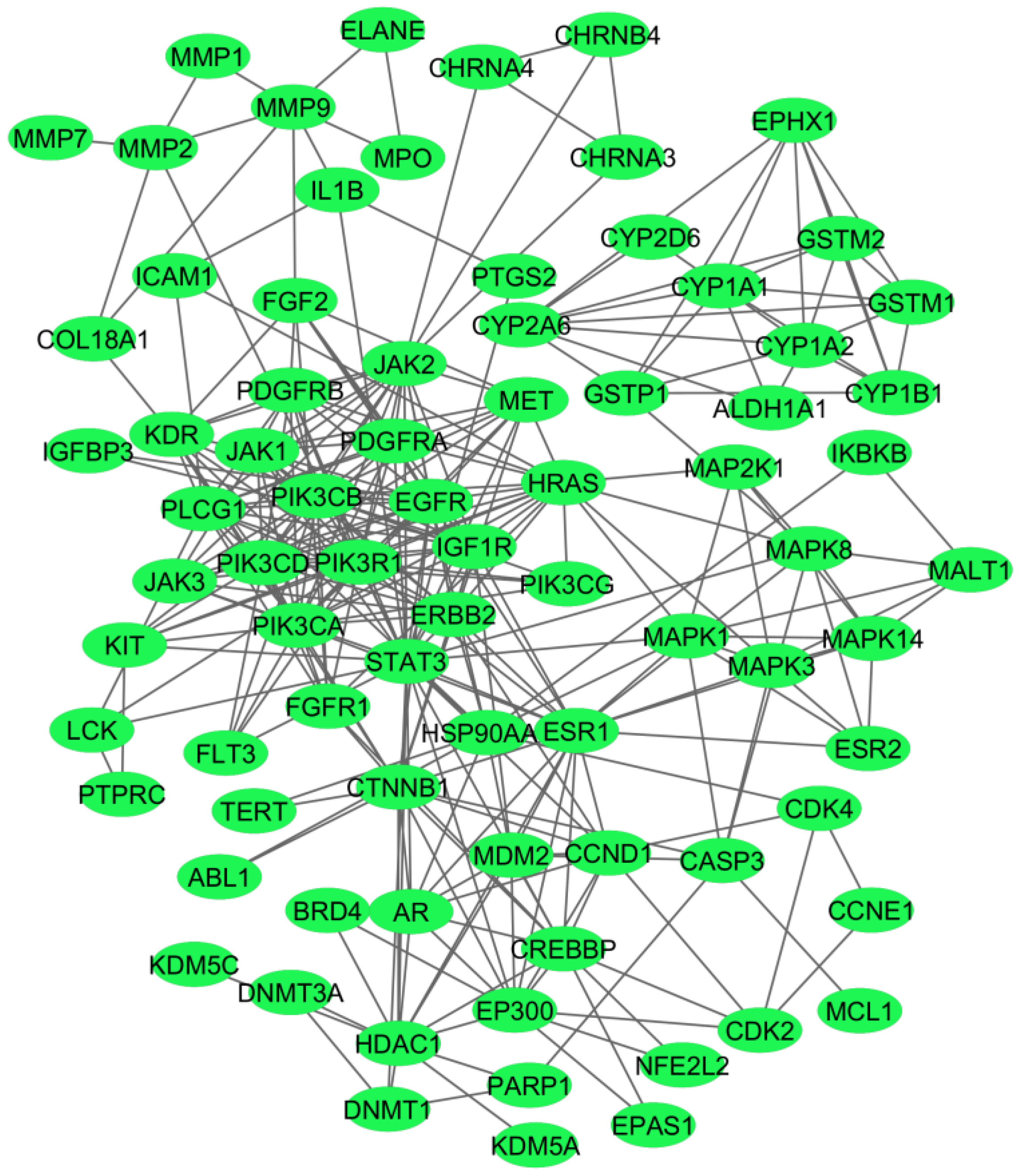
2.1. Protein–Protein Interaction Network at the Interface of Melanoma and Autoimmune Diseases
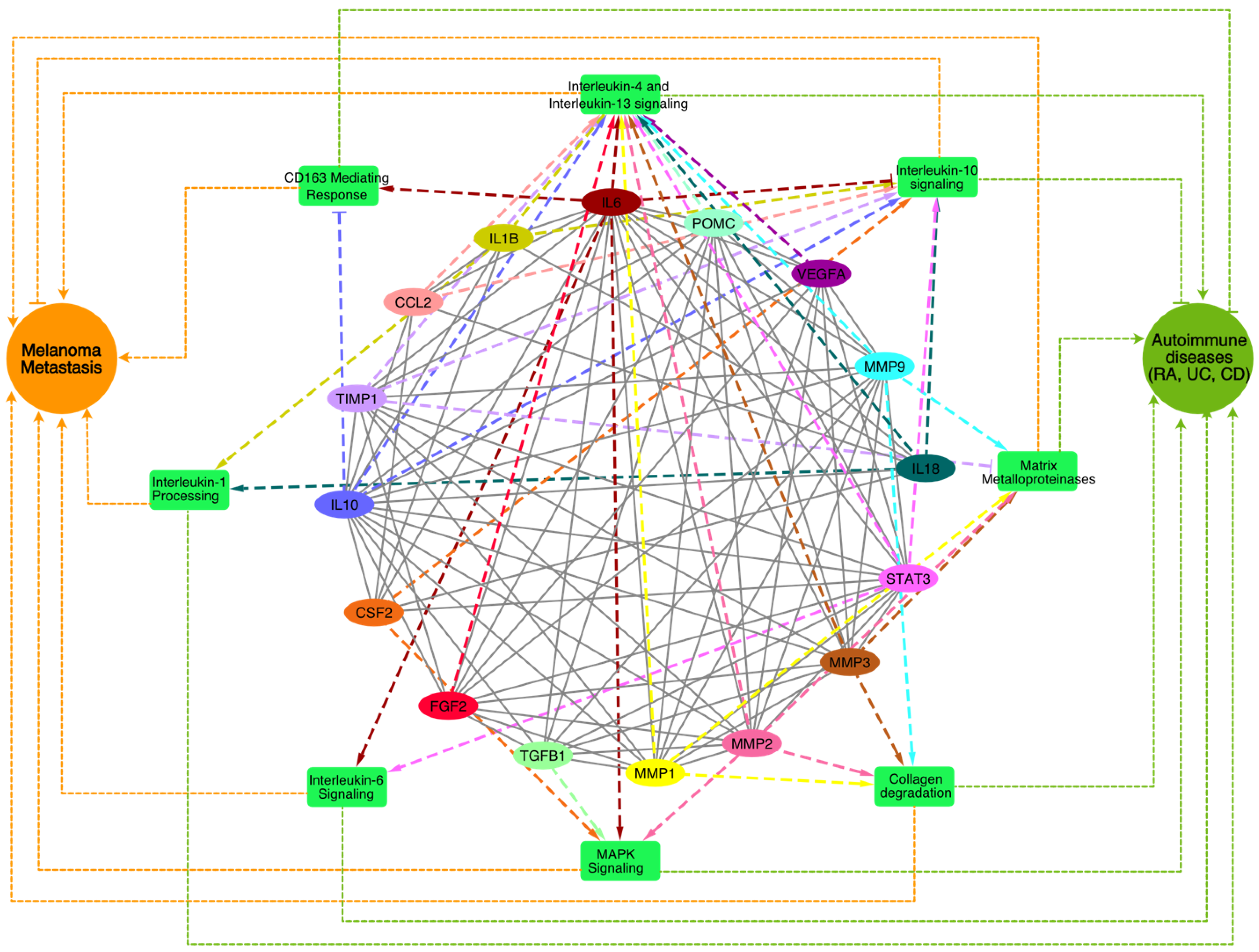
2.2. Identification of the Hub Genes from the PPI Network Associated with Crosstalk between Melanoma and Autoimmune Diseases
2.3. Pathway Enrichment Analysis of the Top MCODE Cluster
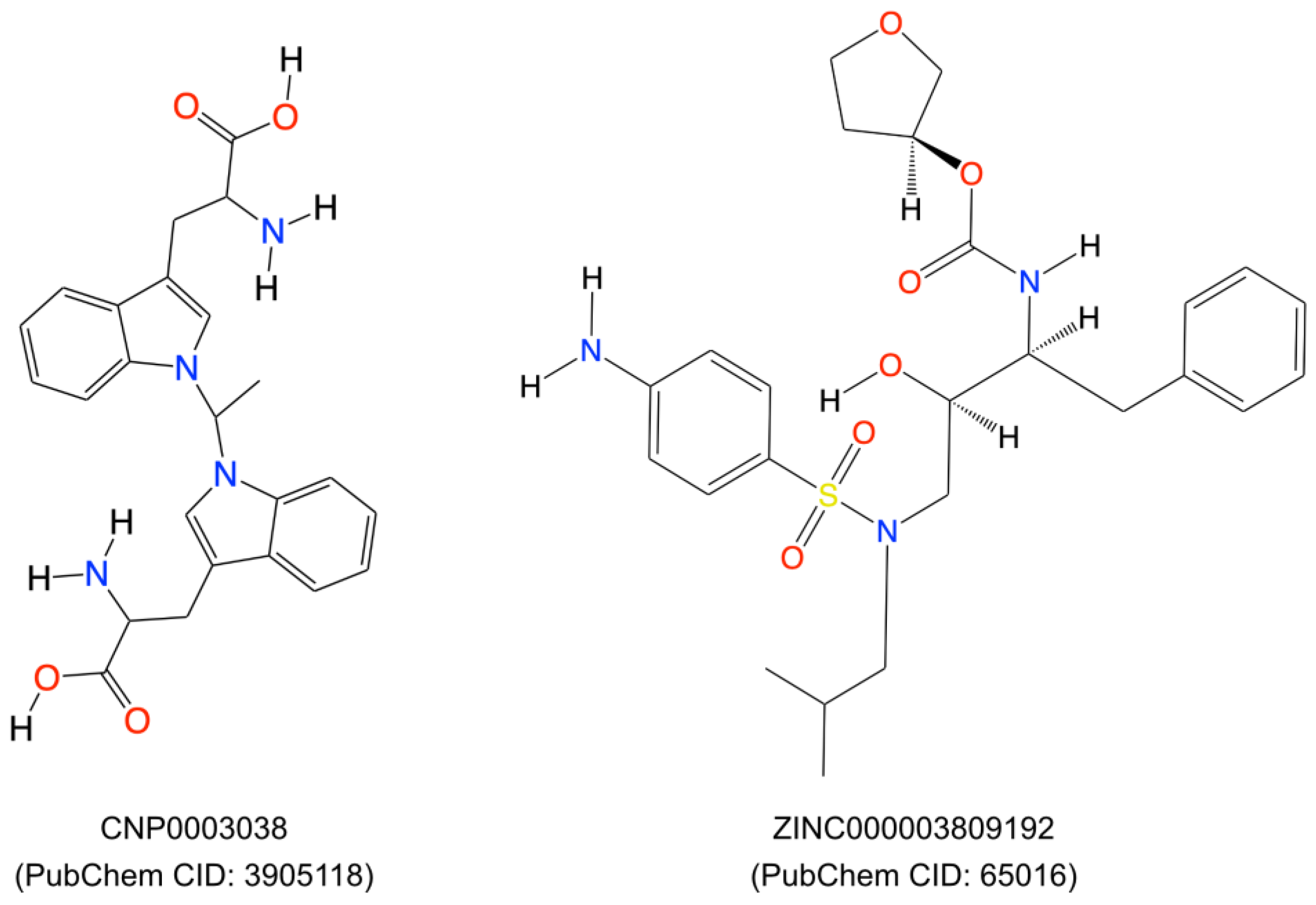
2.4. Identification of Lead Molecule and Molecular Docking
2.5. Molecular Dynamics Simulation
3. Methods and Methodology
3.1. Data Collection and Protein–Protein Interaction (PPI)
3.2. Identification of Highly Interconnected Clusters in the Tumor-Autoimmune PPI Network
3.3. Pathway Enrichment Analysis
3.4. Three-Dimensional Structure Preparation and Screening of the Lead Compounds
3.5. Molecular Dynamic Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowe, L. Metastatic melanoma and rare melanoma variants: A review. Pathology 2023, 55, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.P.; Hastings, K.T. Immune Checkpoint Inhibitors in the Treatment of Melanoma: From Basic Science to Clinical Application. In Cutaneous Melanoma: Etiology and Therapy; AU: Brisbane, Australia, 2017; pp. 121–142. [Google Scholar]
- Shah, V.; Panchal, V.; Shah, A.; Vyas, B.; Agrawal, S.; Bharadwaj, S. Immune checkpoint inhibitors in metastatic melanoma therapy (Review). Med. Int. 2024, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Motofei, I.G. Melanoma and autoimmunity: Spontaneous regressions as a possible model for new therapeutic approaches. Melanoma Res. 2019, 29, 231–236. [Google Scholar] [CrossRef]
- Ibis, B.; Aliazis, K.; Cao, C.; Yenyuwadee, S.; Boussiotis, V.A. Immune-related adverse effects of checkpoint immunotherapy and implications for the treatment of patients with cancer and autoimmune diseases. Front. Immunol. 2023, 14, 1197364. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 12. [Google Scholar] [CrossRef]
- Khan, S.; Gerber, D.E. Autoimmunity, Checkpoint Inhibitor Therapy and Immune-related Adverse Events: A Review. Semin. Cancer Biol. 2020, 64, 93–101. [Google Scholar] [CrossRef]
- Hassel, J.C. Checkpoint blocker induced autoimmunity as an indicator for tumour efficacy in melanoma. Br. J. Cancer 2021, 126, 163–164. [Google Scholar] [CrossRef]
- Portenkirchner, C.; Kienle, P.; Horisberger, K. Checkpoint inhibitor-induced colitis—A clinical overview of incidence, prognostic implications and extension of current treatment options. Pharmaceuticals 2021, 14, 367. [Google Scholar] [CrossRef]
- Lo, C.H.; Khalili, H.; Lochhead, P.; Song, M.; Lopes, E.W.; Burke, K.E.; Richter, J.M.; Chan, A.T.; Ananthakrishnan, A.N. Immune-mediated disease and Risk of Crohn’s Disease or Ulcerative Colitis: A Prospective Cohort Study. Aliment. Pharmacol. Ther. 2021, 53, 598–607. [Google Scholar] [CrossRef]
- Jeurling, S.; Cappelli, L.C. Treatment of immune checkpoint inhibitor-induced inflammatory arthritis. Curr. Opin. Rheumatol. 2020, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Eddy, K.; Shah, R.; Chen, S. Decoding Melanoma Development and Progression: Identification of Therapeutic Vulnerabilities. Front. Oncol. 2021, 10, 626129. [Google Scholar] [CrossRef]
- Napolitano, M.; di Vico, F.; Ruggiero, A.; Fabbrocini, G.; Patruno, C. The hidden sentinel of the skin: An overview on the role of interleukin-13 in atopic dermatitis. Front. Med. 2023, 10, 1165098. [Google Scholar] [CrossRef]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Fong, T.; Shabe, P.; Martineau, D.; Ando, D. Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Investig. 2001, 19, 239–247. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014, 5, 114. [Google Scholar] [CrossRef]
- Iwakura, Y. Roles of IL-1 in the development of rheumatoid arthritis: Consideration from mouse models. Cytokine Growth Factor Rev. 2002, 13, 341–355. [Google Scholar] [CrossRef]
- Silva, F.A.R.; Rodrigues, B.L.; Ayrizono, M.D.L.S.; Leal, R.F. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.B.; Westphal, J.R.; Van Muijen, G.N.P.; Ruiter, D.J. Matrix metalloproteinases in human melanoma. J. Investig. Dermatol. 2000, 115, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, G.; Hritz, I.; Varga, M.Z.; Juhász, M.; Miheller, P.; Cierny, G.; Tulassay, Z.; Herszényi, L. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig. Dis. 2012, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moestrup, S.K. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal. 2013, 18, 2352–2363. [Google Scholar] [CrossRef]
- Miskolczi, Z.; Smith, M.P.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene 2018, 37, 3166–3182. [Google Scholar] [CrossRef]
- Van Kempen, L.C.L.T.; Rijntjes, J.; Mamor-Cornelissen, I.; Vincent-Naulleau, S.; Gerritsen, M.J.P.; Ruiter, D.J.; Van Dijk, M.C.R.F.; Geffrotin, C.; Van Muijen, G.N.P. Type I collagen expression contributes to angiogenesis and the development of deeply invasive cutaneous melanoma. Int. J. Cancer 2008, 122, 1019–1029. [Google Scholar] [CrossRef]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Jarlborg, M.; Gabay, C. Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine 2022, 149, 155742. [Google Scholar] [CrossRef] [PubMed]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal. 2023, 17, 55–74. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Tanaka, R.; Okiyama, N.; Okune, M.; Ishitsuka, Y.; Watanabe, R.; Furuta, J.; Ohtsuka, M.; Otsuka, A.; Maruyama, H.; Fujisawa, Y.; et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J. Dermatol. Sci. 2017, 86, 71–73. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022, 40, 509–523.e6. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Huang, Q.; Zheng, X.; Liu, M. Progress and Challenges of Predictive Biomarkers for Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 617335. [Google Scholar] [CrossRef]
- Khoshmirsafa, M.; Seif, F.; Bagheri, N.; Beshkar, P.; Mousavi, M.; Shirzad, H. Correlation of interleukin 6 and transforming growth factor β1 with peripheral blood regulatory T cells in rheumatoid arthritis patients: A potential biomarker. Cent. J. Immunol. 2018, 43, 281–288. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Cui, T.; Zhang, J. IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac. Exp. Ther. Med. 2021, 22, 890. [Google Scholar] [CrossRef]
- Nikolaus, S.; Waetzig, G.H.; Butzin, S.; Ziolkiewicz, M.; Al-Massad, N.; Thieme, F.; Lövgren, U.; Rasmussen, B.B.; Reinheimer, T.M.; Seegert, D.; et al. Evaluation of interleukin-6 and its soluble receptor components sIL-6R and sgp130 as markers of inflammation in inflammatory bowel diseases. Int. J. Colorectal Dis. 2018, 33, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT online: Collection of Open Natural Products database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, Q.T.; Vo, N.Q.H.; Mai, T.T.; Tran, T.T.N.; Tran, T.D.; Le, M.T.; Trinh, D.T.T.; Minh Thai, K. Structure-based 3D-Pharmacophore modeling to discover novel interleukin 6 inhibitors: An in silico screening, molecular dynamics simulations and binding free energy calculations. PLoS ONE 2022, 17, e0266632. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniyan, S.; Irfan, N.; Umamaheswari, A.; Puratchikody, A. Design and virtual screening of novel fluoroquinolone analogs as effective mutant DNA GyrA inhibitors against urinary tract infection-causing fluoroquinolone resistant Escherichia coli. RSC Adv. 2018, 8, 23629–23647. [Google Scholar] [CrossRef]
- Pal, S.; Kumar, V.; Kundu, B.; Bhattacharya, D.; Preethy, N.; Reddy, M.P.; Talukdar, A. Ligand-based Pharmacophore Modeling, Virtual Screening and Molecular Docking Studies for Discovery of Potential Topoisomerase I Inhibitors. Comput. Struct. Biotechnol. J. 2019, 17, 291–310. [Google Scholar] [CrossRef]
- Smellie, A.; Kahn, S.D.; Teig, S.L. Analysis of Conformational Coverage. 1. Validation and Estimation of Coverage. J. Chem. Inf. Comput. Sci. 1995, 35, 285–294. [Google Scholar] [CrossRef]
- Diller, D.J.; Merz, K.M. High throughput docking for library design and library prioritization. Proteins Struct. Funct. Genet. 2001, 43, 113–124. [Google Scholar] [CrossRef]
- Koska, J.; Spassov, V.Z.; Maynard, A.J.; Yan, L.; Austin, N.; Flook, P.K.; Venkatachalam, C.M. Fully automated molecular mechanics based induced fit protein-ligand docking method. J. Chem. Inf. Model. 2008, 48, 1965–1973. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Love, L.A.; Rader, J.I.; Crofford, L.J.; Raybourne, R.B.; Principato, M.A.; Page, S.W.; Trucksess, M.W.; Smith, M.J.; Dugan, E.M.; Turner, M.L.; et al. Pathological and immunological effects of ingesting L-tryptophan and 1,1′-ethylidenebis (L-tryptophan) in Lewis rats. J. Clin. Investig. 1993, 91, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.A.; Miyasaka, N.; Inuo, G.; Saito, I.; Kolb, J.P.; Fujita, K.; Kashiwazaki, S. 1,1′-Ethylidenebis(tryptophan) (peak E) induces functional activation of human eosinophils and interleukin 5 production from T lymphocytes: Association of eosinophilia-myalgia syndrome with a l-tryptophan contaminant. J. Clin. Immunol. 1994, 14, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.W.; Jarrett, J.L.; Studenberg, S.D.; Humphreys, J.E.; Dennis, S.W.; Brouwer, K.R.; Woolley, J.L. Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm. Res. 1999, 16, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Wang, W.; Sun, H.B.; Zhang, L.L.; Wu, S.L.; Liu, W.T. Insights into effect of the Asp25/Asp25′ protonation states on binding of inhibitors Amprenavir and MKP97 to HIV-1 protease using molecular dynamics simulations and MM-GBSA calculations. SAR QSAR Environ. Res. 2021, 32, 615–641. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Li, T.; Wang, H.; Shi, W.; Qi, P.; Li, C.; Chen, J.; Bao, J.; Huang, G.; et al. Repositioning of amprenavir as a novel extracellular signal-regulated kinase-2 inhibitor and apoptosis inducer in MCF-7 human breast cancer. Int. J. Oncol. 2017, 50, 823–834. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Boulanger, M.J.; Chow, D.-C.; Brevnova, E.E.; Garcia, K.C. Hexameric structure and assembly of the interieukin-6/IL-6 α-receptor/gp130 complex. Science 2003, 300, 2101–2104. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, Á.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.; Stahl, M.; Seehra, J.S. 1.9 A crystal structure of interleukin 6: Implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997, 16, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, B.; Chowdhury, P.; Goswami, N.; Gogoi, N.; Naiya, T.; Chetia, P.; Mahanta, S.; Chetia, D.; Tanti, B.; Borah, P.; et al. Identification of potential plant-based inhibitor against viral proteases of SARS-CoV-2 through molecular docking, MM-PBSA binding energy calculations and molecular dynamics simulation. Mol. Divers. 2021, 25, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Sterling, T.; Irwin, J.J. ZINC 15—Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef]
- Yang, W.; Wang, S.; Zhang, X.; Sun, H.; Zhang, M.; Chen, H.; Cui, J.; Li, J.; Peng, F.; Zhu, M.; et al. New natural compound inhibitors of PDGFRA (platelet-derived growth factor receptor α) based on computational study for high–grade glioma therapy. Front. Neurosci. 2023, 16, 1060012. [Google Scholar] [CrossRef]




| Modules | Nodes | Interaction | MCODE Score | Genes |
|---|---|---|---|---|
| 1 | 16 | 76 | 10.133 | CCL2, CSF2, FGF2, IL10, IL18, IL1B, IL6, MMP1, MMP2, MMP3, MMP9, POMC, STAT3, TGFB1, TIMP1, and VEGFA |
| 2 | 7 | 18 | 6 | CREBBP, EP300, FOXO3, HIF1A, MAPK1, MDM2, and TP53 |
| 3 | 4 | 6 | 4 | AKT1, CD40, CD40LG, and PIK3CG |
| 4 | 8 | 13 | 3.71 | CTNNB1, CXCL10, CXCL8, IL1A, IL4, MYC, NFKB1, and TNF |
| 5 | 7 | 10 | 3.33 | CALM1, CALM2, CALM3, CXCR4, FAS, PIK3CB, and STAT5A |
| 6 | 3 | 3 | 3 | HLA-B, HLA-C, and HLA-DQB1 |
| S. No. | Compound ID | Database | Compound Name | LibDock Score | -CDOCKER Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | CNP0003841 | Coconut | N-[(3-methoxyphenyl)methyl]-3-({5-[(4-phenylpiperazin-1-yl)methyl]-1,2-oxazol-3-yl}methyl)oxetan-3-amine | 127.506 | NA |
| 2 | CNP0004058 | Coconut | 2-chloro-5-hydroxy-N-{[4-hydroxy-5-(hydroxymethyl)-3-{4-[3-(trifluoromethyl)phenyl]piperazin-1-yl}oxolan-2-yl]methyl}benzamide | 126.919 | NA |
| 3 | CNP0004582 | Coconut | 2-{[({3-methyl-4-[(7-methyl-1H-1,3-benzodiazol-2-yl)methyl]-6-(propan-2-yl)cyclohex-2-en-1-yl}methyl)carbamoyl]methoxy}acetic acid | 122.508 | 18.4652 |
| 4 | CNP0004629 | Coconut | 2-{[({3-methyl-4-[(1-methyl-1H-1,3-benzodiazol-2-yl)methyl]-6-(propan-2-yl)cyclohex-2-en-1-yl}methyl)carbamoyl]methoxy}acetic acid | 121.359 | 13.0031 |
| 5 | CNP0000288 | Coconut | 7-methoxy-2-(4-methoxyphenyl)-4-[2-(4-methoxyphenyl)ethyl]-3,4-dihydro-2H-1-benzopyran | 120.936 | 25.4486 |
| 6 | ZINC03809192 | ZINC | [(3S)-oxolan-3-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate | 120.668 | 34.7136 |
| 7 | CNP0004224 | Coconut | 4-(dimethylamino)-N-[5-hydroxy-7a-(2-{[2-(1H-indol-3-yl)ethyl]carbamoyl}ethyl)-3,3,5-trimethyl-octahydro-1H-inden-1-yl]benzamide | 118.314 | NA |
| 8 | CNP0004392 | Coconut | 4-[(2-{3-[2-(pyrrolidin-1-yl)pyridin-4-yl]-1,2,4-oxadiazol-5-yl}pyrrolidin-1-yl)methyl]benzoic acid | 118.034 | NA |
| 9 | CNP0003909 | Coconut | 3-[4-(4-methoxyphenyl)-1H-imidazol-2-yl]-4-[(4-methylphenyl)methyl]morpholine | 117.757 | 17.5344 |
| 10 | ZINC03955219 | ZINC | [(3aS,4R,6aR)-2,3,3a,4,5,6a-hexahydrofuro [2,3-b]furan-4-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate | 117.727 | 18.3056 |
| 11 | CNP0004686 | Coconut | 4-cyano-N-{2,3-dihydroxy-5-[6-(morpholin-4-yl)pyridin-3-yl]cyclopentyl}benzamide | 117.281 | NA |
| 12 | CNP0004257 | Coconut | N-[(2H-1,3-benzodioxol-5-yl)methyl]-3-({5-[(dimethylamino)methyl]-1,2-oxazol-3-yl}methyl)oxetan-3-amine | 116.072 | NA |
| 13 | CNP0003888 | Coconut | 3-[4-(4-chlorophenyl)-1H-imidazol-2-yl]-4-[(1-methyl-1H-imidazol-2-yl)methyl]morpholine | 115.838 | 16.6375 |
| 14 | CNP0004329 | Coconut | N-[(2H-1,3-benzodioxol-4-yl)methyl]-3-({5-[(4-phenylpiperazin-1-yl)methyl]-1,2-oxazol-3-yl}methyl)oxetan-3-amine | 115.688 | NA |
| 15 | CNP0004277 | Coconut | (5-{[(3-{[5-(pyridin-2-yl)-1,2-oxazol-3-yl]methyl}oxetan-3-yl)amino]methyl}furan-2-yl)methanol | 115.539 | NA |
| 16 | CNP0004720 | Coconut | 2-{[(3-{[5-(4-methoxyphenyl)-1,2-oxazol-3-yl]methyl}oxetan-3-yl)amino]methyl}phenol | 115.352 | NA |
| 17 | CNP0003796 | Coconut | N-[(4-methoxyphenyl)methyl]-3-{[5-(pyridin-2-yl)-1,2-oxazol-3-yl]methyl}oxetan-3-amine | 113.167 | NA |
| 18 | CNP0004058 | Coconut | 2-chloro-5-hydroxy-N-{[4-hydroxy-5-(hydroxymethyl)-3-{4-[3-(trifluoromethyl)phenyl]piperazin-1-yl}oxolan-2-yl]methyl}benzamide | 112.619 | NA |
| 19 | CNP0003038 | Coconut | 2-amino-3-(1-{1-[3-(2-amino-2-carboxyethyl)-1H-indol-1-yl]ethyl}-1H-indol-3-yl)propanoic acid | 111.568 | 41.6684 |
| 20 | CNP0005022 | Coconut | 4-({3-[4-(pyridin-4-yl)-1H-imidazol-2-yl]morpholin-4-yl}methyl)benzoic acid | 111.381 | 22.286 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.P.; Singh, A.; Wolkenhauer, O.; Gupta, S.K. Regulatory Role of IL6 in Immune-Related Adverse Events during Checkpoint Inhibitor Treatment in Melanoma. Int. J. Mol. Sci. 2024, 25, 10600. https://doi.org/10.3390/ijms251910600
Singh KP, Singh A, Wolkenhauer O, Gupta SK. Regulatory Role of IL6 in Immune-Related Adverse Events during Checkpoint Inhibitor Treatment in Melanoma. International Journal of Molecular Sciences. 2024; 25(19):10600. https://doi.org/10.3390/ijms251910600
Chicago/Turabian StyleSingh, Krishna P., Anuj Singh, Olaf Wolkenhauer, and Shailendra Kumar Gupta. 2024. "Regulatory Role of IL6 in Immune-Related Adverse Events during Checkpoint Inhibitor Treatment in Melanoma" International Journal of Molecular Sciences 25, no. 19: 10600. https://doi.org/10.3390/ijms251910600






