Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Effects of IGSM on ANIT-Induced Liver Injury in Rats
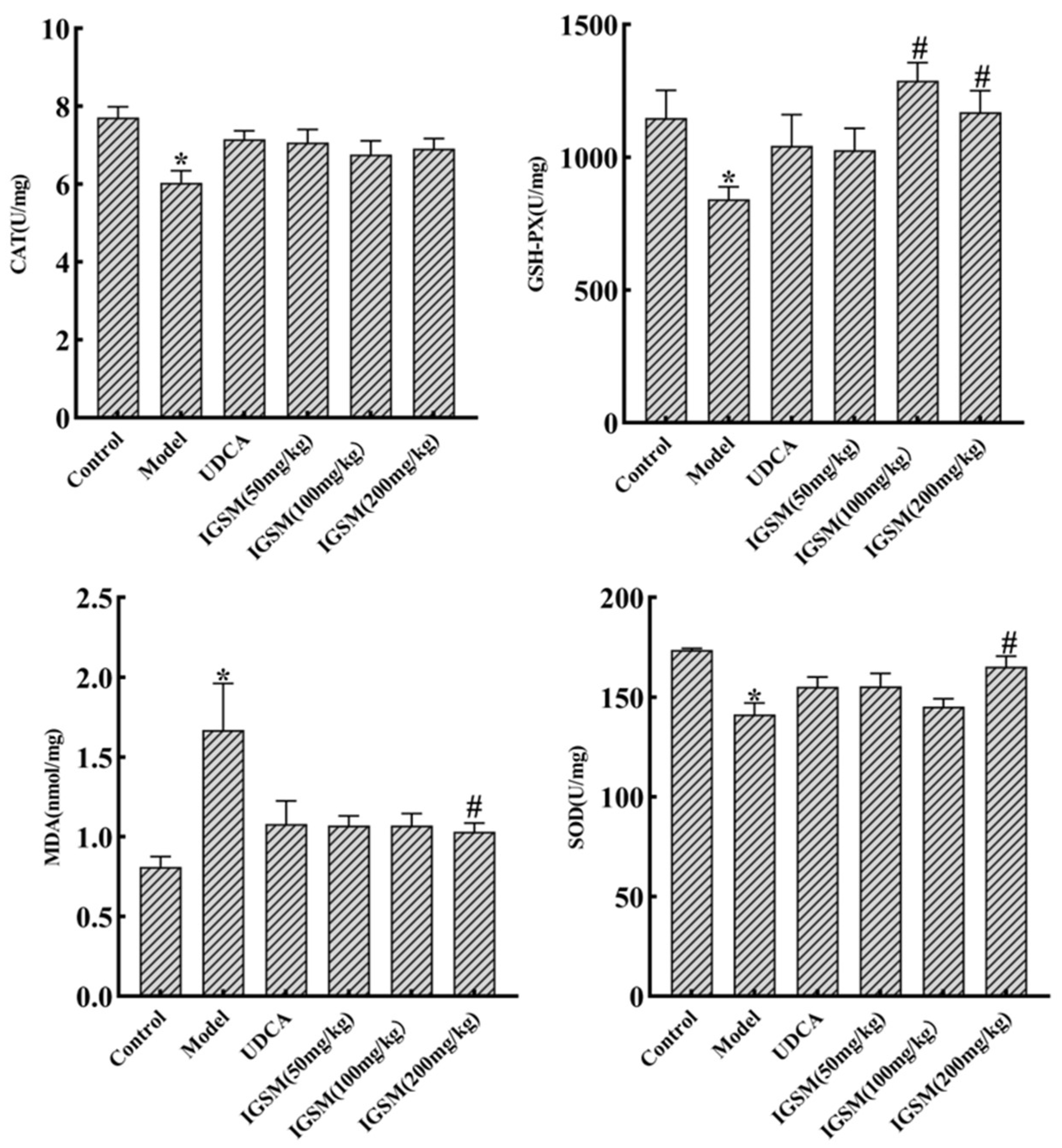
2.2. Effects of IGSM on ANIT-Induced Oxidative Stress in Rats
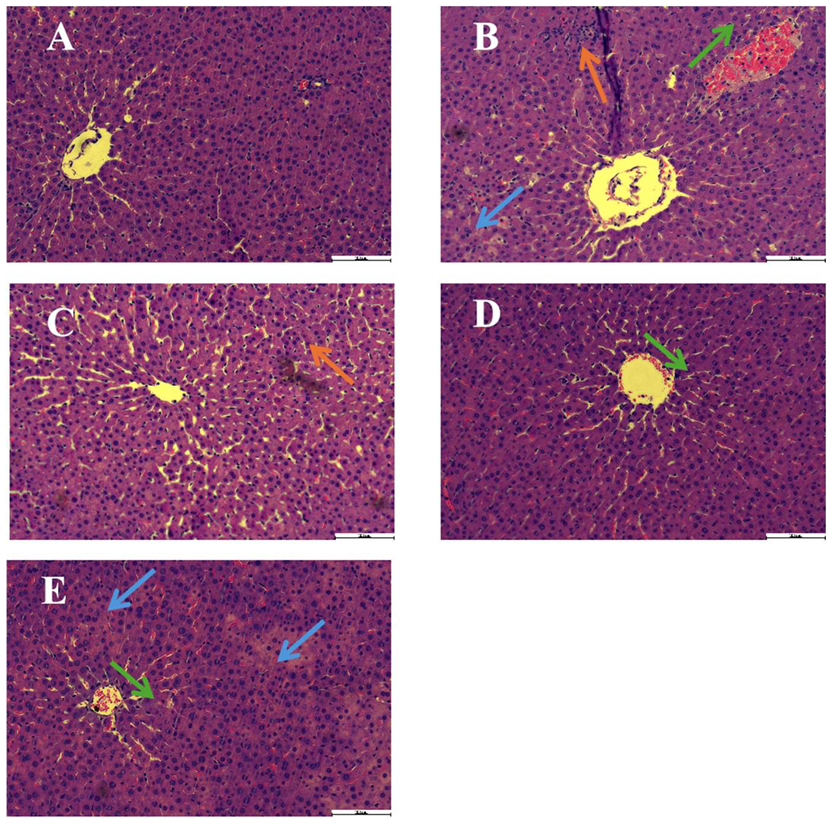
2.3. Effects of IGSM on the Morphology of Rat Livers with ANIT-Induced Liver Injury
2.4. Effects of IGSM on the mRNA Levels of FXR and Related Proteins
2.5. Quantitative Analysis of IGSM
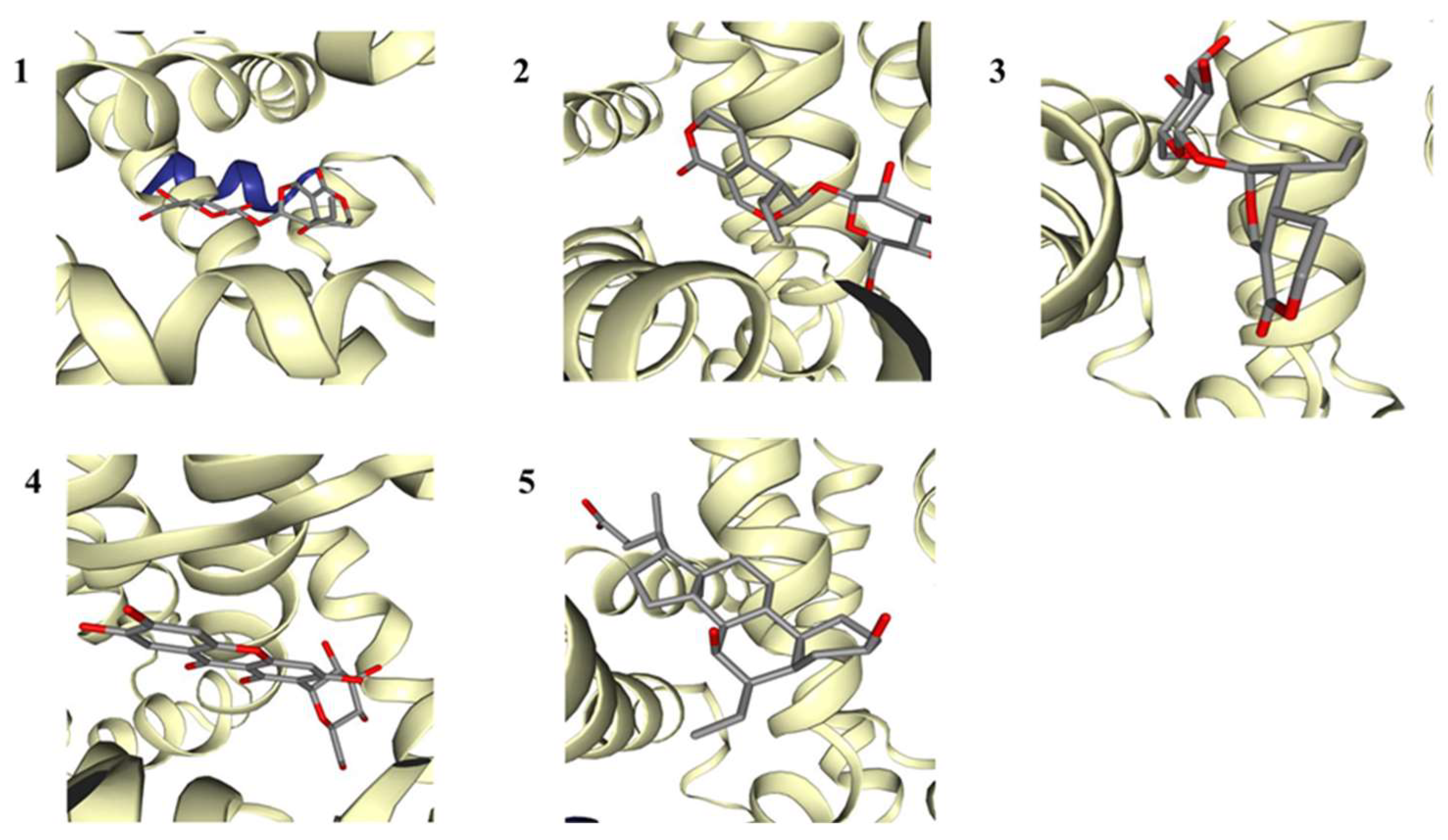
2.6. FXR-Activating Effects of the Main Compounds of IGSM
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Reagents
4.2. Preparation of IGSM
4.3. Animals and Treatments
4.4. Determination of the MDA Level, GSH-Px, CAT, and SOD Activity in Rat Liver Tissues
4.5. HE Staining of Rat Liver Tissues
4.6. Effects of IGSM on Fxr, Ntcp, Bsep, and Mrp2 mRNA Levels
4.7. UPLC Analysis of IGSM
4.8. Molecular Docking Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hegade, V.S.; Jones, D.E.J. Complications of cholestasis. Medicine 2019, 47, 818–821. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Li, F.; Gonzalez, F.J. FXR signaling in the enterohepatic system. Mol. Cell Endocrinol. 2013, 368, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Kimmel, R.; Weinberger, C.; Stroup, D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem. 2000, 275, 10918–10924. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Li, K.W. Role of farnesoid X receptor in cholestasis. J. Dig. Dis. 2016, 17, 501–509. [Google Scholar] [CrossRef]
- Hasegawa, S.; Yoneda, M.; Kurita, Y.; Nogami, A.; Honda, Y.; Hosono, K.; Nakajima, A. Cholestatic Liver Disease: Current Treatment Strategies and New Therapeutic Agents. Drugs 2021, 81, 1181–1192. [Google Scholar] [CrossRef]
- Floreani, A.; Gabbia, D.; De Martin, S. Obeticholic Acid for Primary Biliary Cholangitis. Biomedicines 2022, 10, 2464. [Google Scholar] [CrossRef]
- Meng, X.H.; Chen, D.D.; Zhang, Y.S.; Chen, G.P. Research progress on chemical constituents, pharmacological actions, and clinical applications of Swertia mussotii. Drugs Clin. 2012, 27, 176–179. [Google Scholar]
- Yun, H.; Wu, X.; Ding, Y.; Xiong, W.; Duan, X.; Gao, H.; Yang, Y.; Chen, Z. Exploring the Mechanism of Swertia mussotii Franch. for Hepatoprotective Effects with iTRAQ LC-MS/MS. Comb. Chem. High. Throughput Screen. 2021, 24, 1332–1339. [Google Scholar] [CrossRef]
- Lv, P.; Wei, L.; Du, Y.; Yang, H.; Peng, M. Hepatoprotective and toxic characteristics of the whole herb of traditional Tibetan folk medicine Swertia mussotii Franch. J. Med. Plants Res. 2010, 4, 706–709. [Google Scholar]
- Gao, Y.; Chai, J.; Li, S.; Liu, C.; Cheng, Y.; Liu, X.; Lian, W.; Chen, W. Protective effect of alcohol extract of Swertia mussotii Franch. on lipopolysaccharide-induced cholestatic liver damage in rats. J. Third Mil. Med. Univ. 2014, 36, 769–773. [Google Scholar]
- Li, S.; Li, B.; Guo, Y.; Zhang, S.; Jiang, W.; Cao, L.; Du, X.; Mu, Z.; Yang, Y.; Zhong, G.; et al. Comparison of Pharmacological Actions of Crude Extracts from Six Origin Species of Tibetan Medicine Dida on Mice with Acute Intrahepatic Cholestasis. Tradit. Chin. Drug Res. Pharmacol. 2017, 28, 314–319. [Google Scholar]
- Shi, M.; Tang, J.; Zhang, T.; Han, H. Swertiamarin, an active iridoid glycoside from Swertia pseudochinensis H. Hara, protects against alpha-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and bile acid excretion pathway. J. Ethnopharmacol. 2022, 291, 115164. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Mehta, L.; Malhotra, A.; Kapoor, G.; Nagarajan, K.; Kumar, P.; Chawla, V.; Chawla, P.A. Exploring the Multitarget Potential of Iridoids: Advances and Applications. Curr. Top. Med. Chem. 2023, 23, 371–388. [Google Scholar]
- Bridi, R.; von Poser, G.L.; de Carvalho Meirelles, G. Iridoids as a Potential Hepatoprotective Class: A Review. Mini Rev. Med. Chem. 2023, 23, 452–479. [Google Scholar]
- Wei, X.; Ma, Y.; Dong, Z.; Wang, G.; Lan, X.; Liao, Z.; Chen, M. Dehydrodiconiferyl alcohol, a lignan from Herpetospermum pedunculosum, alleviates cholestasis by activating pathways associated with the farnesoid X receptor. Phytomedicine 2021, 80, 153378. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, F.; Gao, Q.; Xing, R.; Chen, S. A Review on the Ethnomedicinal Usage, Phytochemistry, and Pharmacological Properties of Gentianeae (Gentianaceae) in Tibetan Medicine. Plants 2021, 10, 2383. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, G.; Hao, H.; Wang, H. FXR agonists for MASH therapy: Lessons and perspectives from obeticholic acid. Med. Res. Rev. 2024, 44, 568–586. [Google Scholar] [CrossRef]
- Kossor, D.C.; Goldstein, R.S.; Ngo, W.; DeNicola, D.B.; Leonard, T.B.; Dulik, D.M.; Meunier, P.C. Biliary epithelial cell proliferation following alpha-naphthylisothiocyanate (ANIT) treatment: Relationship to bile duct obstruction. Fundam. Appl. Toxicol. 1995, 26, 51–62. [Google Scholar] [CrossRef]
- Cui, Y.J.; Aleksunes, L.M.; Tanaka, Y.; Goedken, M.J.; Klaassen, C.D. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol. Sci. 2009, 110, 47–60. [Google Scholar] [CrossRef]
- Kodali, P.; Wu, P.; Lahiji, P.A.; Brown, E.J.; Maher, J.J. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G355–G363. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Jaeschke, H.; Klaassen, C.D. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010, 30, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Guihua, L.; Jianping, Z.; Haijuan, C.; Zhi, C. Protective Effect of Ethyl of Swertia musstii Franch on Mice with Acute Hepatic Injury. J. Sichuan Tradit. Chin. Med. 2008, 26, 29–30. [Google Scholar]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Li, G.; Xue, J.; Chen, Z.L.; Jin, F.; Luo, L.; Zhou, X.; Ma, Q.; Cai, X.; et al. Effects of corilagin on alleviating cholestasis via farnesoid X receptor-associated pathways in vitro and in vivo. Br. J. Pharmacol. 2018, 175, 810–829. [Google Scholar] [CrossRef]
- Ren, T.; Pang, L.; Dai, W.; Wu, S.; Kong, J. Regulatory mechanisms of the bile salt export pump (BSEP/ABCB11) and its role in related diseases. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101641. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, W.A.; Gill, R.K. Bile acid transporters: Structure, function, regulation and pathophysiological implications. Pharm. Res. 2007, 24, 1803–1823. [Google Scholar] [CrossRef]
- Zollner, G.; Wagner, M.; Fickert, P.; Geier, A.; Fuchsbichler, A.; Silbert, D.; Gumhold, J.; Zatloukal, K.; Kaser, A.; Tilg, H.; et al. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G798–G805. [Google Scholar] [CrossRef]
- Eller, C.; Heydmann, L.; Colpitts, C.C.; Verrier, E.R.; Schuster, C.; Baumert, T.F. The functional role of sodium taurocholate cotransporting polypeptide NTCP in the life cycle of hepatitis B, C and D viruses. Cell Mol. Life Sci. 2018, 75, 3895–3905. [Google Scholar] [CrossRef]
- Yun, H.; Chen, Z. Research progress of Swertia mussotii. West China J. Pharm. Sci. 2020, 35, 567–571. [Google Scholar]
- Tian, C.; Zhang, T.; Wang, L.; Shan, Q.; Jiang, L. The hepatoprotective effect and chemical constituents of total iridoids and xanthones extracted from Swertia mussotii Franch. J. Ethnopharmacol. 2014, 154, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, Q.; Yang, F.; Gong, J.; Han, H.; Yang, L.; Wang, Z. Target profiling analyses of bile acids in the evaluation of hepatoprotective effect of gentiopicroside on ANIT-induced cholestatic liver injury in mice. J. Ethnopharmacol. 2016, 194, 63–71. [Google Scholar] [CrossRef]
- Han, H.; Xu, L.; Xiong, K.; Zhang, T.; Wang, Z. Exploration of Hepatoprotective Effect of Gentiopicroside on Alpha-Naphthylisothiocyanate-Induced Cholestatic Liver Injury in Rats by Comprehensive Proteomic and Metabolomic Signatures. Cell Physiol. Biochem. 2018, 49, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.L.; Yang, F.; Gong, J.T.; Tang, X.W.; Wang, G.Y.; Wang, Z.T.; Yang, L. Sweroside ameliorates α-naphthylisothiocyanate-induced cholestatic liver injury in mice by regulating bile acids and suppressing pro-inflammatory responses. Acta Pharmacol. Sin. 2016, 37, 1218–1228. [Google Scholar] [CrossRef]
- Gong, J.; Yang, F.; Yang, Q.; Tang, X.; Shu, F.; Xu, L.; Wang, Z.; Yang, L. Sweroside ameliorated carbon tetrachloride (CCl4)-induced liver fibrosis through FXR-miR-29a signaling pathway. J. Nat. Med. 2020, 74, 17–25. [Google Scholar] [CrossRef]


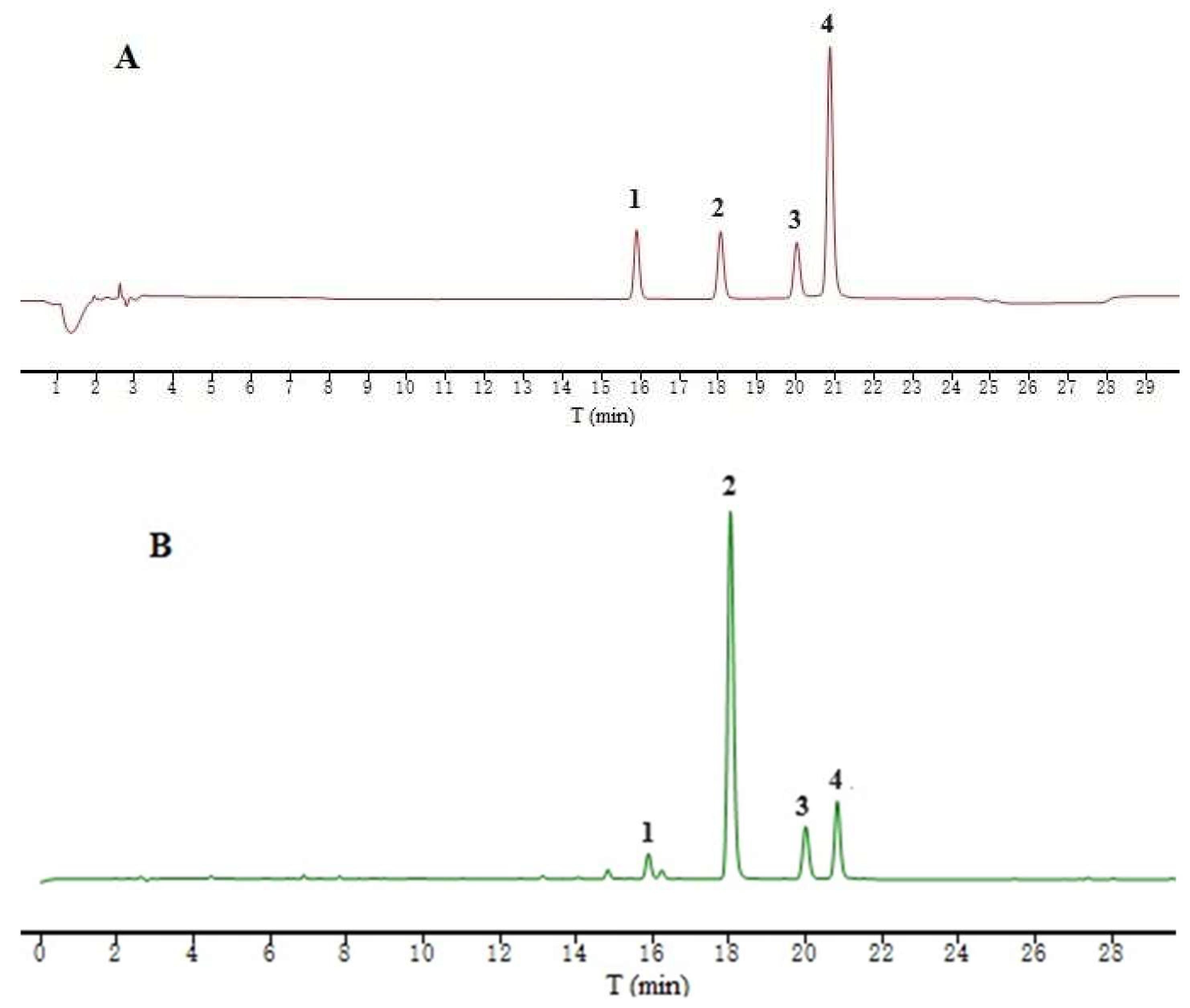
| NO. | Rt (min) | Compounds | Content in the Sample (%) | Regression Equation | Correlation Coefficient | Linear Range (μg) |
|---|---|---|---|---|---|---|
| 1 | 15.899 | Swertiamarin | 2.92 | y = 2297.3x − 0.1483 | 1 | 0.25–0.025 |
| 2 | 18.059 | gentiopicroside | 41.39 | y = 2628.4x + 9.931 | 1 | 1.2–0.24 |
| 3 | 20.019 | sweroside | 6.33 | y = 2667.0x + 0.7405 | 0.9999 | 0.22–0.022 |
| 4 | 20.872 | mangiferin | 2.97 | y = 11,278x − 14.361 | 0.9999 | 0.22–0.022 |
| Protein Name | PDB ID | Compound Name | PubChem CID | Affinity (kcal/mol) |
|---|---|---|---|---|
| FXR | 3DCT | 1: Swertiamarin | 442,435 | −7.8 |
| 2: Gentiopicroside | 88,708 | −7.7 | ||
| 3: Sweroside | 161,036 | −7.7 | ||
| 4: Mangiferin | 5,281,647 | −9.0 | ||
| 5: Obeticholic acid | 447,715 | −8.9 |
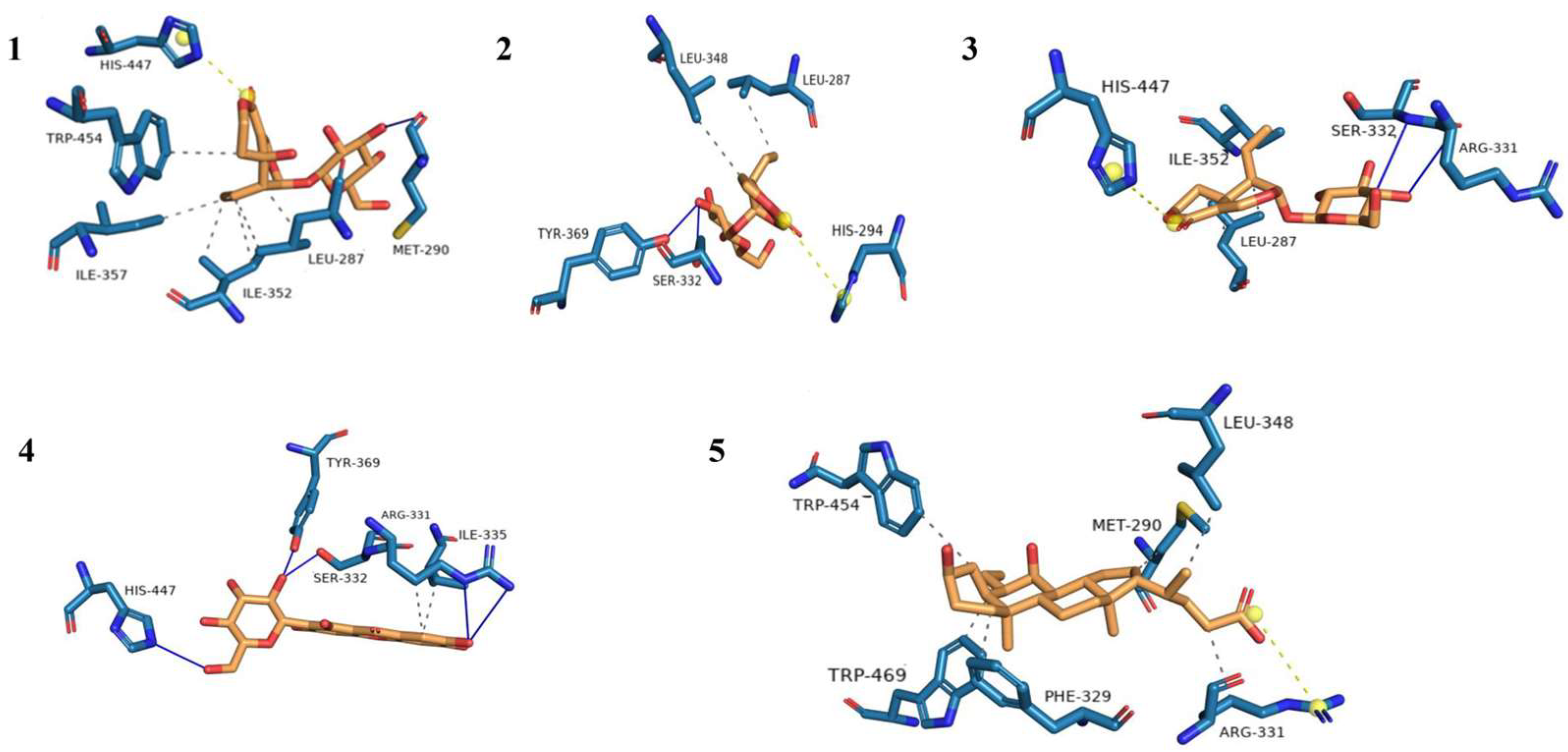
| NO. | Hydrophobic Interactions | Hydrogen Bonds | Other Forces |
|---|---|---|---|
| 1 | LEU-287, ILE-352, ILE-357, and TRP-454 | MET-290 | HIS-447 |
| 2 | LEU-287 and LEU-348 | SER-332 and TYR-369 | HIS-294 |
| 3 | LEU-287 and ILE-352 | ARG-331 and SER-332 | HIS-447 |
| 4 | ARG-331 and ILE-335 | ARG-331, SER-332, TYR-369, and HIS-447 | - |
| 5 | MET-290, PHE-329, ARG-331, LEU-348, TRP-454, and TRP-469 | ARG-331 | - |
| Gene | Sequence (5′->3′) | |
|---|---|---|
| Ntcp | Sense | GGACATGAACCTCAGCATCGT |
| Antisense | TTGTAGGGCACCTTGTCCTT | |
| Mrp2 | Sense | GTAGAGAATGAGGCGCCCTG |
| Antisense | GGCCGATACCGCACTTGATA | |
| Bsep | Sense | TCCTCTGAGCCAAAAGCTGA |
| Antisense | CTGCACTCAACAACCCTTTGC | |
| Fxr | Sense | CGAGGGCTGCAAAGGTTTCT |
| Antisense | TCCCATCTCTCTGCACTTCCT | |
| GAPDH | Sense | CCGCATCTTCTTGTGCAGTG |
| Antisense | CCGATACGGCCAAATCCGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Wang, Z.; Hu, N.; Tie, F.; Liu, Z.; Sun, Y.; Wang, Y.; Tan, N.; Wang, H. Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 10607. https://doi.org/10.3390/ijms251910607
Dong Q, Wang Z, Hu N, Tie F, Liu Z, Sun Y, Wang Y, Tan N, Wang H. Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress. International Journal of Molecular Sciences. 2024; 25(19):10607. https://doi.org/10.3390/ijms251910607
Chicago/Turabian StyleDong, Qi, Zhenhua Wang, Na Hu, Fangfang Tie, Zenggen Liu, Ying Sun, Yue Wang, Nixia Tan, and Honglun Wang. 2024. "Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress" International Journal of Molecular Sciences 25, no. 19: 10607. https://doi.org/10.3390/ijms251910607






