Unravelling the Role of Gut and Oral Microbiota in the Pediatric Population with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Type 1 Diabetes Mellitus
3. Gut and Oral Microbiota
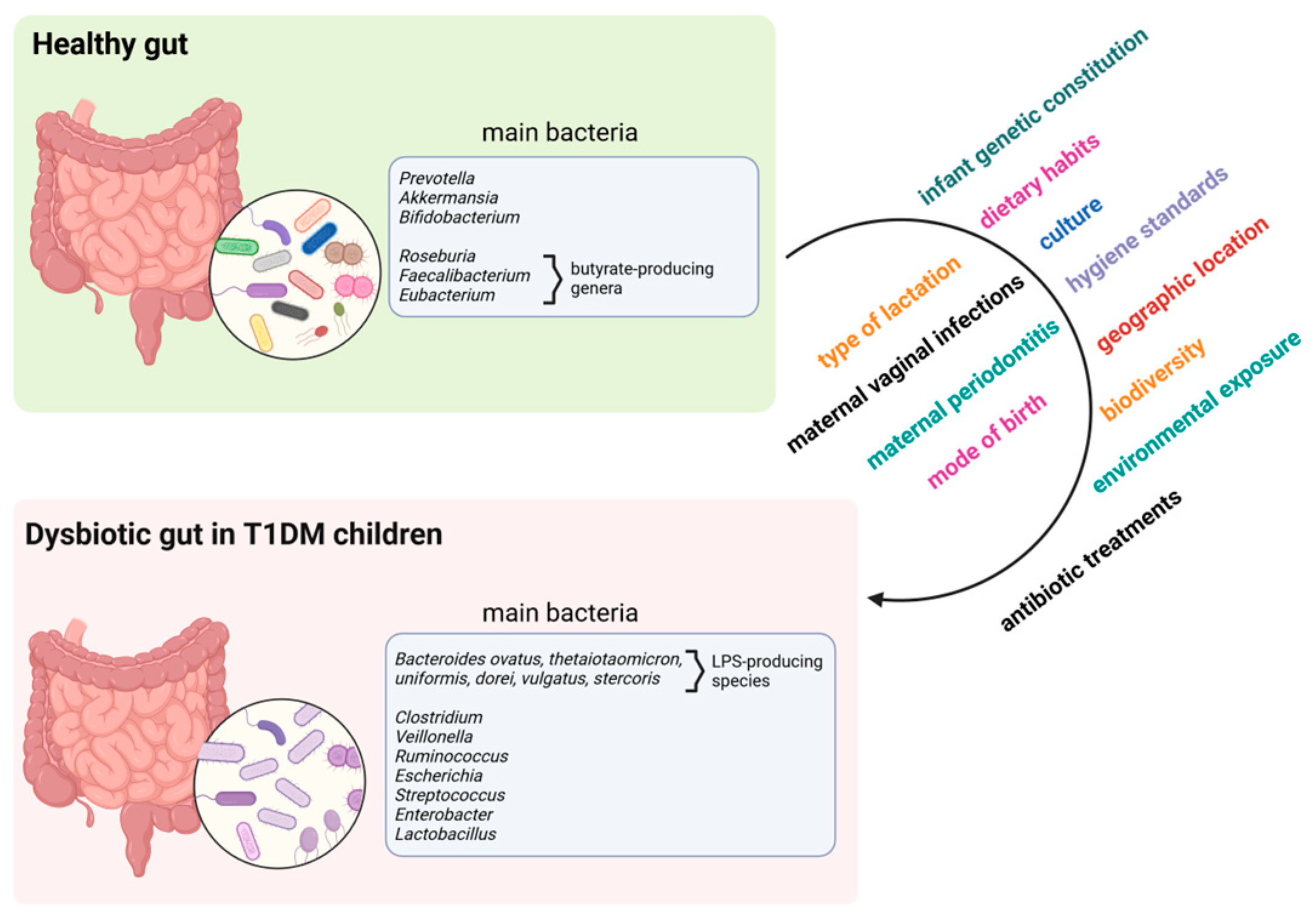
4. Gut Microbiome in T1DM
5. SCFAs in T1DM
6. Oral Microbiome in T1DM
7. The Interplay between Gut and Oral Microbiota in T1DM
8. Possible Therapeutic Approaches
8.1. Prebiotics, Probiotics, and Synbiotics
8.2. Faecal Microbiome Transplantation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Quattrin, T.; Mastrandrea, L.D.; Walker, L.S.K. Type 1 Diabetes. Lancet 2023, 401, 2149–2162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, Y.; Gong, T. The Interplay between Oral Microbiota, Gut Microbiota and Systematic Diseases. J. Oral. Microbiol. 2023, 15, 2213112. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Kim, C.; Banerjee, T.; Lee, J.M. Fluctuations in the Incidence of Type 1 Diabetes in the United States from 2001 to 2015: A Longitudinal Study. BMC Med. 2017, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Harding, J.L.; Wander, P.L.; Zhang, X.; et al. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and Regional Estimates and Projections of Diabetes-Related Health Expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Type I Diabetes Mellitus. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Hummel, M.; Schenker, M.; Bonifacio, E. Autoantibody Appearance and Risk for Development of Childhood Diabetes in Offspring of Parents with Type 1 Diabetes: The 2-Year Analysis of the German BABYDIAB Study. Diabetes 1999, 48, 460–468. [Google Scholar] [CrossRef]
- Regnell, S.E.; Lernmark, Å. Early Prediction of Autoimmune (Type 1) Diabetes. Diabetologia 2017, 60, 1370–1381. [Google Scholar] [CrossRef]
- Kawasaki, E. Anti-Islet Autoantibodies in Type 1 Diabetes. Int. J. Mol. Sci. 2023, 24, 10012. [Google Scholar] [CrossRef] [PubMed]
- Minniakhmetov, I.; Yalaev, B.; Khusainova, R.; Bondarenko, E.; Melnichenko, G.; Dedov, I.; Mokrysheva, N. Genetic and Epigenetic Aspects of Type 1 Diabetes Mellitus: Modern View on the Problem. Biomedicines 2024, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Li, Z.; Zhou, Z. Gut Microbiome in Type 1 Diabetes: A Comprehensive Review. Diabetes Metab. Res. Rev. 2018, 34, e3043. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Ludvigsson, J. Environmental Risk Factors for Type 1 Diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The Gut Ecosystem and Immune Tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Engevik, A.C.; Engevik, M.A. Exploring the Impact of Intestinal Ion Transport on the Gut Microbiota. Comput. Struct. Biotechnol. J 2021, 19, 134–144. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting Family Members Share Microbiota with One Another and with Their Dogs. Elife 2013, 2, e00458. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Yin, H.; Yu, J.; Wu, W.; Li, X.; Hu, R. Analysis of the Microbiome in Maternal, Intrauterine and Fetal Environments Based on 16S rRNA Genes Following Different Durations of Membrane Rupture. Sci. Rep. 2023, 13, 15010. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Martínez, C.; Santaella-Pascual, M.; Yagüe-Guirao, G.; Martínez-Graciá, C. Infant Gut Microbiota Colonization: Influence of Prenatal and Postnatal Factors, Focusing on Diet. Front. Microbiol. 2023, 14, 1236254. [Google Scholar] [CrossRef]
- Araos, R.; D’Agata, E.M.C. The Human Microbiota and Infection Prevention. Infect. Control. Hosp. Epidemiol. 2019, 40, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the Healthy “Core Microbiome” of Oral Microbial Communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L. Genetics of Taste Lab Citizen Scientists Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Tomás, I.; Cabrera-Rubio, R.; Catalan, M.D.; Nyvad, B.; Mira, A. Microbial Geography of the Oral Cavity. J. Dent. Res. 2013, 92, 616–621. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Wu, Y.; Chi, X.; Zhang, Q.; Chen, F.; Deng, X. Characterization of the Salivary Microbiome in People with Obesity. PeerJ 2018, 6, e4458. [Google Scholar] [CrossRef]
- Ogawa, T.; Hirose, Y.; Honda-Ogawa, M.; Sugimoto, M.; Sasaki, S.; Kibi, M.; Kawabata, S.; Ikebe, K.; Maeda, Y. Composition of Salivary Microbiota in Elderly Subjects. Sci. Rep. 2018, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Zanotta, N.; Campisciano, G.; Zerbato, V.; Di Bella, S.; Cason, C.; Luzzati, R.; Confalonieri, M.; Palamara, A.T.; Comar, M. Profiling of Oral Microbiota and Cytokines in COVID-19 Patients. Front. Microbiol. 2021, 12, 671813. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A Review of Saliva: Normal Composition, Flow, and Function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Sakata, S.; Ma, J.; Asakawa, M.; Takeshita, T.; Furuta, M.; Ninomiya, T.; Yamashita, Y. High-Resolution Detection of Translocation of Oral Bacteria to the Gut. J. Dent. Res. 2023, 102, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive Transmission of Microbes along the Gastrointestinal Tract. Elife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Sorini, C.; Cosorich, I.; Lo Conte, M.; De Giorgi, L.; Facciotti, F.; Lucianò, R.; Rocchi, M.; Ferrarese, R.; Sanvito, F.; Canducci, F.; et al. Loss of Gut Barrier Integrity Triggers Activation of Islet-Reactive T Cells and Autoimmune Diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 15140–15149. [Google Scholar] [CrossRef]
- Antonini, M.; Lo Conte, M.; Sorini, C.; Falcone, M. How the Interplay Between the Commensal Microbiota, Gut Barrier Integrity, and Mucosal Immunity Regulates Brain Autoimmunity. Front. Immunol. 2019, 10, 1937. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin Upregulation Is Associated with Increased Gut Permeability in Subjects with Type 1 Diabetes and Their Relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef]
- Carratù, R.; Secondulfo, M.; de Magistris, L.; Iafusco, D.; Urio, A.; Carbone, M.G.; Pontoni, G.; Cartenì, M.; Prisco, F. Altered Intestinal Permeability to Mannitol in Diabetes Mellitus Type I. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 264–269. [Google Scholar] [CrossRef]
- Maffeis, C.; Martina, A.; Corradi, M.; Quarella, S.; Nori, N.; Torriani, S.; Plebani, M.; Contreas, G.; Felis, G.E. Association between Intestinal Permeability and Faecal Microbiota Composition in Italian Children with Beta Cell Autoimmunity at Risk for Type 1 Diabetes. Diabetes Metab. Res. Rev. 2016, 32, 700–709. [Google Scholar] [CrossRef]
- Gavin, P.G.; Mullaney, J.A.; Loo, D.; Cao, K.-A.L.; Gottlieb, P.A.; Hill, M.M.; Zipris, D.; Hamilton-Williams, E.E. Intestinal Metaproteomics Reveals Host-Microbiota Interactions in Subjects at Risk for Type 1 Diabetes. Diabetes Care 2018, 41, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Dore, M.P.; Pes, G.M.; Delitala, G.; Delitala, A.P. Is There a Role for Gut Microbiota in Type 1 Diabetes Pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 14650. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in Endocrinology: Gut Microbiota in Patients with Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia Is Associated with an Increased Risk of Incident Diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef]
- Bedi, S.; Richardson, T.M.; Jia, B.; Saab, H.; Brinkman, F.S.L.; Westley, M. Similarities between Bacterial GAD and Human GAD65: Implications in Gut Mediated Autoimmune Type 1 Diabetes. PLoS ONE 2022, 17, e0261103. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Park, S.H.; Jialal, I. Increased Levels of Ligands of Toll-like Receptors 2 and 4 in Type 1 Diabetes. Diabetologia 2009, 52, 1665–1668. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, R.; Han, B.; Sun, C.; Chen, R.; Wei, H.; Chen, L.; Du, H.; Li, G.; Yang, Y.; et al. Functional and Metabolic Alterations of Gut Microbiota in Children with New-Onset Type 1 Diabetes. Nat. Commun. 2022, 13, 6356. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Hildebrand, D.; Sahr, A.; Wölfle, S.J.; Heeg, K.; Kubatzky, K.F. Regulation of Toll-like Receptor 4-Mediated Immune Responses through Pasteurella Multocida Toxin-Induced G Protein Signalling. Cell Commun. Signal. 2012, 10, 22. [Google Scholar] [CrossRef]
- Traversi, D.; Rabbone, I.; Ignaccolo, M.G.; Carletto, G.; Racca, I.; Vallini, C.; Andriolo, V.; Cadario, F.; Savastio, S.; Siliquini, R.; et al. Gut Microbiota Diversity and T1DM Onset: Preliminary Data of a Case-Control Study. Hum. Microbiome J. 2017, 5–6, 11–13. [Google Scholar] [CrossRef]
- Mejía-León, M.E.; Petrosino, J.F.; Ajami, N.J.; Domínguez-Bello, M.G.; de la Barca, A.M.C. Fecal Microbiota Imbalance in Mexican Children with Type 1 Diabetes. Sci. Rep. 2014, 4, 3814. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward Defining the Autoimmune Microbiome for Type 1 Diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- de Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal Microbiota Composition Differs between Children with β-Cell Autoimmunity and Those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The Dynamics of the Human Infant Gut Microbiome in Development and in Progression toward Type 1 Diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Endesfelder, D.; zu Castell, W.; Ardissone, A.; Davis-Richardson, A.G.; Achenbach, P.; Hagen, M.; Pflueger, M.; Gano, K.A.; Fagen, J.R.; Drew, J.C.; et al. Compromised Gut Microbiota Networks in Children with Anti-Islet Cell Autoimmunity. Diabetes 2014, 63, 2006–2014. [Google Scholar] [CrossRef]
- Russell, J.T.; Roesch, L.F.W.; Ördberg, M.; Ilonen, J.; Atkinson, M.A.; Schatz, D.A.; Triplett, E.W.; Ludvigsson, J. Genetic Risk for Autoimmunity Is Associated with Distinct Changes in the Human Gut Microbiome. Nat. Commun. 2019, 10, 3621. [Google Scholar] [CrossRef]
- Cinek, O.; Kramna, L.; Mazankova, K.; Odeh, R.; Alassaf, A.; Ibekwe, M.U.; Ahmadov, G.; Elmahi, B.M.E.; Mekki, H.; Lebl, J.; et al. The Bacteriome at the Onset of Type 1 Diabetes: A Study from Four Geographically Distant African and Asian Countries. Diabetes Res. Clin. Pract. 2018, 144, 51–62. [Google Scholar] [CrossRef]
- Kemppainen, K.M.; Ardissone, A.N.; Davis-Richardson, A.G.; Fagen, J.R.; Gano, K.A.; León-Novelo, L.G.; Vehik, K.; Casella, G.; Simell, O.; Ziegler, A.G.; et al. Early Childhood Gut Microbiomes Show Strong Geographic Differences among Subjects at High Risk for Type 1 Diabetes. Diabetes Care 2015, 38, 329–332. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Alkanani, A.K.; Hara, N.; Gottlieb, P.A.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Alterations in Intestinal Microbiota Correlate with Susceptibility to Type 1 Diabetes. Diabetes 2015, 64, 3510–3520. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- d’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems 2017, 2, e00046-17. [Google Scholar] [CrossRef] [PubMed]
- Fedulovs, A.; Pahirko, L.; Jekabsons, K.; Kunrade, L.; Valeinis, J.; Riekstina, U.; Pīrāgs, V.; Sokolovska, J. Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes. Biomedicines 2023, 11, 3269. [Google Scholar] [CrossRef]
- Lo Conte, M.; Cosorich, I.; Ferrarese, R.; Antonini Cencicchio, M.; Nobili, A.; Palmieri, V.; Massimino, L.; Lamparelli, L.A.; Liang, W.; Riba, M.; et al. Alterations of the Intestinal Mucus Layer Correlate with Dysbiosis and Immune Dysregulation in Human Type 1 Diabetes. eBioMedicine 2023, 91, 104567. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut Microbiota in Children with Type 1 Diabetes Differs from That in Healthy Children: A Case-Control Study. BMC Med 2013, 11, 46. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Qi, C.-J.; Zhang, Q.; Yu, M.; Xu, J.-P.; Zheng, J.; Wang, T.; Xiao, X.-H. Imbalance of Fecal Microbiota at Newly Diagnosed Type 1 Diabetes in Chinese Children. Chin. Med. J. 2016, 129, 1298–1304. [Google Scholar] [CrossRef]
- Davis-Richardson, A.G.; Ardissone, A.N.; Dias, R.; Simell, V.; Leonard, M.T.; Kemppainen, K.M.; Drew, J.C.; Schatz, D.; Atkinson, M.A.; Kolaczkowski, B.; et al. Bacteroides dorei Dominates Gut Microbiome Prior to Autoimmunity in Finnish Children at High Risk for Type 1 Diabetes. Front. Microbiol. 2014, 5, 678. [Google Scholar] [CrossRef]
- Matos, J.; Matos, I.; Calha, M.; Santos, P.; Duarte, I.; Cardoso, Y.; Faleiro, M.L. Insights from Bacteroides Species in Children with Type 1 Diabetes. Microorganisms 2021, 9, 1436. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; May, P.; Laczny, C.C.; Lebrun, L.A.; Bellora, C.; Krishna, A.; Wampach, L.; Schneider, J.G.; Hogan, A.; de Beaufort, C.; et al. Integrated Multi-Omics of the Human Gut Microbiome in a Case Study of Familial Type 1 Diabetes. Nat. Microbiol. 2016, 2, 16180. [Google Scholar] [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The Human Gut Microbiome in Early-Onset Type 1 Diabetes from the TEDDY Study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Li, H.-L.; Ma, C.-Y.; Shi, T.-Y.; Wang, T.-Y.; Yan, D.; Tang, H.; Lin, H.; Deng, K.-J. Predicting the Role of the Human Gut Microbiome in Type 1 Diabetes Using Machine-Learning Methods. Brief Funct. Genom. 2024, 23, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, J.; Li, X.; Huang, J.; Zhou, Z. Emerging Trends and Focus on the Link between Gut Microbiota and Type 1 Diabetes: A Bibliometric and Visualization Analysis. Front. Microbiol. 2023, 14, 1137595. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Edreira, D.; Liñares-Blanco, J.; Fernandez-Lozano, C. Machine Learning Analysis of the Human Infant Gut Microbiome Identifies Influential Species in Type 1 Diabetes. Expert Syst. Appl. 2021, 185, 115648. [Google Scholar] [CrossRef]
- Biassoni, R.; Di Marco, E.; Squillario, M.; Barla, A.; Piccolo, G.; Ugolotti, E.; Gatti, C.; Minuto, N.; Patti, G.; Maghnie, M.; et al. Gut Microbiota in T1DM-Onset Pediatric Patients: Machine-Learning Algorithms to Classify Microorganisms as Disease Linked. J. Clin. Endocrinol. Metab. 2020, 105, dgaa407. [Google Scholar] [CrossRef]
- Tan, H.; Shi, Y.; Yue, T.; Zheng, D.; Luo, S.; Weng, J.; Zheng, X. Machine Learning Approach Reveals Microbiome, Metabolome, and Lipidome Profiles in Type 1 Diabetes. J. Adv. Res. 2023, 64, 213–221. [Google Scholar] [CrossRef]
- Mokhtari, P.; Jambal, P.; Metos, J.M.; Shankar, K.; Anandh Babu, P.V. Microbial Taxonomic and Functional Shifts in Adolescents with Type 1 Diabetes Are Associated with Clinical and Dietary Factors. eBioMedicine 2023, 93, 104641. [Google Scholar] [CrossRef]
- Endesfelder, D.; Engel, M.; Davis-Richardson, A.G.; Ardissone, A.N.; Achenbach, P.; Hummel, S.; Winkler, C.; Atkinson, M.; Schatz, D.; Triplett, E.; et al. Towards a Functional Hypothesis Relating Anti-Islet Cell Autoimmunity to the Dietary Impact on Microbial Communities and Butyrate Production. Microbiome 2016, 4, 17. [Google Scholar] [CrossRef]
- Dedrick, S.; Sundaresh, B.; Huang, Q.; Brady, C.; Yoo, T.; Cronin, C.; Rudnicki, C.; Flood, M.; Momeni, B.; Ludvigsson, J.; et al. The Role of Gut Microbiota and Environmental Factors in Type 1 Diabetes Pathogenesis. Front. Endocrinol. 2020, 11, 78. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.A.; Mannerla, M.M.; Frimodt-Møller, M.; Persson, F.; Hansen, T.W.; Lehto, M.; Hörkkö, S.; Blaut, M.; Forsblom, C.; Groop, P.-H.; et al. Faecal Biomarkers in Type 1 Diabetes with and without Diabetic Nephropathy. Sci. Rep. 2021, 11, 15208. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut. Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Visekruna, A. Short-Chain Fatty Acids: Bacterial Messengers Modulating the Immunometabolism of T Cells. Eur. J. Immunol. 2019, 49, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut Microbiota-Derived Butyrate Regulates Gut Mucus Barrier Repair by Activating the Macrophage/WNT/ERK Signaling Pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Badami, E.; Sorini, C.; Coccia, M.; Usuelli, V.; Molteni, L.; Bolla, A.M.; Scavini, M.; Mariani, A.; King, C.; Bosi, E.; et al. Defective Differentiation of Regulatory FoxP3+ T Cells by Small-Intestinal Dendritic Cells in Patients with Type 1 Diabetes. Diabetes 2011, 60, 2120–2124. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Li, X.; Li, J.; Zheng, T.; Xie, L.; Li, C.; Tang, Y.; Guo, K.; Huang, J.; et al. Distinct Signatures of Gut Microbiota and Metabolites in Different Types of Diabetes: A Population-Based Cross-Sectional Study. EClinicalMedicine 2023, 62, 102132. [Google Scholar] [CrossRef]
- Bell, K.J.; Saad, S.; Tillett, B.J.; McGuire, H.M.; Bordbar, S.; Yap, Y.A.; Nguyen, L.T.; Wilkins, M.R.; Corley, S.; Brodie, S.; et al. Metabolite-Based Dietary Supplementation in Human Type 1 Diabetes Is Associated with Microbiota and Immune Modulation. Microbiome 2022, 10, 9. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A Proposed Model Linking Inflammation to Obesity, Diabetes, and Periodontal Infections. J. Periodontol. 2005, 76, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Størling, J.; Pociot, F. Type 1 Diabetes Candidate Genes Linked to Pancreatic Islet Cell Inflammation and Beta-Cell Apoptosis. Genes 2017, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Al-Khabbaz, A.K.; Al-Shammari, K.F.; Hasan, A.; Abdul-Rasoul, M. Periodontal Health of Children with Type 1 Diabetes Mellitus in Kuwait: A Case-Control Study. Med. Princ. Pract. 2013, 22, 144–149. [Google Scholar] [CrossRef]
- Carneiro, V.L.; Fraiz, F.C.; Ferreira, F.D.M.; Pintarelli, T.P.; Oliveira, A.C.B.; Boguszewski, M.C. da S. The Influence of Glycemic Control on the Oral Health of Children and Adolescents with Diabetes Mellitus Type 1. Arch. Endocrinol. Metab. 2015, 59, 535–540. [Google Scholar] [CrossRef]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The Power of Saliva: Antimicrobial and Beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef]
- Torrungruang, K.; Jitpakdeebordin, S.; Charatkulangkun, O.; Gleebbua, Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia Co-Infection Are Associated with Severe Periodontitis in a Thai Population. PLoS ONE 2015, 10, e0136646. [Google Scholar] [CrossRef]
- Plemmenos, G.; Piperi, C. Pathogenic Molecular Mechanisms in Periodontitis and Peri-Implantitis: Role of Advanced Glycation End Products. Life 2022, 12, 218. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Selway, C.A.; Jensen, E.D.; Pena, A.S.; Smart, G.; Weyrich, L.S. Type 1 Diabetes, Periodontal Health, and a Familial History of Hyperlipidaemia Is Associated with Oral Microbiota in Children: A Cross-Sectional Study. BMC Oral. Health 2023, 23, 15. [Google Scholar] [CrossRef]
- de Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct Fecal and Oral Microbiota Composition in Human Type 1 Diabetes, an Observational Study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef] [PubMed]
- Pachoński, M.; Koczor-Rozmus, A.; Mocny-Pachońska, K.; Łanowy, P.; Mertas, A.; Jarosz-Chobot, P. Oral Microbiota in Children with Type 1 Diabetes Mellitus. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 100–108. [Google Scholar] [CrossRef]
- Abola, I.; Gudra, D.; Ustinova, M.; Fridmanis, D.; Emulina, D.E.; Skadins, I.; Brinkmane, A.; Lauga-Tunina, U.; Gailite, L.; Auzenbaha, M. Oral Microbiome Traits of Type 1 Diabetes and Phenylketonuria Patients in Latvia. Microorganisms 2023, 11, 1471. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, M.; Nassar, M.; Moriel, N.; Cher, A.; Faibis, S.; Ram, D.; Zangen, D.; Yassour, M.; Steinberg, D. Characterization of the Oral Microbiome Among Children with Type 1 Diabetes Compared With Healthy Children. Front. Microbiol. 2021, 12, 756808. [Google Scholar] [CrossRef] [PubMed]
- Babatzia, A.; Papaioannou, W.; Stavropoulou, A.; Pandis, N.; Kanaka-Gantenbein, C.; Papagiannoulis, L.; Gizani, S. Clinical and Microbial Oral Health Status in Children and Adolescents with Type 1 Diabetes Mellitus. Int. Dent. J. 2020, 70, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Carelli, M.; Maguolo, A.; Zusi, C.; Olivieri, F.; Emiliani, F.; De Grandi, G.; Unali, I.; Zerman, N.; Signoretto, C.; Maffeis, C. Oral Microbiota in Children and Adolescents with Type 1 Diabetes Mellitus: Novel Insights into the Pathogenesis of Dental and Periodontal Disease. Microorganisms 2023, 11, 668. [Google Scholar] [CrossRef]
- Silvestre, F.-J.; Miralles, L.; Llambes, F.; Bautista, D.; Solá-Izquierdo, E.; Hernández-Mijares, A. Type 1 Diabetes Mellitus and Periodontal Disease: Relationship to Different Clinical Variables. Med. Oral. Patol. Oral. Cir. Bucal. 2009, 14, E175–E179. [Google Scholar]
- Yuan, X.; Wu, J.; Chen, R.; Chen, Z.; Su, Z.; Ni, J.; Zhang, M.; Sun, C.; Zhang, F.; Liu, Y.; et al. Characterization of the Oral Microbiome of Children with Type 1 Diabetes in the Acute and Chronic Phases. J. Oral. Microbiol. 2022, 14, 2094048. [Google Scholar] [CrossRef]
- Kunath, B.J.; Hickl, O.; Queirós, P.; Martin-Gallausiaux, C.; Lebrun, L.A.; Halder, R.; Laczny, C.C.; Schmidt, T.S.B.; Hayward, M.R.; Becher, D.; et al. Alterations of Oral Microbiota and Impact on the Gut Microbiome in Type 1 Diabetes Mellitus Revealed by Integrated Multi-Omic Analyses. Microbiome 2022, 10, 243. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Roles of Oral Microbiota and Oral-Gut Microbial Transmission in Hypertension. J. Adv. Res. 2023, 43, 147–161. [Google Scholar] [CrossRef]
- Lam, G.A.; Albarrak, H.; McColl, C.J.; Pizarro, A.; Sanaka, H.; Gomez-Nguyen, A.; Cominelli, F.; Paes Batista da Silva, A. The Oral-Gut Axis: Periodontal Diseases and Gastrointestinal Disorders. Inflamm. Bowel Dis. 2023, 29, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the Adult Digestive Tract Bacterial Microbiome Based on Seven Mouth Surfaces, Tonsils, Throat and Stool Samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Schloss, P.D. Dynamics and Associations of Microbial Community Types across the Human Body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yang, X.; Xu, Z.; Li, J.; Sun, C.; Chen, R.; Wei, H.; Chen, L.; Du, H.; Li, G.; et al. The Profile of Blood Microbiome in New-Onset Type 1 Diabetes Children. iScience 2024, 27, 110252. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, C.; Wang, R.; Wang, J.; Yang, Z.; Wang, X. A Pilot Study on the Characterization and Correlation of Oropharyngeal and Intestinal Microbiota in Children with Type 1 Diabetes Mellitus. Front. Pediatr. 2024, 12, 1382466. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef]
- Khor, B.; Snow, M.; Herrman, E.; Ray, N.; Mansukhani, K.; Patel, K.A.; Said-Al-Naief, N.; Maier, T.; Machida, C.A. Interconnections Between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance Between Systemic Health and Disease. Microorganisms 2021, 9, 496. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Liu, Y.; Shi, M.; Zhang, M.; Zhang, H.; Chen, J. Advances in Fecal Microbiota Transplantation for the Treatment of Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2024, 14, 1370999. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Donaldson, J.; Jachimowicz, K. The Role of Nutritional Factors in the Modulation of the Composition of the Gut Microbiota in People with Autoimmune Diabetes. Nutrients 2022, 14, 2498. [Google Scholar] [CrossRef]
- Manning, T.S.; Gibson, G.R. Microbial-Gut Interactions in Health and Disease. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. The Crucial Role of Early-Life Gut Microbiota in the Development of Type 1 Diabetes. Acta Diabetol. 2021, 58, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Spall, M.; Evans-Molina, C.; DiMeglio, L.A. Evaluating the Effect of Prebiotics on the Gut Microbiome Profile and β Cell Function in Youth with Newly Diagnosed Type 1 Diabetes: Protocol of a Pilot Randomized Controlled Trial. Pilot Feasibility Stud. 2023, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schick, A.; Meddings, J.; Reimer, R.A.; Huang, C. Effect of Prebiotic on Microbiota, Intestinal Permeability, and Glycemic Control in Children with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, Nutrition, and the Small Intestine. Curr. Gastroenterol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Dovi, K.S.; Bajinka, O.; Conteh, I. Evidence and Possible Mechanisms of Probiotics in the Management of Type 1 Diabetes Mellitus. J. Diabetes Metab. Disord. 2022, 21, 1081–1094. [Google Scholar] [CrossRef]
- Homayouni-Rad, A.; Soroush, A.-R.; Khalili, L.; Norouzi-Panahi, L.; Kasaie, Z.; Ejtahed, H.-S. Diabetes Management by Probiotics: Current Knowledge and Future Pespective. Int. J. Vitam. Nutr. Res. 2016, 86, 215–227. [Google Scholar] [CrossRef]
- Neiva, L.P.; Lopez, L.C.; Pasiani, R.O.; Serra, M.J.R.; Rullo, V.E.V. Use of Probiotics and Similar in Pediatric Patients with Type 1 Diabetes Mellitus: A Systematic Review. Rev. Paul. Pediatr. 2024, 42, e2023097. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Rohilla, L.; Jacob, N.; Yadav, J.; Sachdeva, N. A High Potency Multi-Strain Probiotic Improves Glycemic Control in Children with New-Onset Type 1 Diabetes Mellitus: A Randomized, Double-Blind, and Placebo-Controlled Pilot Study. Pediatr. Diabetes 2021, 22, 1014–1022. [Google Scholar] [CrossRef]
- Lokesh, M.N.; Kumar, R.; Jacob, N.; Sachdeva, N.; Rawat, A.; Yadav, J.; Dayal, D. Supplementation of High-Strength Oral Probiotics Improves Immune Regulation and Preserves Beta Cells among Children with New-Onset Type 1 Diabetes Mellitus: A Randomised, Double-Blind Placebo Control Trial. Indian J. Pediatr. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Groele, L.; Szajewska, H.; Szalecki, M.; Świderska, J.; Wysocka-Mincewicz, M.; Ochocińska, A.; Stelmaszczyk-Emmel, A.; Demkow, U.; Szypowska, A. Lack of Effect of Lactobacillus Rhamnosus GG and Bifidobacterium Lactis Bb12 on Beta-Cell Function in Children with Newly Diagnosed Type 1 Diabetes: A Randomised Controlled Trial. BMJ Open Diabetes Res. Care 2021, 9, e001523. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, Prebiotics, and Synbiotics—Approaching a Definition. Am. J. Clin. Nutr. 2001, 73, 361S–364S. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Qiu, X.; Wen, Q.; Liu, M.; Zhou, D.; Chen, Q. Probiotics, Pre-Biotics and Synbiotics in the Treatment of Pre-Diabetes: A Systematic Review of Randomized Controlled Trials. Front. Public Health 2021, 9, 645035. [Google Scholar] [CrossRef] [PubMed]
- Baroni, I.; Fabrizi, D.; Luciani, M.; Magon, A.; Conte, G.; De Angeli, G.; Paglione, G.; Ausili, D.; Caruso, R. Probiotics and Synbiotics for Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2024, 43, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Fuhri Snethlage, C.M.; de Wit, D.; Wortelboer, K.; Rampanelli, E.; Hanssen, N.M.J.; Nieuwdorp, M. Can Fecal Microbiota Transplantations Modulate Autoimmune Responses in Type 1 Diabetes? Immunol. Rev. 2024, 325, 46–63. [Google Scholar] [CrossRef]
- de Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal Microbiota Transplantation Halts Progression of Human New-Onset Type 1 Diabetes in a Randomised Controlled Trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef]
- Zou, B.; Liu, S.-X.; Li, X.-S.; He, J.-Y.; Dong, C.; Ruan, M.-L.; Xu, L.; Bai, T.; Huang, Z.-H.; Shu, S.-N. Long-Term Safety and Efficacy of Fecal Microbiota Transplantation in 74 Children: A Single-Center Retrospective Study. Front. Pediatr. 2022, 10, 964154. [Google Scholar] [CrossRef]
- He, L.; Chen, R.; Zhang, B.; Zhang, S.; Khan, B.A.; Zhu, D.; Wu, Z.; Xiao, C.; Chen, B.; Chen, F.; et al. Fecal Microbiota Transplantation Treatment of Autoimmune-Mediated Type 1 Diabetes Mellitus. Front. Immunol. 2022, 13, 930872. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut Microbiota in the Pathogenesis and Therapeutic Approaches of Diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]


| Author | Ref. | Main Findings in T1DM Individuals |
|---|---|---|
| Devaraj et al. (2009) | [47] | ↑ Circulating LPS |
| Yuan et al. (2022) | [48] | ↑ Gene-related LPS biosynthesis |
| Traversi et al. (2017) Mejía-Leon et al. (2014) | [51,52] | Loss of α-diversity and β-diversity |
| Cinek et al. (2018) Leiva-Gea et al. (2018) | [58,60] | ↓ Firmicutes/Bacteroidetes ratio |
| Brown et al. (2011) | [67] | ↑ Bacteroides, Clostridium, Veillonella, Ruminococcus, Escherichia, Streptococcus, Enterobacter, Lactobacillus ↓ Prevotella, Akkermansia, Bifidobacterium, Roseburia, Faecalibacterium, Eubacterium |
| Giongo et al. (2011) | [53] | ↑ Bacteroides ovatus, B. thetaiotaomicron, B. uniformis ↓ B. fragilis, B. vulgatus |
| Davis-Richardson et al. (2014) | [69] | ↑ Bacteroides dorei and vulgatus |
| Matos et al. (2021) | [70] | Some Bacteroides dorei strains invade and damage intestinal epithelial TJs |
| Vatanen et al. (2018) TEDDY Study | [72] | No significant taxonomic differences in gut microbiota between T1DM patients and controls |
| Biassoni et al. (2020) | [76] | ↑ Bacteroides stercoris |
| Endesfelder et al. (2016) | [79] | Presence of three bacterial communities prior to the development of islet autoantibodies |
| Author | Ref. | Main Findings in T1DM Individuals |
|---|---|---|
| Vila et al. (2019) | [96] | ↓ saliva ↑ pathogenic and acidogenic bacteria (Streptococcus mutans and Lactobacillus) |
| Torrungruang et al. (2015) | [97] | ↑ periodontopathogenic bacteria (Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans) |
| Chen et al. (2020) | [99] | ↑ Streptococcus mutans, Lactobacillus, and Actinomyces |
| de Groot et al. (2017) | [101] | ↑ Actinobacteria and Firmicutes (Streptococcus spp., Actinomyces spp., Rothia spp.) ↓ Bacteroidetes and Proteobacteria |
| Moskovitz et al. (2021) | [104] | ↑ Streptococcus genus ↓ Mogibacterium genus |
| Carelli et al. (2023) Silvestre et al. (2009) | [106] [107] | ↑ S. mitis, S. oralis, S. anginosus, S. gordonii |
| Yuan et al. (2022) | [108] | ↑ opportunistic pathogens (Streptococcus, Granulicatella, Rothia and Rhodococcus) ↓ Veillonella and Prevotella |
| Kunath et al. (2022) | [109] | ↑ S. mutans ↓ S. salivarius |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luppi, S.; Aldegheri, L.; Azzalini, E.; Pacetti, E.; Barucca Sebastiani, G.; Fabiani, C.; Robino, A.; Comar, M. Unravelling the Role of Gut and Oral Microbiota in the Pediatric Population with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 10611. https://doi.org/10.3390/ijms251910611
Luppi S, Aldegheri L, Azzalini E, Pacetti E, Barucca Sebastiani G, Fabiani C, Robino A, Comar M. Unravelling the Role of Gut and Oral Microbiota in the Pediatric Population with Type 1 Diabetes Mellitus. International Journal of Molecular Sciences. 2024; 25(19):10611. https://doi.org/10.3390/ijms251910611
Chicago/Turabian StyleLuppi, Stefania, Luana Aldegheri, Eros Azzalini, Emanuele Pacetti, Giulia Barucca Sebastiani, Carolina Fabiani, Antonietta Robino, and Manola Comar. 2024. "Unravelling the Role of Gut and Oral Microbiota in the Pediatric Population with Type 1 Diabetes Mellitus" International Journal of Molecular Sciences 25, no. 19: 10611. https://doi.org/10.3390/ijms251910611






