Ursolic Acid Regulates Immune Balance, Modulates Gut Microbial Metabolism, and Improves Liver Health in Mice
Abstract
1. Introduction
2. Results
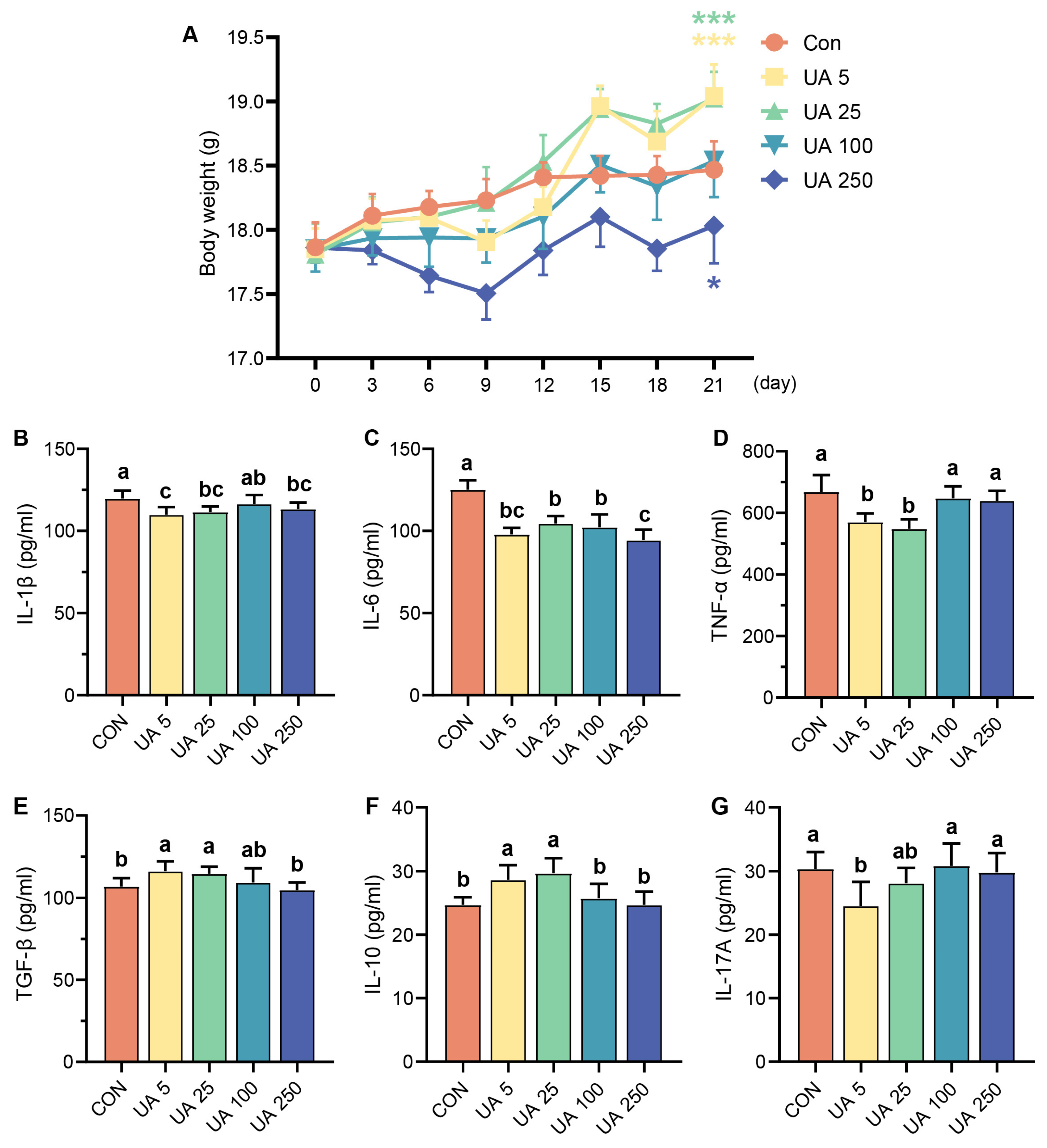
2.1. Effect of UA on Immune-Related Cytokines in Mice
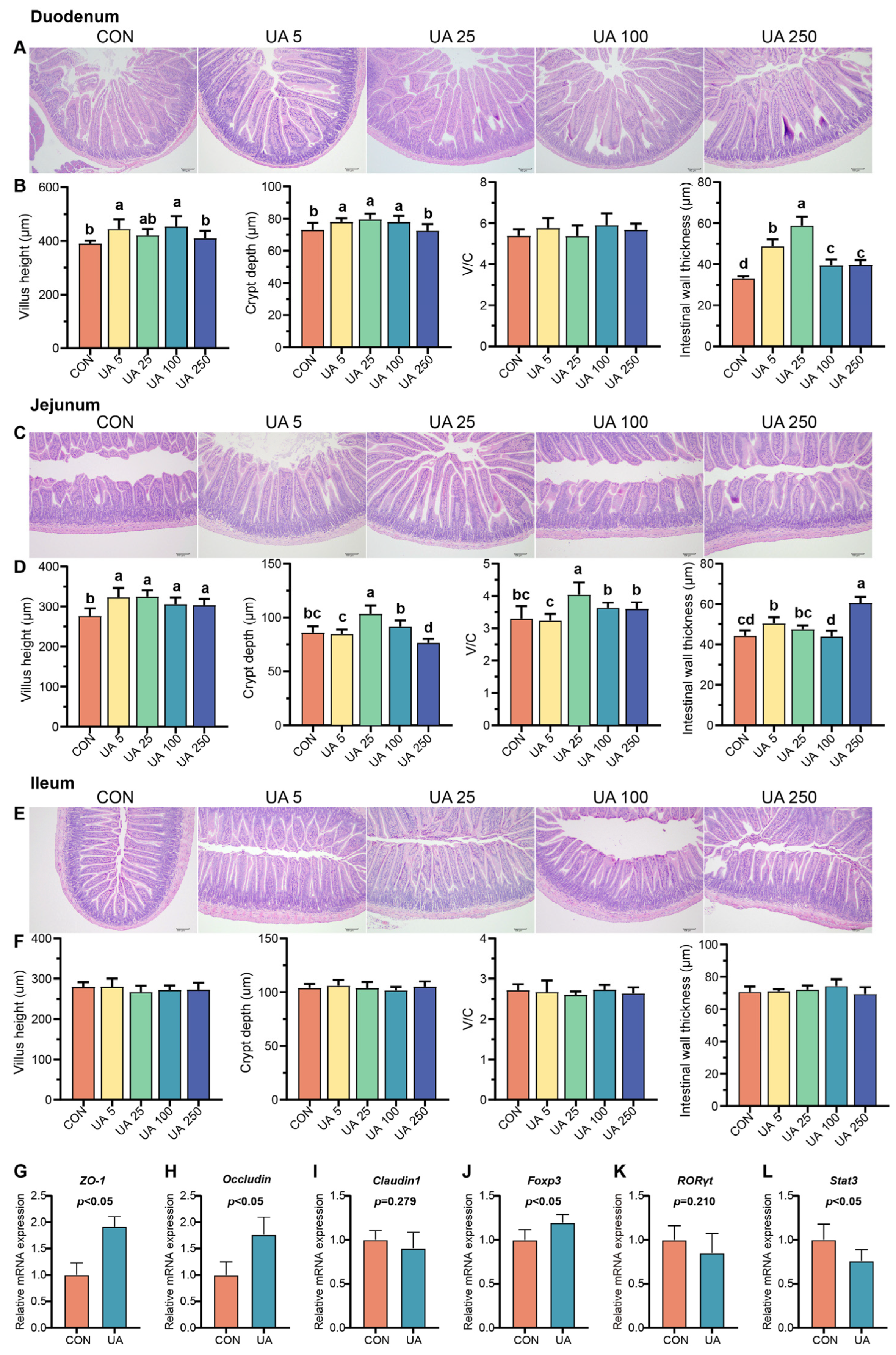
2.2. UA Promotes Intestinal Development, Intestinal Epithelial Barrier Integrity, and Immune Balance in Mice
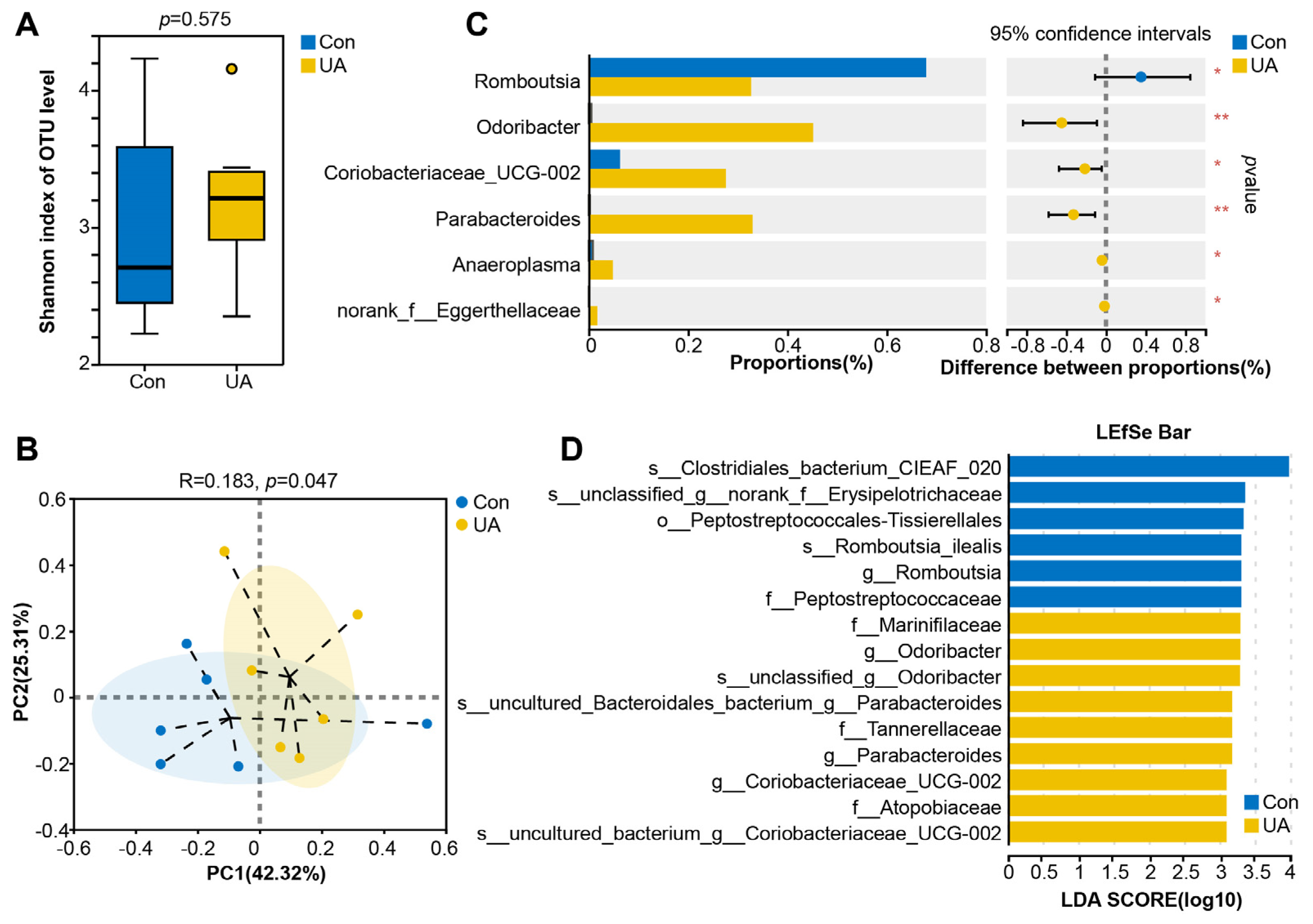
2.3. UA Changes the Composition and Structure of the Gut Microbiota in Mice
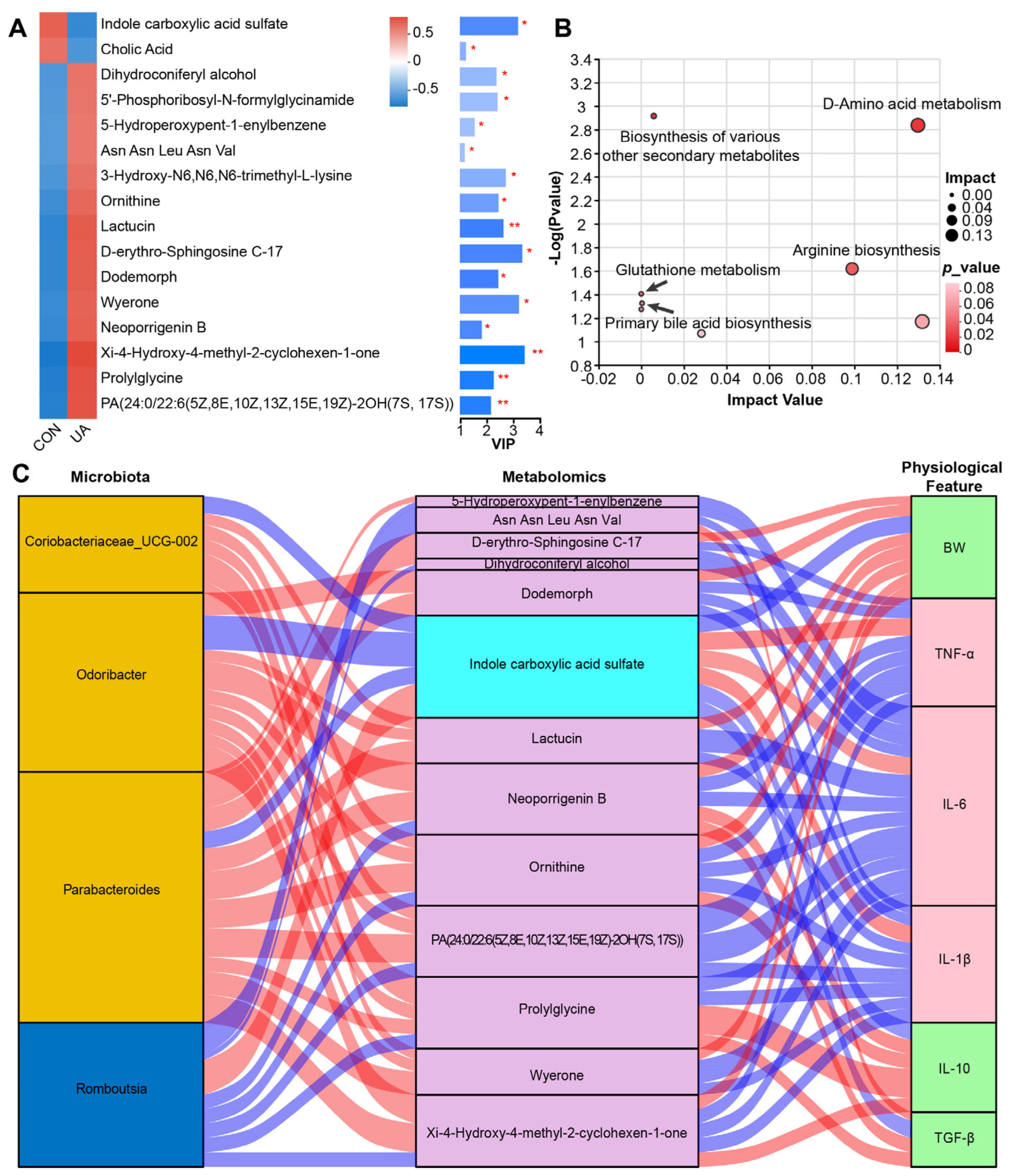
2.4. UA Also Altered Metabolism of Gut Microbiota
2.5. UA Treatment Alters the Gut Microbiota and Its Metabolic Characteristics and Affects the Host Phenotype
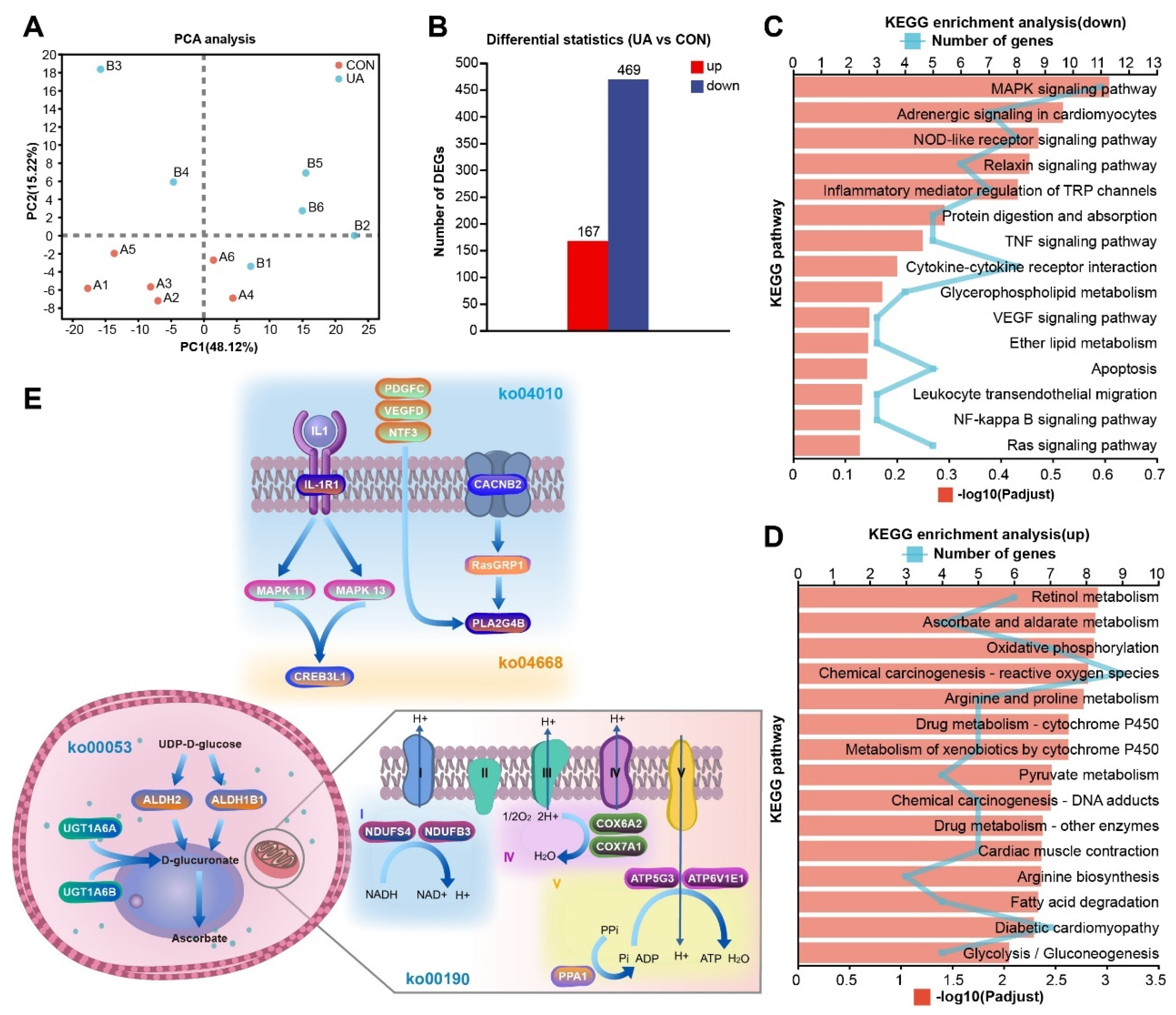
2.6. UA Alters the Liver Transcriptome
2.7. Multi-Omics Integrated Analysis Reveals the Possible Regulatory Mechanism of UA
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Experimental Design
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Histological Analysis
4.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.6. 16S rRNA Gene Sequencing
4.7. Microbiota Data Analysis
4.8. Liquid Chromatograph–Mass Spectrometer Analysis (LC–MS Analysis)
4.9. RNA Extraction, Library Construction, and Sequencing
4.10. Correlation Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Akhtar, M.; Pan, H.; Liu, Q.; Chen, Y.; Zhou, X.; You, Y.; Shi, D.; Liu, H. Fecal microbiota transplantation improves chicken growth performance by balancing jejunal Th17/Treg cells. Microbiome 2023, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, T.; He, S.; Zhang, Y.; Tan, Y.; Bai, Y.; Shi, J.; Deng, W.; Qiu, J.; Wang, Z.; et al. Ursolic acid reduces oxidative stress injury to ameliorate experimental autoimmune myocarditis by activating Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2023, 14, 1189372. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Luo, N.; Yu, B.; Wu, H.; Wu, S.; Tian, C.; Guo, J.; Ning, H.; Bi, C.; Wei, H.; et al. Ursolic Acid Derivative UA232 Promotes Tumor Cell Apoptosis by Inducing Endoplasmic Reticulum Stress and Lysosomal Dysfunction. Int. J. Biol. Sci. 2022, 18, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, W.; Huang, C.; Jian, J.; Zhang, Y.; Liu, Q.; Chen, P.; Zhu, X. Ursolic acid alleviates Kupffer cells pyroptosis in liver fibrosis by the NOX2/NLRP3 inflammasome signaling pathway. Int. Immunopharmacol. 2022, 113, 109321. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, F.; Tang, Z.; Yang, X.; Liu, Y.; Wang, F.; Chen, B. Anti-inflammatory and antioxidant activity of ursolic acid: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1256946. [Google Scholar] [CrossRef]
- Zhang, B.C.; Lai, C.M.; Luo, B.Y.; Shao, J.W. Triterpenoids-templated self-assembly nanosystem for biomimetic delivery of CRISPR/Cas9 based on the synergy of TLR-2 and ICB to enhance HCC immunotherapy. Acta Pharm. Sin. B 2024, 14, 3205–3217. [Google Scholar] [CrossRef]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef]
- Cheng, H.; Guan, X.; Chen, D.; Ma, W. The Th17/Treg Cell Balance: A Gut Microbiota-Modulated Story. Microorganisms 2019, 7, 583. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, J.Y.; Xing, Y.; Wu, P.; Jin, Y.W.; Wei, W.; Zhao, L.; Yang, J.; Chen, G.C.; Qin, L.Q. Lactobacillus rhamnosus GG alleviates radiation-induced intestinal injury by modulating intestinal immunity and remodeling gut microbiota. Microbiol. Res. 2024, 286, 127821. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-B.; Luo, M.-M.; Chen, Z.-Y.; He, B.-H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ren, X.; Liu, X.; Wang, Y.; Ma, J.; Song, R.; Wang, X.; Dong, Y.; Fan, Q.; Wei, J.; et al. Dietary Ursolic Acid Prevents Alcohol-Induced Liver Injury via Gut–Liver Axis Homeostasis Modulation: The Key Role of Microbiome Manipulation. J. Agric. Food Chem. 2021, 69, 7074–7083. [Google Scholar] [CrossRef]
- Sheng, Q.; Li, F.; Chen, G.; Li, J.; Li, J.; Wang, Y.; Lu, Y.; Li, Q.; Li, M.; Chai, K. Ursolic Acid Regulates Intestinal Microbiota and Inflammatory Cell Infiltration to Prevent Ulcerative Colitis. J. Immunol. Res. 2021, 2021, 6679316. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, Q.; Zhang, W.; Wan, Y.; Huang, C.; Zhu, X. Ursolic acid reverses liver fibrosis by inhibiting NOX4/NLRP3 inflammasome pathways and bacterial dysbiosis. Gut Microbes 2021, 13, 1972746. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef]
- Chen, C.; Gao, K.; Chen, Z.; Zhang, Q.; Ke, X.; Mao, B.; Fan, Q.; Li, Y.; Chen, S. The supplementation of the multi-strain probiotics WHHPRO™ alleviates high-fat diet-induced metabolic symptoms in rats via gut-liver axis. Front. Nutr. 2023, 10, 1324691. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Yan, S.; Liu, M.; Sun, B.; Lu, Y.; Shao, Y. Ursolic acid alleviates inflammation and against diabetes-induced nephropathy through TLR4-mediated inflammatory pathway. Mol. Med. Rep. 2018, 18, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Y.; Liu, Y.; Luo, Z.; Law, B.Y.K.; Zheng, Y.; Huang, X.; Li, W. Ursolic acid enhances the antitumor effects of sorafenib associated with Mcl-1-related apoptosis and SLC7A11-dependent ferroptosis in human cancer. Pharmacol. Res. 2022, 182, 106306. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tang, B.; Wang, F.C.; Tang, L.; Lei, Y.Y.; Luo, Y.; Huang, S.J.; Yang, M.; Wu, L.Y.; Wang, W.; et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225–5241. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, W.; Xiong, Y.; Li, Y.; Wan, Q.; Zhou, W.; Zhao, H.; Xiao, Q.; Liu, D. Astragaloside IV alleviates ulcerative colitis by regulating the balance of Th17/Treg cells. Phytomedicine 2022, 104, 154287. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wan, Y.Y. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell. Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Hao, W.; Kwek, E.; He, Z.; Zhu, H.; Liu, J.; Zhao, Y.; Ma, K.Y.; He, W.S.; Chen, Z.Y. Ursolic acid alleviates hypercholesterolemia and modulates the gut microbiota in hamsters. Food Funct. 2020, 11, 6091–6103. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.B.; Pires, S.; Guo, C.J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A–Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2021, 162, 166–178. [Google Scholar] [CrossRef]
- Xing, C.; Wang, M.; Ajibade, A.A.; Tan, P.; Fu, C.; Chen, L.; Zhu, M.; Hao, Z.Z.; Chu, J.; Yu, X.; et al. Microbiota regulate innate immune signaling and protective immunity against cancer. Cell Host Microbe 2021, 29, 959–974.E7. [Google Scholar] [CrossRef] [PubMed]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef]
- Koh, G.Y.; Kane, A.V.; Wu, X.; Crott, J.W. Parabacteroides distasonis attenuates tumorigenesis, modulates inflammatory markers and promotes intestinal barrier integrity in azoxymethane-treated A/J mice. Carcinogenesis 2020, 41, 909–917. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Cuffaro, B.; Assohoun, A.L.W.; Boutillier, D.; Súkeníková, L.; Desramaut, J.; Boudebbouze, S.; Salomé-Desnoulez, S.; Hrdý, J.; Waligora-Dupriet, A.J.; Maguin, E.; et al. In Vitro Characterization of Gut Microbiota-Derived Commensal Strains: Selection of Parabacteroides distasonis Strains Alleviating TNBS-Induced Colitis in Mice. Cells 2020, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Qi, H.; Li, Y.; Yun, H.; Zhang, T.; Huang, Y.; Zhou, J.; Yan, H.; Wei, J.; Liu, Y.; Zhang, Z.; et al. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun. Biol. 2019, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wu, X.; Zou, N.; Zhang, Y.; Yuan, J.; Gao, Y.; Chen, W.; Yao, J.; Li, C.; Hou, J.; et al. Cichorium pumilum Jacq Extract Inhibits LPS-Induced Inflammation via MAPK Signaling Pathway and Protects Rats from Hepatic Fibrosis Caused by Abnormalities in the Gut-Liver Axis. Front. Pharmacol. 2021, 12, 683613. [Google Scholar] [CrossRef]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Xue, B.; Yu, C.; Wang, L.; Deng, R.; Liu, H.; Chen, Z.; Zhang, Y.; Fan, S.; et al. RasGRP1 promotes the acute inflammatory response and restricts inflammation-associated cancer cell growth. Nat. Commun. 2022, 13, 7001. [Google Scholar] [CrossRef] [PubMed]
- Pandian, V.D.; Giovannucci, D.R.; Vazquez, G.; Kumarasamy, S. CACNB2 is associated with aberrant RAS-MAPK signaling in hypertensive Dahl Salt-Sensitive rats. Biochem. Biophys. Res. Commun. 2019, 513, 760–765. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, W.; Sun, Y.; Ren, X.; Yan, C.; Song, S.; Ai, C. Fucoidan ameliorates alcohol-induced liver injury in mice through Parabacteroides distasonis-mediated regulation of the gut-liver axis. Int. J. Biol. Macromol. 2024, 279, 135309. [Google Scholar] [CrossRef]
- Zhao, Q.; Dai, M.Y.; Huang, R.Y.; Duan, J.Y.; Zhang, T.; Bao, W.M.; Zhang, J.Y.; Gui, S.Q.; Xia, S.M.; Dai, C.T.; et al. Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat. Commun. 2023, 14, 1829. [Google Scholar] [CrossRef]
- Shu, Y.; Huang, Y.; Dong, W.; Fan, X.; Sun, Y.; Chen, G.; Zeng, X.; Ye, H. The polysaccharides from Auricularia auricula alleviate non-alcoholic fatty liver disease via modulating gut microbiota and bile acids metabolism. Int. J. Biol. Macromol. 2023, 246, 125662. [Google Scholar] [CrossRef]
- Canbay, A.; Sowa, J.P. L-Ornithine L-Aspartate (LOLA) as a Novel Approach for Therapy of Non-alcoholic Fatty Liver Disease. Drugs 2019, 79 (Suppl. 1), 39–44. [Google Scholar] [CrossRef]
- Ding, Y.; Koda, Y.; Shashni, B.; Takeda, N.; Zhang, X.; Tanaka, N.; Nishikawa, Y.; Nagasaki, Y. An orally deliverable ornithine-based self-assembling polymer nanomedicine ameliorates hyperammonemia in acetaminophen-induced acute liver injury. Acta Biomater. 2023, 168, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Imam, K.M.S.U.; Tian, Y.; Xin, F.; Xie, Y.; Wen, B. Lactucin, a Bitter Sesquiterpene from Cichorium intybus, Inhibits Cancer Cell Proliferation by Downregulating the MAPK and Central Carbon Metabolism Pathway. Molecules 2022, 27, 7358. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Zheng, G.; Zhang, Q.; Jin, P.; Zhang, H.; Su, L.; Qin, D.; Yao, G. Sesquiterpenoids with diverse carbon skeletons from the roots of Cichorium glandulosum and their anti-inflammatory activities. Fitoterapia 2019, 136, 104170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, H.; Huang, L.; Sun, C.; Yue, Y.; Cao, X.; Jia, H.; Wang, C.; Gao, Y. Disulfiram with Cu2+ alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Theranostics 2023, 13, 2879–2895. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Cui, Y.; Wang, F.; Wu, F.; Li, C.; Liu, S.; Chen, B. Ursolic Acid Regulates Immune Balance, Modulates Gut Microbial Metabolism, and Improves Liver Health in Mice. Int. J. Mol. Sci. 2024, 25, 10623. https://doi.org/10.3390/ijms251910623
Zhao M, Cui Y, Wang F, Wu F, Li C, Liu S, Chen B. Ursolic Acid Regulates Immune Balance, Modulates Gut Microbial Metabolism, and Improves Liver Health in Mice. International Journal of Molecular Sciences. 2024; 25(19):10623. https://doi.org/10.3390/ijms251910623
Chicago/Turabian StyleZhao, Man, Yali Cui, Fengxia Wang, Fengyang Wu, Chong Li, Shudong Liu, and Baojiang Chen. 2024. "Ursolic Acid Regulates Immune Balance, Modulates Gut Microbial Metabolism, and Improves Liver Health in Mice" International Journal of Molecular Sciences 25, no. 19: 10623. https://doi.org/10.3390/ijms251910623
APA StyleZhao, M., Cui, Y., Wang, F., Wu, F., Li, C., Liu, S., & Chen, B. (2024). Ursolic Acid Regulates Immune Balance, Modulates Gut Microbial Metabolism, and Improves Liver Health in Mice. International Journal of Molecular Sciences, 25(19), 10623. https://doi.org/10.3390/ijms251910623








