The Evolution and Biological Activity of Metazoan Mixed Lineage Kinase Domain-Like Protein (MLKL)
Abstract
1. Introduction
2. Results
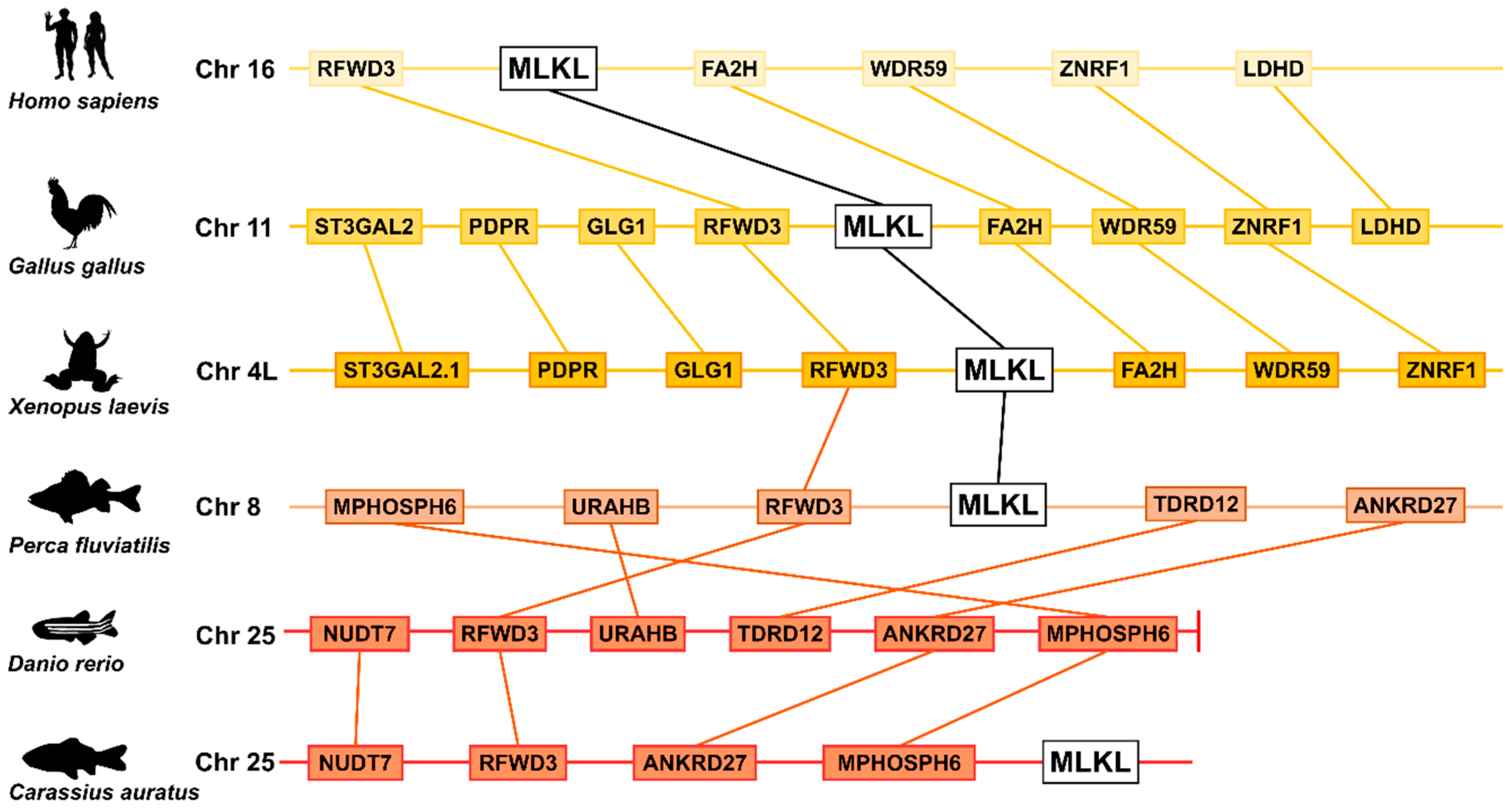
2.1. Divergent Evolution of MLKL in Invertebrates and Vertebrates
2.2. Variation in MLKL Gene Number in Invertebrates and Vertebrates
2.3. Tissue Specific Expression Profiles of Vertebrate MLKL
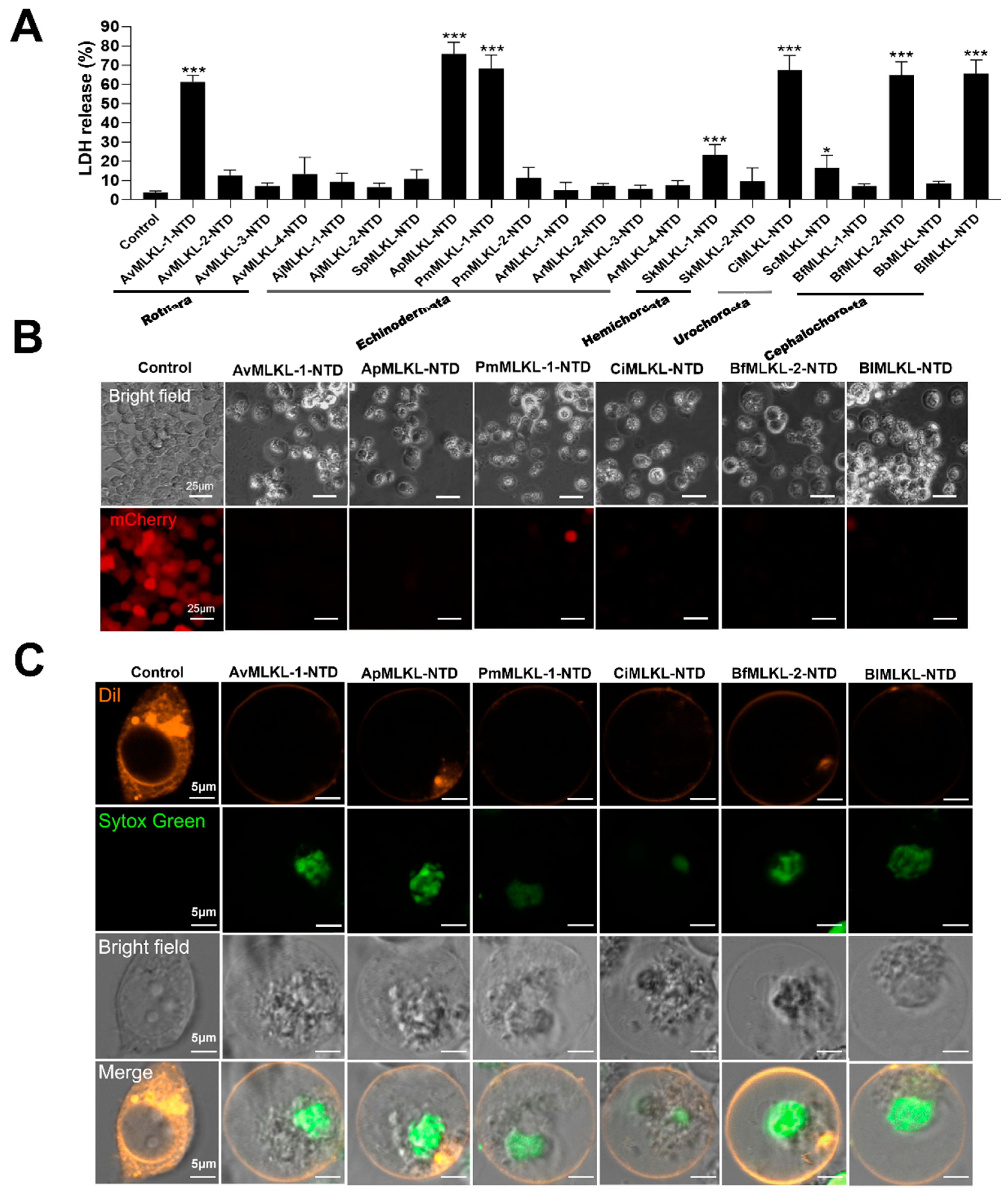
2.4. Necroptosis-Inducing Abilities of Invertebrate MLKL
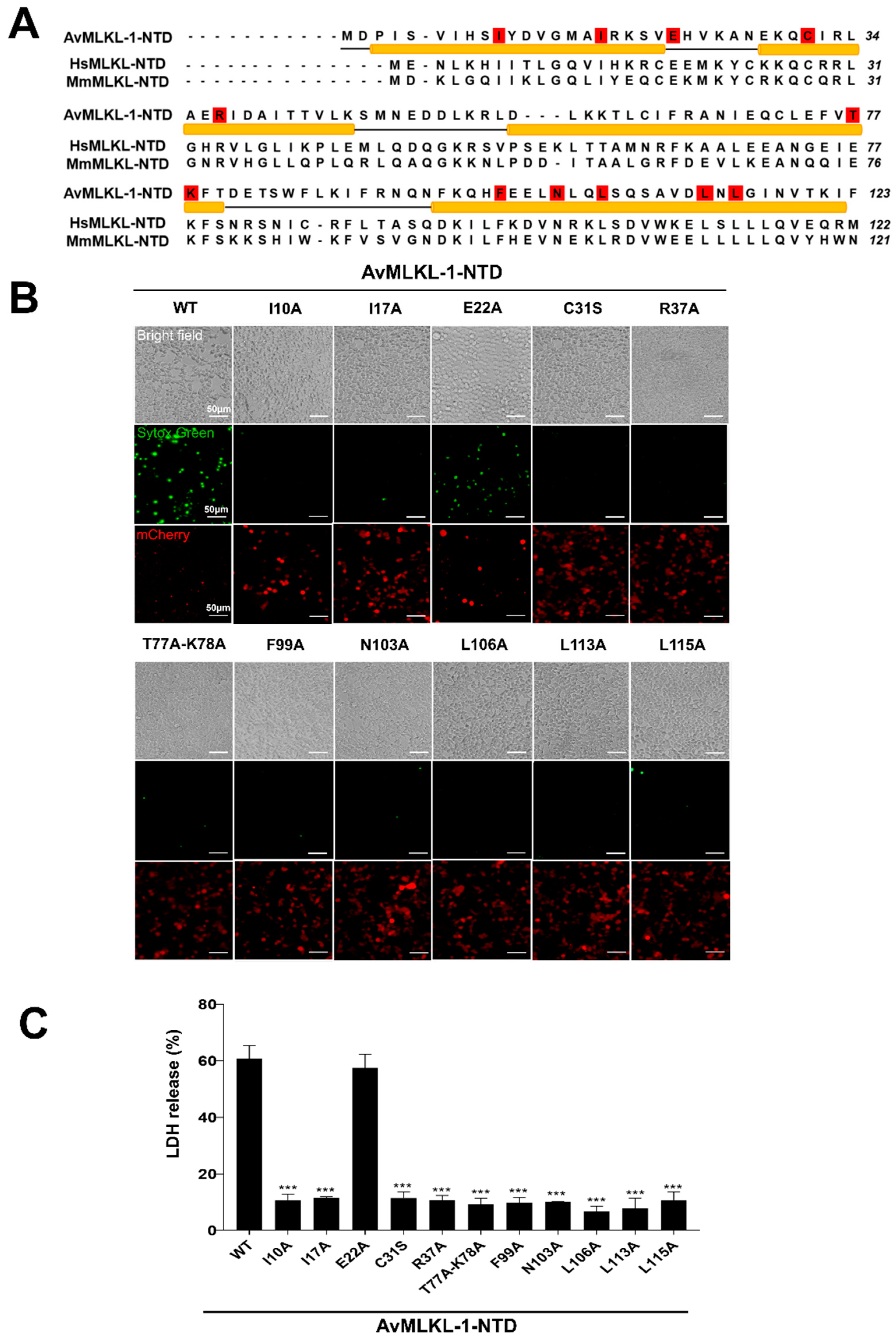
2.5. Identification of Key Residues Required for the Necroptotic Activity of AvMLKL-1
3. Discussion
4. Materials and Methods
4.1. Sequence Collection
4.2. Phylogeny and Synteny
4.3. Transcriptome Analysis
4.4. Gene Cloning and Mutagenesis
4.5. Lactate Dehydrogenase (LDH) Assay
4.6. Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rickard, J.A.; Anderton, H.; Etemadi, N.; Nachbur, U.; Darding, M.; Peltzer, N.; Lalaoui, N.; Lawlor, K.E.; Vanyai, H.; Hall, C. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. Elife 2014, 3, e03464. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.A.; O’Donnell, J.A.; Evans, J.M.; Lalaoui, N.; Poh, A.R.; Rogers, T.; Vince, J.E.; Lawlor, K.E.; Ninnis, R.L.; Anderton, H. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 2014, 157, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.M.; Kauppi, M.; Majewski, I.J.; Liu, Z.; Cox, A.J.; Miyake, S.; Petrie, E.J.; Silk, M.A.; Li, Z.; Tanzer, M.C. A missense mutation in the MLKL brace region promotes lethal neonatal inflammation and hematopoietic dysfunction. Nat. Commun. 2020, 11, 3150. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Van Eeckhoutte, H.P.; Liu, G.; Nair, P.M.; Jones, B.; Gillis, C.M.; Nalkurthi, B.C.; Verhamme, F.; Buyle-Huybrecht, T.; Vandenabeele, P. Necroptosis signaling promotes inflammation, airway remodeling, and emphysema in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 204, 667–681. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, Y.; Chen, Z.; Huang, J.; Xiao, J.; Zou, J.; Feng, H. RIPK3 collaborates with RIPK1 to inhibit MAVS-mediated signaling during black carp antiviral innate immunity. Fish Shellfish Immunol. 2021, 115, 142–149. [Google Scholar] [CrossRef]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012, 11, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.W.; Kaiser, W.J. DAI another way: Necroptotic control of viral infection. Cell Host Microbe 2017, 21, 290–293. [Google Scholar] [CrossRef]
- He, S.; Liang, Y.; Shao, F.; Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3–mediated pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 20054–20059. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013, 288, 31268–31279. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.-G. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5322–5327. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Osorio, V.; Abdelwahab, Y.; Ros, U. The many faces of MLKL, the executor of necroptosis. Int. J. Mol. Sci. 2023, 24, 10108. [Google Scholar] [CrossRef]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.-G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Wang, X.; Zhang, Y.; Wan, H.; Song, Y.; Chen, X.; Shao, J.; Han, J. Distinct roles of RIP1–RIP3 hetero-and RIP3–RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014, 21, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Weinlich, R.; Oberst, A.; Beere, H.M.; Green, D.R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 127–136. [Google Scholar] [CrossRef]
- Xia, B.; Fang, S.; Chen, X.; Hu, H.; Chen, P.; Wang, H.; Gao, Z. MLKL forms cation channels. Cell Res. 2016, 26, 517–528. [Google Scholar] [CrossRef]
- Rodriguez, D.; Weinlich, R.; Brown, S.; Guy, C.; Fitzgerald, P.; Dillon, C.; Oberst, A.; Quarato, G.; Low, J.; Cripps, J.G.; et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016, 23, 76–88. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef]
- Su, L.; Quade, B.; Wang, H.; Sun, L.; Wang, X.; Rizo, J. A plug release mechanism for membrane permeation by MLKL. Structure 2014, 22, 1489–1500. [Google Scholar] [CrossRef]
- Quarato, G.; Guy, C.S.; Grace, C.R.; Llambi, F.; Nourse, A.; Rodriguez, D.A.; Wakefield, R.; Frase, S.; Moldoveanu, T.; Green, D.R. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol. Cell 2016, 61, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, Y.; Hulpiau, P.; Saeys, Y.; Bertrand, M.J.; Vandenabeele, P. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 2016, 26, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, L.K.; Huang, M.; Zhang, X.; Nakano, R.T.; Kopp, L.B.; Saur, I.M.; Jacob, F.; Kovacova, V.; Lapin, D.; Parker, J.E.; et al. Discovery of a family of mixed lineage kinase domain-like proteins in plants and their role in innate immune signaling. Cell Host Microbe 2020, 28, 813–824.e6. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.; Hogan, D.; Berman, J. Do fungi undergo apoptosis-like programmed cell death? mBio 2018, 9, e00948-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, D.; Orchard, R.C.; Hancks, D.C.; Reese, T.A.J.N. Norovirus MLKL-like protein initiates cell death to induce viral egress. Nature 2023, 616, 152–158. [Google Scholar] [CrossRef]
- Hildebrand, J.M.; Tanzer, M.C.; Lucet, I.S.; Young, S.N.; Spall, S.K.; Sharma, P.; Pierotti, C.; Garnier, J.-M.; Dobson, R.C.; Webb, A.I.; et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl. Acad. Sci. USA 2014, 111, 15072–15077. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Guo, X.; Litman, G.W.; Dishaw, L.J.; Zhang, G. Massive expansion and functional divergence of innate immune genes in a protostome. Sci. Rep. 2015, 5, 8693. [Google Scholar] [CrossRef]
- Palmer, W.J.; Jiggins, F.M. Comparative genomics reveals the origins and diversity of arthropod immune systems. Mol. Biol. Evol. 2015, 32, 2111–2129. [Google Scholar] [CrossRef]
- Gladyshev, E.A.; Meselson, M.; Arkhipova, I.R. Massive horizontal gene transfer in bdelloid rotifers. Science 2008, 320, 1210–1213. [Google Scholar] [CrossRef]
- Eyres, I.; Boschetti, C.; Crisp, A.; Smith, T.P.; Fontaneto, D.; Tunnacliffe, A.; Barraclough, T.G. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biol. 2015, 13, 90. [Google Scholar] [CrossRef]
- Henderson, S.; Dunne, E.M.; Fasey, S.A.; Giles, S. The early diversification of ray-finned fishes (Actinopterygii): Hypotheses, challenges and future prospects. Biol. Rev. 2023, 98, 284–315. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Van de Peer, Y. From 2R to 3R: Evidence for a fish-specific genome duplication (FSGD). Bioessays 2005, 27, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Wright, J., Jr.; May, B. Linkage relationships reflecting ancestral tetraploidy in salmonid fish. Genetics 1987, 116, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Muramoto, J.; Christian, L.; Atkin, N.B. Diploid-tetraploid relationship among old-world members of the fish family Cyprinidae. Chromosoma 1967, 23, 1–9. [Google Scholar] [CrossRef]
- Linardopoulou, E.V.; Williams, E.M.; Fan, Y.; Friedman, C.; Young, J.M.; Trask, B.J. Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature 2005, 437, 94–100. [Google Scholar] [CrossRef]
- Vasilikos, L.; Spilgies, L.M.; Knop, J.; Wong, W.W.L. Regulating the balance between necroptosis, apoptosis and inflammation by inhibitors of apoptosis proteins. Immunol. Cell Biol. 2017, 95, 160–165. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, M.; Cao, D.; Yang, C.; Jin, J.; Wu, L.; Hong, X.; Li, W.; Lu, L.; Li, J.; et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci. Adv. 2021, 7, eabf6290. [Google Scholar] [CrossRef]
- Martens, S.; Bridelance, J.; Roelandt, R.; Vandenabeele, P.; Takahashi, N. MLKL in cancer: More than a necroptosis regulator. Cell Death Differ. 2021, 28, 1757–1772. [Google Scholar] [CrossRef]
- Tanzer, M.C.; Matti, I.; Hildebrand, J.M.; Young, S.; Wardak, A.; Tripaydonis, A.; Petrie, E.J.; Mildenhall, A.L.; Vaux, D.; Vince, J.E. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ. 2016, 23, 1185–1197. [Google Scholar] [CrossRef]
- Murphy, J.M. The killer pseudokinase mixed lineage kinase domain-like protein (MLKL). Cold Spring Harb. Perspect. Biol. 2020, 12, a036376. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Z.; Sun, L. The evolutionary diversification and antimicrobial potential of MPEG1 in Metazoa. Comput. Struct. Biotechnol. J. 2023, 21, 5818–5828. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jiang, S.; Qin, K.; Sun, L. New insights into the evolutionary dynamic and lineage divergence of gasdermin E in metazoa. Front. Cell Dev. Biol. 2022, 10, 952015. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-J.; Kanda, M.; Koyanagi, R.; Hisata, K.; Akiyama, T.; Sakamoto, H.; Sakamoto, T.; Satoh, N. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nat. Ecol. Evol. 2018, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.; Potter, S.C.; Finn, R.D. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Qin, K.; Jiang, S.; Xu, H.; Yuan, Z.; Sun, L.J.C.R. Pyroptotic gasdermin exists in Mollusca and is vital to eliminating bacterial infection. Cell Rep. 2023, 42, 112414. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, Z.; Sun, Y.; Zhang, T.; Sun, L. Coral gasdermin triggers pyroptosis. Sci. Immunol. 2020, 5, eabd2591. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yuan, Z.; Xu, H.; Chen, Y.; Sun, L. The Evolution and Biological Activity of Metazoan Mixed Lineage Kinase Domain-Like Protein (MLKL). Int. J. Mol. Sci. 2024, 25, 10626. https://doi.org/10.3390/ijms251910626
Wang Q, Yuan Z, Xu H, Chen Y, Sun L. The Evolution and Biological Activity of Metazoan Mixed Lineage Kinase Domain-Like Protein (MLKL). International Journal of Molecular Sciences. 2024; 25(19):10626. https://doi.org/10.3390/ijms251910626
Chicago/Turabian StyleWang, Qingyue, Zihao Yuan, Hang Xu, Yuan Chen, and Li Sun. 2024. "The Evolution and Biological Activity of Metazoan Mixed Lineage Kinase Domain-Like Protein (MLKL)" International Journal of Molecular Sciences 25, no. 19: 10626. https://doi.org/10.3390/ijms251910626
APA StyleWang, Q., Yuan, Z., Xu, H., Chen, Y., & Sun, L. (2024). The Evolution and Biological Activity of Metazoan Mixed Lineage Kinase Domain-Like Protein (MLKL). International Journal of Molecular Sciences, 25(19), 10626. https://doi.org/10.3390/ijms251910626





