Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair
Abstract
:1. Introduction
2. Results
2.1. In Vivo Experiment
2.1.1. Histological Assessment of Cartilage Repair
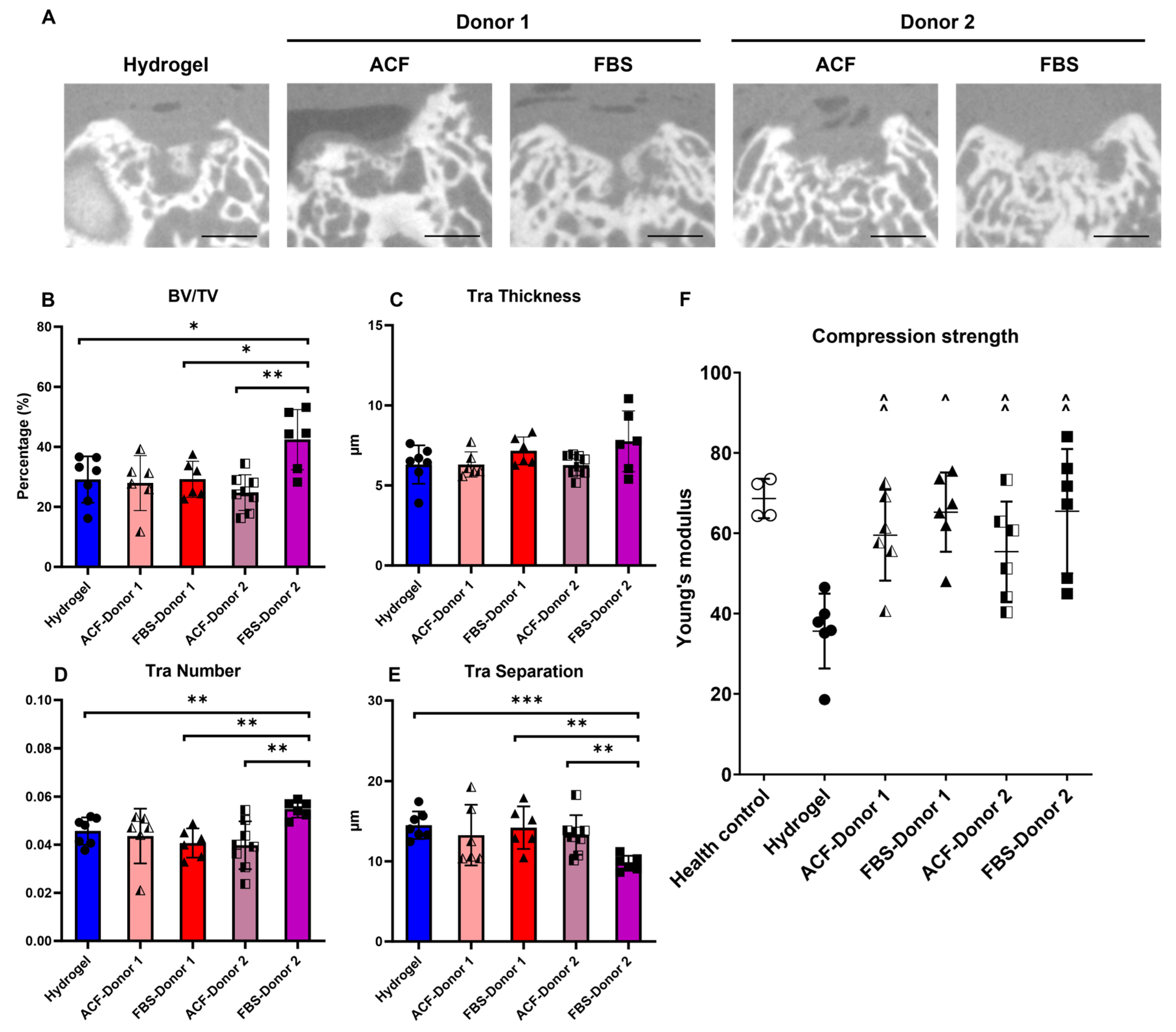
2.1.2. Micro-CT Assessment of Subchondral Bone Repair and Mechanical Strength of Repair Tissue
2.2. In Vitro Experiment
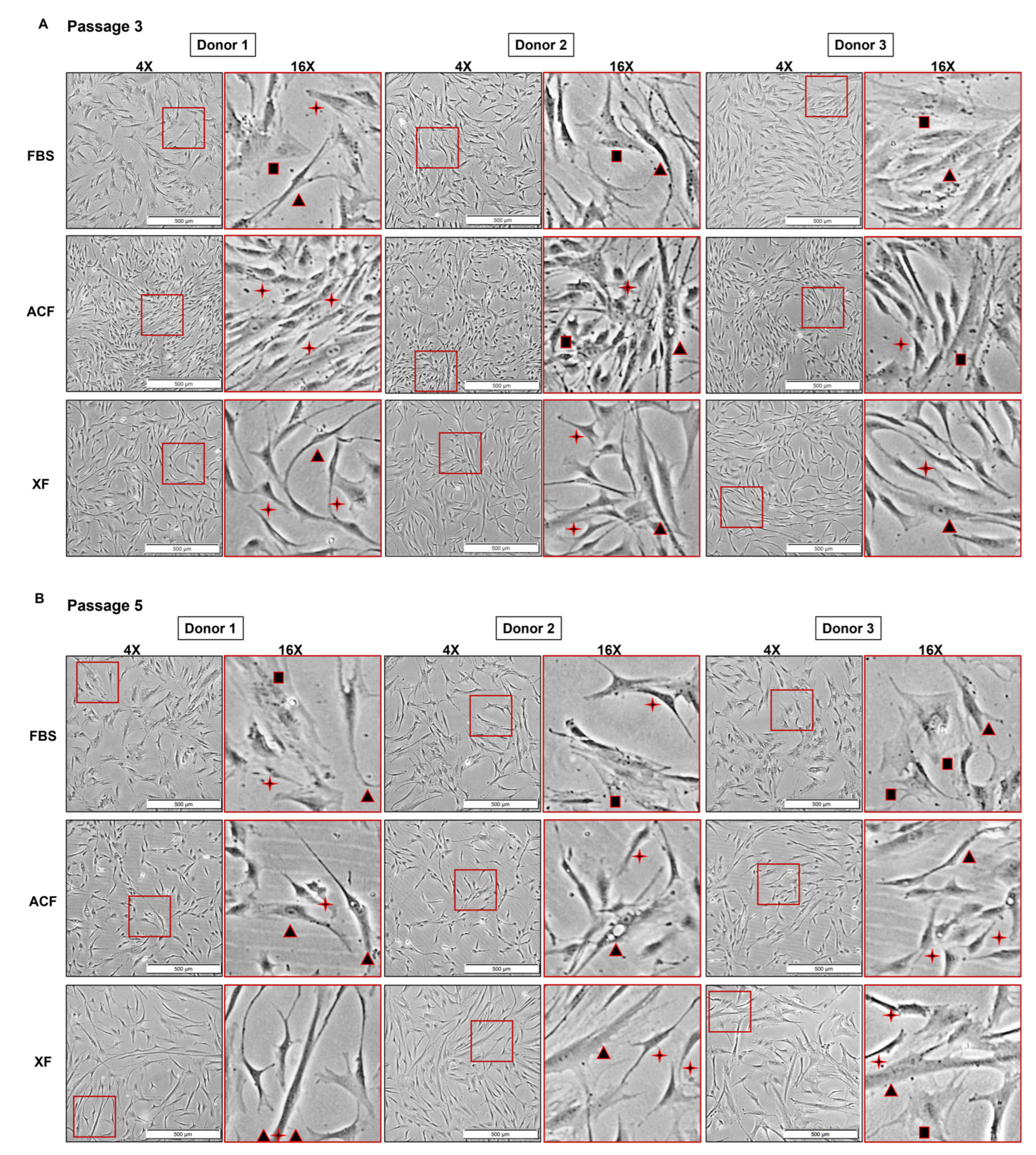
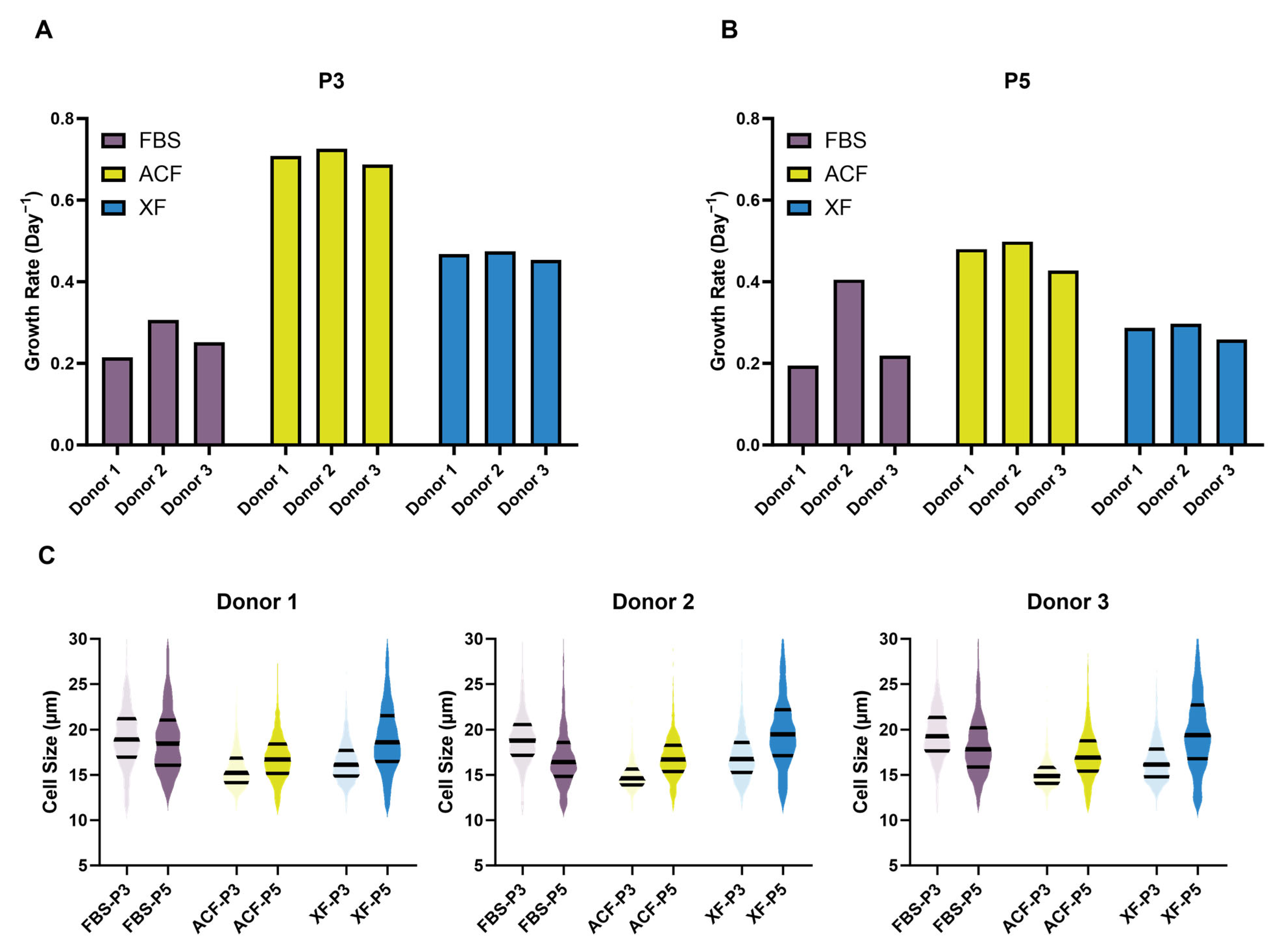
2.2.1. SF Medium Reduced Donor–Donor Variability in Terms of Growth Rate and Cell Morphology
2.2.2. SF Medium Preserved Expression of MSC Characterization Markers
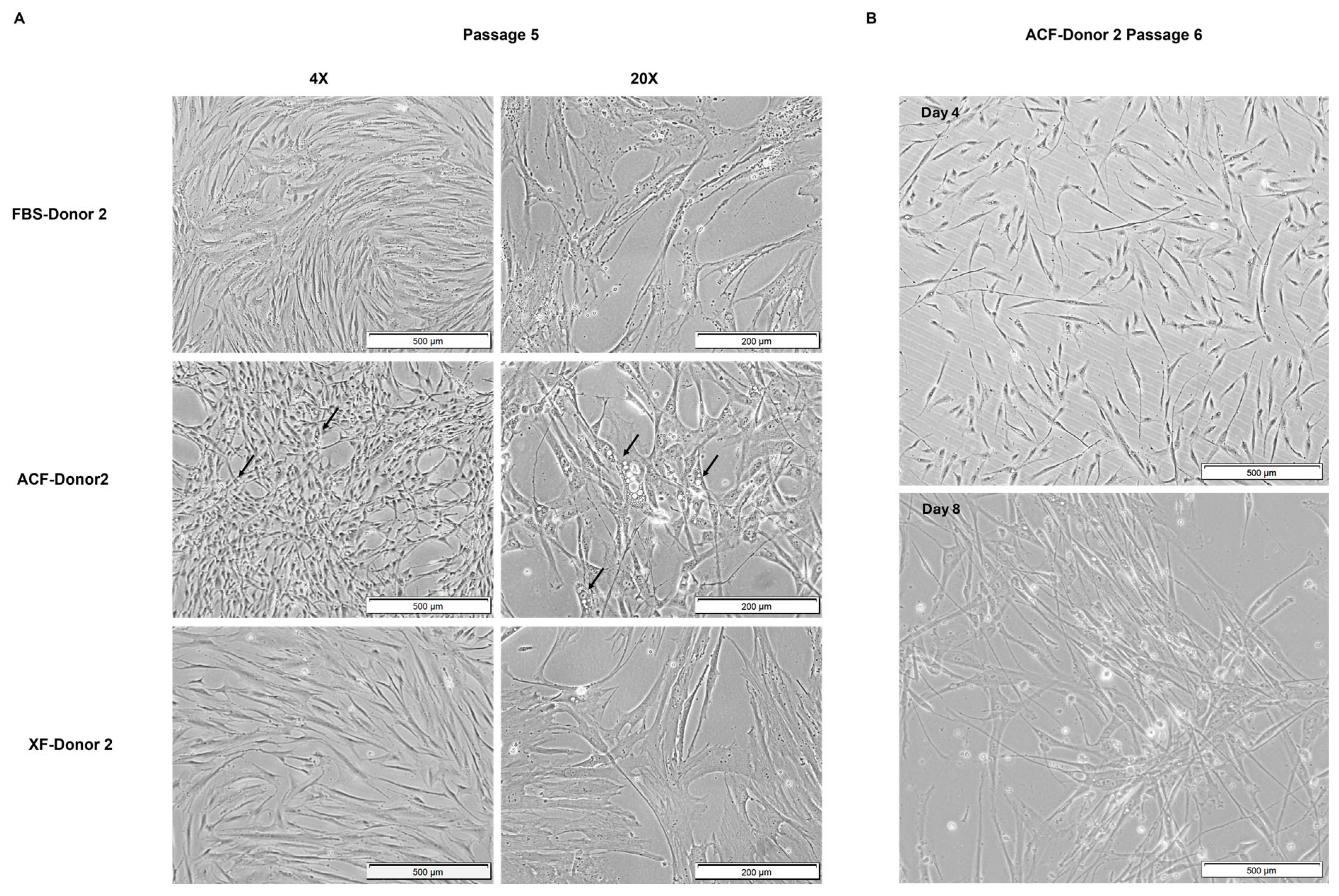
2.2.3. MSC Morphology at Higher Confluency and Later Passage
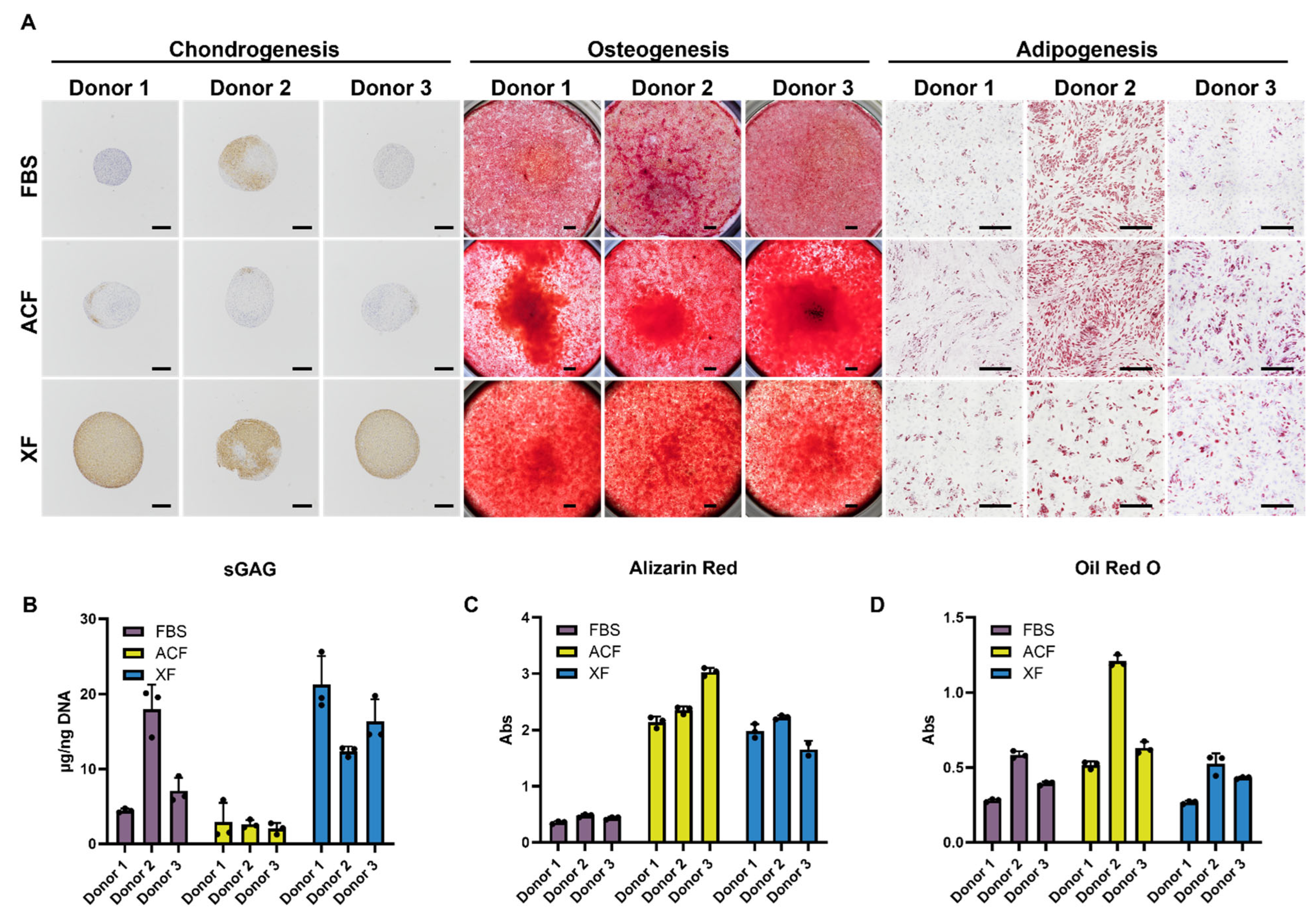
2.2.4. Trilineage Differentiation Assay
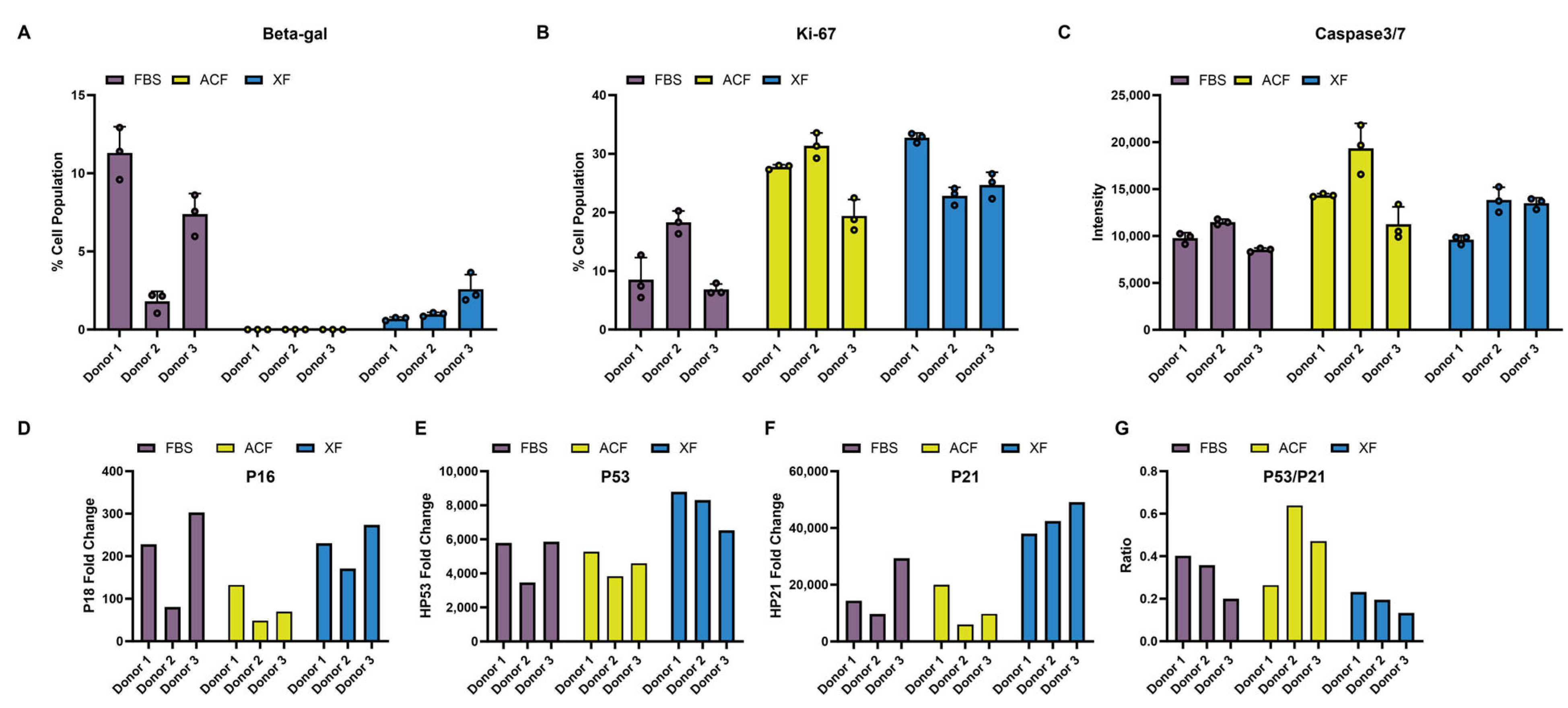
2.2.5. Comparison of MSC Proliferation, Senescence, and Apoptosis Markers
3. Discussion
4. Materials and Methods
4.1. MSC Cell Culture
4.2. Animal Experiment
4.3. Mechanical Strength Test
4.4. Micro-Computed Tomography (Micro-CT)
4.5. Histological and Immunohistochemical Staining
4.6. Trilineage Differentiation
4.7. Real-Time Polymerase Chain Reaction (qPCR)
4.8. Flow Cytometry
4.9. Ki-67 Immunofluorescence Staining
4.10. Beta-Gal Staining
4.11. Caspase-3/7 Assay
4.12. Image and Statistical Analysis
4.13. Selection of Articles for Review Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzella, M.; Walker, K.; Cormier, C.; Kapanowski, M.; Ishmakej, A.; Saifee, A.; Govind, Y.; Chaudhry, G.R. Regulation of self-renewal and senescence in primitive mesenchymal stem cells by Wnt and TGFβ signaling. Stem Cell Res. Ther. 2023, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; Denslin, V.; Ren, X.; Lee, C.S.; Yap, F.L.; Lee, E.H. Repair of Osteochondral Defects With Predifferentiated Mesenchymal Stem Cells of Distinct Phenotypic Character Derived From a Nanotopographic Platform. Am. J. Sports Med. 2020, 48, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Chi, Y.; Sun, T.; Gao, Y.; Dou, X.; Han, Z.; Xue, F.; Li, H.; Liu, W.; et al. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells in the treatment of refractory immune thrombocytopenia: A prospective, single arm, phase I trial. Signal Transduct. Target. Ther. 2024, 9, 102. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef]
- Yari, H.; Mikhailova, M.V.; Mardasi, M.; Jafarzadehgharehziaaddin, M.; Shahrokh, S.; Thangavelu, L.; Ahmadi, H.; Shomali, N.; Yaghoubi, Y.; Zamani, M.; et al. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: A groundbreaking cell-free approach. Stem Cell Res. Ther. 2022, 13, 423. [Google Scholar] [CrossRef]
- Bogliotti, Y.; Vander Roest, M.; Mattis, A.N.; Gish, R.G.; Peltz, G.; Anwyl, R.; Kivlighn, S.; Schuur, E.R. Clinical Application of Induced Hepatocyte-like Cells Produced from Mesenchymal Stromal Cells: A Literature Review. Cells 2022, 11, 1998. [Google Scholar] [CrossRef]
- Jin, X.; Liang, W.; Zhang, L.; Cao, S.; Yang, L.; Xie, F. Economic and Humanistic Burden of Osteoarthritis: An Updated Systematic Review of Large Sample Studies. PharmacoEconomics 2023, 41, 1453–1467. [Google Scholar] [CrossRef]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.Q.A.; Wong, K.L.; Shen, L.; Lim, J.Y.; Toh, W.S.; Lee, E.H.; Hui, J.H.P. Equivalent 10-Year Outcomes After Implantation of Autologous Bone Marrow–Derived Mesenchymal Stem Cells Versus Autologous Chondrocyte Implantation for Chondral Defects of the Knee. Am. J. Sports Med. 2019, 47, 2881–2887. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Li, J. Isolation, culture, and delivery considerations for the use of mesenchymal stem cells in potential therapies for acute liver failure. Front. Immunol. 2023, 14, 1243220. [Google Scholar] [CrossRef] [PubMed]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Schepici, G.; Gugliandolo, A.; Mazzon, E. Serum-Free Cultures: Could They Be a Future Direction to Improve Neuronal Differentiation of Mesenchymal Stromal Cells? Int. J. Mol. Sci. 2022, 23, 6391. [Google Scholar] [CrossRef]
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Yong, T.K.; Chan, A.; et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, M.H.; Jang, J.E.; Lee, J.E.; Yang, Y.; Choi, J.Y.; Kang, H.S.; Lee, U.; Choung, J.W.; Jung, H.; et al. Comparative analysis of mesenchymal stem cells cultivated in serum free media. Sci. Rep. 2022, 12, 8620. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.T.H.; Nguyen, L.T.; Than, U.T.T. Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application. Tissue Eng. Regen. Med. 2021, 18, 15–23. [Google Scholar] [CrossRef]
- Thamarath, S.S.; Tee, C.A.; Neo, S.H.; Yang, D.; Othman, R.; Boyer, L.A.; Han, J. Rapid and Live-Cell Detection of Senescence in Mesenchymal Stem Cells by Micro Magnetic Resonance Relaxometry. Stem Cells Transl. Med. 2023, 12, 266–280. [Google Scholar] [CrossRef]
- Lee, W.C.; Shi, H.; Poon, Z.; Nyan, L.M.; Kaushik, T.; Shivashankar, G.V.; Chan, J.K.; Lim, C.T.; Han, J.; Van Vliet, K.J. Multivariate biophysical markers predictive of mesenchymal stromal cell multipotency. Proc. Natl. Acad. Sci. USA 2014, 111, E4409–E4418. [Google Scholar] [CrossRef] [PubMed]
- Poon, Z.; Lee, W.C.; Guan, G.; Nyan, L.M.; Lim, C.T.; Han, J.; Van Vliet, K.J. Bone Marrow Regeneration Promoted by Biophysically Sorted Osteoprogenitors From Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2014, 4, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Makhija, E.; Zheng, Y.; Jiahao, W.; Leong, H.; Othman, R.; Ng, E.; Lee, E.; Kellogg, L.; Lee, Y.; Yu, H.; et al. Topological defects in self-assembled patterns of mesenchymal stromal cells in vitro are predictive attributes of condensation and chondrogenesis. PLoS ONE 2024, 19, e0297769. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Neo, S.H.; Yang, D.; Jeon, H.; Tee, C.A.; Denslin, V.; Lin, D.J.; Lee, E.H.; Boyer, L.A.; et al. Size-Based Microfluidic-Enriched Mesenchymal Stem Cell Subpopulations Enhance Articular Cartilage Repair. Am. J. Sports Med. 2024, 52, 503–515. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Muschler, G.F. Improved biological performance of human cartilage-derived progenitors in platelet lysate xenofree media in comparison to fetal bovine serum media. Curr. Res. Transl. Med. 2022, 70, 103353. [Google Scholar] [CrossRef]
- Chen, S.; Meng, L.; Wang, S.; Xu, Y.; Chen, W.; Wei, J. Effect assessment of a type of xeno-free and serum-free human adipose-derived mesenchymal stem cells culture medium by proliferation and differentiation capacities. Cytotechnology 2023, 75, 403–420. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Boonyuen, S.; Apinyauppatham, K.; Pripatnanont, P. Effects of Oral Cavity Stem Cell Sources and Serum-Free Cell Culture on Hydrogel Encapsulation of Mesenchymal Stem Cells for Bone Regeneration: An In Vitro Investigation. Bioengineering 2024, 11, 59. [Google Scholar] [CrossRef]
- Pinto, D.S.; Ahsan, T.; Serra, J.; Fernandes-Platzgummer, A.; Cabral, J.M.S.; da Silva, C.L. Modulation of the in vitro angiogenic potential of human mesenchymal stromal cells from different tissue sources. J. Cell. Physiol. 2020, 235, 7224–7238. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.; Fernandes-Platzgummer, A.; Carmelo, J.G.; Swiech, K.; Covas, D.T.; Cabral, J.M.; da Silva, C.L. Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells. Biotechnol. J. 2016, 11, 1048–1059. [Google Scholar] [CrossRef]
- Santos, F.; Andrade, P.Z.; Abecasis, M.M.; Gimble, J.M.; Chase, L.G.; Campbell, A.M.; Boucher, S.; Vemuri, M.C.; Silva, C.L.; Cabral, J.M. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: A microcarrier-based culture system under xeno-free conditions. Tissue Eng. Part C Methods 2011, 17, 1201–1210. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, J.; Park, S.R.; Min, B.H.; Choi, B.H. Characterization of Human Fetal Cartilage Progenitor Cells During Long-Term Expansion in a Xeno-Free Medium. Tissue Eng. Regen. Med. 2018, 15, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Wuchter, P.; Vetter, M.; Saffrich, R.; Diehlmann, A.; Bieback, K.; Ho, A.D.; Horn, P. Evaluation of GMP-compliant culture media for in vitro expansion of human bone marrow mesenchymal stromal cells. Exp. Hematol. 2016, 44, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.G.; Yang, S.; Zachar, V.; Yang, Z.; Lakshmipathy, U.; Bradford, J.; Boucher, S.E.; Vemuri, M.C. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 750–758. [Google Scholar] [CrossRef]
- Naung, N.Y.; Duncan, W.; Silva, R.; Coates, D. Localization and characterization of human palatal periosteum stem cells in serum-free, xeno-free medium for clinical use. Eur. J. Oral Sci. 2019, 127, 99–111. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, N.; Shao, Q.; Zhu, D.; Feng, Y.; Wang, Y.; Yu, M.; Ren, L.; Chen, Q.; Yang, G. Light-controlled scaffold- and serum-free hard palatal-derived mesenchymal stem cell aggregates for bone regeneration. Bioeng. Transl. Med. 2023, 8, e10334. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef]
- Thamm, K.; Möbus, K.; Towers, R.; Baertschi, S.; Wetzel, R.; Wobus, M.; Segeletz, S. A chemically defined biomimetic surface for enhanced isolation efficiency of high-quality human mesenchymal stromal cells under xenogeneic/serum-free conditions. Cytotherapy 2022, 24, 1049–1059. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Stolzing, A.; Fabian, C.; Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Kara, B.; Hewitt, C.J. Serum-free process development: Improving the yield and consistency of human mesenchymal stromal cell production. Cytotherapy 2015, 17, 1524–1535. [Google Scholar] [CrossRef]
- Heathman, T.R.; Glyn, V.A.; Picken, A.; Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Kara, B.; Hewitt, C.J. Expansion, harvest and cryopreservation of human mesenchymal stem cells in a serum-free microcarrier process. Biotechnol. Bioeng. 2015, 112, 1696–1707. [Google Scholar] [CrossRef]
- Aussel, C.; Busson, E.; Vantomme, H.; Peltzer, J.; Martinaud, C. Quality assessment of a serum and xenofree medium for the expansion of human GMP-grade mesenchymal stromal cells. PeerJ 2022, 10, e13391. [Google Scholar] [CrossRef]
- Lensch, M.; Muise, A.; White, L.; Badowski, M.; Harris, D. Comparison of Synthetic Media Designed for Expansion of Adipose-Derived Mesenchymal Stromal Cells. Biomedicines 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Gottipamula, S.; Ashwin, K.M.; Muttigi, M.S.; Kannan, S.; Kolkundkar, U.; Seetharam, R.N. Isolation, expansion and characterization of bone marrow-derived mesenchymal stromal cells in serum-free conditions. Cell Tissue Res. 2014, 356, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Saqi, S.H.; Saliem, M.; Quezada, H.C.; Ekblad, Å.; Jonasson, A.F.; Hovatta, O.; Götherström, C. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2015, 16, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Al-Saqi, S.H.; Saliem, M.; Asikainen, S.; Quezada, H.C.; Ekblad, A.; Hovatta, O.; Le Blanc, K.; Jonasson, A.F.; Götherström, C. Defined serum-free media for in vitro expansion of adipose-derived mesenchymal stem cells. Cytotherapy 2014, 16, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Monitoring the biology stability of human umbilical cord-derived mesenchymal stem cells during long-term culture in serum-free medium. Cell Tissue Bank. 2014, 15, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Hashimoto, Y.; Tensho, K.; Wakitani, S.; Takagi, M. Xeno-free proliferation of human bone marrow mesenchymal stem cells. Cytotechnology 2012, 64, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, E.; Lv, W.; Dong, X.; He, L.; Wang, Y.; Zhang, Y. A Chemically Defined Serum-Free Culture System for Spontaneous Human Mesenchymal Stem Cell Spheroid Formation. Stem Cells Int. 2020, 2020, 1031985. [Google Scholar] [CrossRef]
- Krutty, J.D.; Koesser, K.; Schwartz, S.; Yun, J.; Murphy, W.L.; Gopalan, P. Xeno-Free Bioreactor Culture of Human Mesenchymal Stromal Cells on Chemically Defined Microcarriers. ACS Biomater. Sci. Eng. 2021, 7, 617–625. [Google Scholar] [CrossRef]
- Lembong, J.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T. Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering 2020, 7, 73. [Google Scholar] [CrossRef]
- Yi, X.; Chen, F.; Liu, F.; Peng, Q.; Li, Y.; Li, S.; Du, J.; Gao, Y.; Wang, Y. Comparative separation methods and biological characteristics of human placental and umbilical cord mesenchymal stem cells in serum-free culture conditions. Stem Cell Res. Ther. 2020, 11, 183. [Google Scholar] [CrossRef]
- Moreira, F.; Mizukami, A.; de Souza, L.E.B.; Cabral, J.M.S.; da Silva, C.L.; Covas, D.T.; Swiech, K. Successful Use of Human AB Serum to Support the Expansion of Adipose Tissue-Derived Mesenchymal Stem/Stromal Cell in a Microcarrier-Based Platform. Front. Bioeng. Biotechnol. 2020, 8, 307. [Google Scholar] [CrossRef]
- Fabre, H.; Ducret, M.; Degoul, O.; Rodriguez, J.; Perrier-Groult, E.; Aubert-Foucher, E.; Pasdeloup, M.; Auxenfans, C.; McGuckin, C.; Forraz, N.; et al. Characterization of Different Sources of Human MSCs Expanded in Serum-Free Conditions with Quantification of Chondrogenic Induction in 3D. Stem Cells Int. 2019, 2019, 2186728. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, J.; Han, L.; Jiang, X.; Yan, L.; Hao, J.; Wang, H. Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem Cell Res. Ther. 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Phermthai, T.; Tungprasertpol, K.; Julavijitphong, S.; Pokathikorn, P.; Thongbopit, S.; Wichitwiengrat, S. Successful derivation of xeno-free mesenchymal stem cell lines from endometrium of infertile women. Reprod. Biol. 2016, 16, 261–268. [Google Scholar] [CrossRef]
- Wu, X.; Kang, H.; Liu, X.; Gao, J.; Zhao, K.; Ma, Z. Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord. Cell Prolif. 2016, 49, 579–588. [Google Scholar] [CrossRef]
- Riordan, N.H.; Madrigal, M.; Reneau, J.; de Cupeiro, K.; Jiménez, N.; Ruiz, S.; Sanchez, N.; Ichim, T.E.; Silva, F.; Patel, A.N. Scalable efficient expansion of mesenchymal stem cells in xeno free media using commercially available reagents. J. Transl. Med. 2015, 13, 232. [Google Scholar] [CrossRef]
- Kirsch, M.; Rach, J.; Handke, W.; Seltsam, A.; Pepelanova, I.; Strauß, S.; Vogt, P.; Scheper, T.; Lavrentieva, A. Comparative Analysis of Mesenchymal Stem Cell Cultivation in Fetal Calf Serum, Human Serum, and Platelet Lysate in 2D and 3D Systems. Front. Bioeng. Biotechnol. 2020, 8, 598389. [Google Scholar] [CrossRef]
- Cowper, M.; Frazier, T.; Wu, X.; Curley, L.; Ma, M.H.; Mohiuddin, O.A.; Dietrich, M.; McCarthy, M.; Bukowska, J.; Gimble, J.M. Human Platelet Lysate as a Functional Substitute for Fetal Bovine Serum in the Culture of Human Adipose Derived Stromal/Stem Cells. Cells 2019, 8, 724. [Google Scholar] [CrossRef]
- Laitinen, A.; Oja, S.; Kilpinen, L.; Kaartinen, T.; Möller, J.; Laitinen, S.; Korhonen, M.; Nystedt, J. A robust and reproducible animal serum-free culture method for clinical-grade bone marrow-derived mesenchymal stromal cells. Cytotechnology 2016, 68, 891–906. [Google Scholar] [CrossRef]
- Mojica-Henshaw, M.P.; Jacobson, P.; Morris, J.; Kelley, L.; Pierce, J.; Boyer, M.; Reems, J.A. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy 2013, 15, 1458–1468. [Google Scholar] [CrossRef]
- Shih, D.T.; Chen, J.C.; Chen, W.Y.; Kuo, Y.P.; Su, C.Y.; Burnouf, T. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion 2011, 51, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.T.; Tanavde, V.M.; Hui, J.H.; Lee, E.H. Upregulation of Adipogenesis and Chondrogenesis in MSC Serum-Free Culture. Cell Med. 2011, 2, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, P.; Balasubramanian, S.; Majumdar, A.S.; Ta, M. Isolation, characterization, and gene expression analysis of Wharton’s jelly-derived mesenchymal stem cells under xeno-free culture conditions. Stem Cells Cloning 2011, 4, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Bernabeu, C.; Smadja, D.M. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-β. Front. Med. 2019, 6, 10. [Google Scholar] [CrossRef]
- Joshkon, A.; Heim, X.; Dubrou, C.; Bachelier, R.; Traboulsi, W.; Stalin, J.; Fayyad-Kazan, H.; Badran, B.; Foucault-Bertaud, A.; Leroyer, A.S.; et al. Role of CD146 (MCAM) in Physiological and Pathological Angiogenesis-Contribution of New Antibodies for Therapy. Biomedicines 2020, 8, 633. [Google Scholar] [CrossRef]
- Valdivia, A.; Avalos, A.M.; Leyton, L. Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: Insights into adhesomes, mechanical forces, and signaling pathways. Front. Cell Dev. Biol. 2023, 11, 1221306. [Google Scholar] [CrossRef]
- Brohlin, M.; Kelk, P.; Wiberg, M.; Kingham, P.J. Effects of a defined xeno-free medium on the growth and neurotrophic and angiogenic properties of human adult stem cells. Cytotherapy 2017, 19, 629–639. [Google Scholar] [CrossRef]
- Cimino, M.; Gonçalves, R.M.; Bauman, E.; Barroso-Vilares, M.; Logarinho, E.; Barrias, C.C.; Martins, M.C.L. Optimization of the use of a pharmaceutical grade xeno-free medium for in vitro expansion of human mesenchymal stem/stromal cells. J. Tissue Eng. Regen. Med. 2018, 12, e1785–e1795. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Elango, R.; Manikandan, M.; Siyal, A.-A.; Ali, D.; Al-Rikabi, A.; Hamam, D.; Hamam, R.; Benabdelkamel, H.; Masood, A.; et al. MicroRNA-3148 acts as molecular switch promoting malignant transformation and adipocytic differentiation of immortalized human bone marrow stromal cells via direct targeting of the SMAD2/TGFβ pathway. Cell Death Discov. 2020, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; Wan, D.C.; Glotzbach, J.P.; Hyun, J.; Januszyk, M.; Montoro, D.; Sorkin, M.; James, A.W.; Nelson, E.R.; Li, S.; et al. CD105 Protein Depletion Enhances Human Adipose-derived Stromal Cell Osteogenesis through Reduction of Transforming Growth Factor β1 (TGF-β1) signaling. J. Biol. Chem. 2011, 286, 39497–39509. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Q.; Zhang, N.; Du, X.; Xu, G.; Yan, X. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Signal Transduct. Target. Ther. 2020, 5, 148. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Lee, S.S.; Vũ, T.T.; Weiss, A.S.; Yeo, G.C. Stress-induced senescence in mesenchymal stem cells: Triggers, hallmarks, and current rejuvenation approaches. Eur. J. Cell Biol. 2023, 102, 151331. [Google Scholar] [CrossRef]
- Gutu, N.; Binish, N.; Keilholz, U.; Herzel, H.; Granada, A.E. p53 and p21 dynamics encode single-cell DNA damage levels, fine-tuning proliferation and shaping population heterogeneity. Commun. Biol. 2023, 6, 1196. [Google Scholar] [CrossRef]
- Dexheimer, V.; Frank, S.; Richter, W. Proliferation as a Requirement for In Vitro Chondrogenesis of Human Mesenchymal Stem Cells. Stem Cells Dev. 2012, 21, 2160–2169. [Google Scholar] [CrossRef]
- Haasters, F.; Prall, W.C.; Anz, D.; Bourquin, C.; Pautke, C.; Endres, S.; Mutschler, W.; Docheva, D.; Schieker, M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J. Anat. 2009, 214, 759–767. [Google Scholar] [CrossRef]
- Mosna, F.; Sensebé, L.; Krampera, M. Human bone marrow and adipose tissue mesenchymal stem cells: A user’s guide. Stem Cells Dev 2010, 19, 1449–1470. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Yang, Z.; Chi, Y.; Meng, L.; Mao, A.; Yan, S.; Hu, S.; Zhang, J.; Zhang, Y.; et al. Human mesenchymal stem cells possess different biological characteristics but do not change their therapeutic potential when cultured in serum free medium. Stem Cell Res. Ther. 2014, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.R.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef]
- de Araújo Farias, V.; Carrillo-Gálvez, A.B.; Martín, F.; Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, K.-H.; Young, T.-H.; Cheng, N.-C. The influence of fibroblast growth factor 2 on the senescence of human adipose-derived mesenchymal stem cells during long-term culture. Stem Cells Transl. Med. 2020, 9, 518–530. [Google Scholar] [CrossRef]

| SF/XF Medium | MSC Source | MSC Characterization Marker | Chondrogenesis | Osteo | Adipo | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Trend | Method | End-Point | ||||||
| StemPro™ MSC SFM, Gibco | hADSC | +ve: CD73, CD90, CD105 −ve: CD11b, CD19, CD34, CD45, HLA-DR | - | 3D aggregate chondrogenic induction, followed by histological alcian blue staining | 21 days | - | [26] | |
| hBFP, hLP, and hDP derived MSCs | +ve: CD73, CD90, CD105 −ve: CD11b, CD19, CD34, CD45, HLA-DR | NA | NA | NA | ↑ | NA | [27] | |
| hBMSC, hADSC, hUCSC | +ve: CD73, CD90 and CD105 −ve: CD14, CD19, CD31, CD34, CD45, CD80, HLA-DR | - | 3D aggregate chondrogenic induction, brightfield image examination | 14 days | - | - | [28] | |
| hUCSC | +ve: CD73, CD90, CD105 −ve: CD31, CD80, HLA-DR | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | 15 days | - | - | [29] | |
| hADSC, hBMSC | +ve: CD73, CD90, CD105 −ve: CD31, CD80, HLA-DR | - | High-density micromass cultures, followed by alcian blue staining | 14 days | - | - | [30] | |
| hFCPC | +ve: CD44, CD73, CD90, CD105, CD166 −ve: SSEA-4 and TRA-1-60 | - | 3D aggregate chondrogenic induction, followed by histological safranin O staining | 14 days | - | - | [31] | |
| hBMSC | +ve: CD73, CD90, CD105, CD146 −ve: CD14, CD19, CD34, CD45, HLA-DR | ↑ | High-density micromass cultures, followed by alcian blue histological staining | 21 days | - | - | [32] | |
| hBMSC | +ve: CD73, CD90, CD105 −ve: CD14, CD19, CD34, CD45, HLA-DR | ↑ | 3D aggregate chondrogenic induction, followed by immunohistochemical COLII staining | 14–20 days | - | - | [33] | |
| CellCor, X Cell Therapeutics; StemPro™ MSC SFM, Gibco; Mesencult™-XF, Stem Cell Technologies | hBMSC, hADSC, hUCSC | +ve: CD73, CD90, CD105, CD146 −ve: CD14, CD34, CD45, HLA-DR | - | 2D induction culture followed by alcian blue staining | NA | ↑ | - | [18] |
| E8 medium, ThermoFisher | hPLMSC | +ve: CD73, CD90, CD105 | - | High-density micromass cultures, followed by alcian blue staining | 16 days | - | - | [34] |
| MSC NutriStem® XF Medium, Biological Industry | mPMSC | +ve: CD29, CD44, CD90 −ve: CD34, CD45, CD146 | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | 7 days | - | - | [35] |
| MSC NutriStem® XF Medium + 0.5% hPL, Biological Industry | hCMSC | +ve: CD73, CD90, CD105 | ↑ | 3D aggregate chondrogenic induction, followed by alcian blue staining | 14 days | ↑ | ↑ | [25] |
| RoosterNourish XF/Low Serum, RoosterBio; StemMACS™ MSC Expansion Media XF, Miltenyi; PLTMax Human Platelet Lysate, Sigma; StemXVivo™, Lonza | hBMSC | +ve: CD73, CD90, CD105 −ve: CD34, CD45, HLA-DR | - | 3D aggregate chondrogenic induction | NA | - | - | [36] |
| PRIME-XV MSC Expansion XSFM medium, FUJIFILM Irvine Scientific | hBMSC | +ve: CD44, CD73, CD90, CD105, CD146, CD166 −ve: CD11b, CD14, CD34, CD45, | - | 2D induction culture followed by alcian blue staining | 28 days | - | - | [37] |
| hBMSC | +ve: CD73, CD90, CD105 −ve: CD34, HLA-DR | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | 21 days | - | - | [38] | |
| hBMSC | +ve: CD73, CD90, CD105 −ve: CD34, HLA-DR | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | 21 days | - | - | [39] | |
| PowerStem MSC1 Medium, PAN Biotech; StemMACS™ MSC Expansion Media XF, Miltenyi | hBMSC | +ve: CD73, CD90, CD105 −ve: CD34, HLA-DR | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | 21 days | - | - | [39] |
| MSC-Brew GMP Medium, Miltenyi | hBMSC | +ve: CD73, CD90, CD105, and CD146 −ve: CD34, CD45, HLA-DR | NA | NA | NA | ↑ | ↑ | [40] |
| StemMACS™ MSC Expansion Media XF, Miltenyi; PLTMax Human Platelet Lysate, Sigma; MesenCult-hPL media, StemCell Technologies | hADSC | +ve: CD73, CD90, CD105 | NA | NA | NA | - | - | [41] |
| BD Mosaic™ Mesenchymal Stem Cell SF media, ThermoFisher; MesenCult™-XF, Stem Cell Technologies | hBMSC | +ve: CD73, CD90, CD105, CD166 −ve: CD14, CD19, CD34, CD45, CD133, HLA-DR | - | High-density micromass cultures, followed by safronin O staining | NA | ↑ | - | [42] |
| MesenCult™-XF, Stem Cell Technologies | hBMSC, hADSC | +ve: CD73, CD90, CD105, HLA-I −ve: CD3, CD14, CD31, CD34, CD45, CD80 | NA | NA | NA | - | - | [43] |
| hBMSC, hADSC | +ve: CD73, CD105, CD90, HIL-A-I −ve: CD3, CD14, CD34, CD45, HIL-A-II, CD80 | NA | NA | NA | ↑ | - | [44] | |
| hUCSC | +ve: CD73, CD90, CD105 −ve: CD14, CD34, CD45, CD79a, HLA-DR | - | High-density micromass cultures, followed by alcian blue staining | 14 days or longer | - | - | [45] | |
| hBMSC | +ve: CD90, CD166 | ↑ | 3D aggregate chondrogenic induction, followed by aggrecan gene expression via RT-PCR | 21 days | NA | NA | [46] | |
| TeSR-E8 medium, StemCell Technologies | hADSC, hBMSC, hDPMSC | +ve: CD73, CD90, CD105, CD44 | NA | NA | NA | ↑ | ↓ | [47] |
| RoosterNourish MSC-XF xeno-free media, Roosterbio | hBMSC | NA | NA | NA | NA | - | - | [48] |
| High Performance XF Media Kit, RoosterBio | hBMSC | +ve: CD73, CD90, CD105, CD166 −ve: CD14, CD34, CD45 | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | NA | - | - | [49] |
| SF medium sc-82,013-G, Haoyang | hPDSC | +ve: CD73, CD90, CD105, CD44 | NA | NA | NA | - | - | [50] |
| Human AB serum | hADSC | +ve: CD54, CD73, CD90, CD105 −ve: CF14, CD19, CD34, CD45, CD80 and HLA-DR | - | High-density micromass cultures, followed by alcian blue staining | 15 days | - | - | [51] |
| SF SPE-IV defined medium, ABCell-Bio | hADSC, hBMSC, hUCSC, hDPMSC | +ve: CD10, CD13, CD29, CD44, CD73, CD90, CD105, CD166, D7-Fib, HLA-ABC, CD15, CD49a, CD56, CD106, CD146, CD340 −ve: CD14, CD31, CD33, Cd34, CD45, CD79a, CD133, CD184, HLA-DR, HLA-G, CD271, alpha10ITG, Stro-1, MSCA-1 | - | 3D aggregate chondrogenic induction, followed by immunohistochemical COLII staining | 28 days | - | - | [52] |
| MSCGM-CD, Lonza | hPDSC | +ve: CD73, CD90, CD105 −ve: CD14, CD19, CD34, CD45, HLA-DR | - | 3D aggregate chondrogenic induction, followed by histological alcian blue staining | 21 days | - | - | [53] |
| Allogenic human umbilical cord serum | hESC | +ve: CD29, CD34, CD73, CD90, CD105 −ve: CD45 | NA | NA | NA | - | - | [54] |
| Homemade chemically defined medium | hUCSC | +ve: CD13, CD29, CD44, CD73, CD90, CD105, CD166, HLA-ABC −ve: CD14, CD19, CD34, CD45, HLA-DR | NA | NA | NA | ↑ | - | [55] |
| XcytePLUS™ media | hWJMSC | +ve: CD73, CD90 and CD105 −ve: CD34, CD45 | - | 3D aggregate chondrogenic induction, followed by alcian blue staining | 21 days | ↑ | ↑ | [56] |
| hPL | hADSC | NA | ↑ | 2D induction culture followed by alcian blue staining | 7, 14, and 21 days | ↑ | NA | [57] |
| hADSC | +ve: CD29, CD73, CD90, CD105 −ve: CD34, CD45, CD31 | ↑ | 3D aggregate chondrogenic induction, followed by histological alcian blue staining and total RNA quantification for COLI, COLII, aggrecan and matrilin 1 | 14 days | ↑ | ↓ | [58] | |
| hBMSC | +ve: CD13, CD29, CD44, CD49e, CD73, CD90, CD105, HLA-ABC −ve:CD14, CD19, CD45, HLA-DR | - | 3D aggregate chondrogenic induction, followed by alcian blue histological staining | 14 days | - | - | [59] | |
| Human MSC, Lonza | +ve: CD73, CD90, CD105 −ve: CD34, CD45, HLA-DR | - | High-density micromass cultures, followed by alcian blue staining | 21 days | ↑ | - | [60] | |
| hADSC | +ve: CD29, CDD44, CD73, CD90, CD105, CD166, HLA-I −ve: CD31, HLA-DR | ↑ | High-density micromass cultures, followed by alcian blue staining | 47 h | - | - | [61] | |
| Knockout™ Serum Replacement, Invitrogen | hBMSC | +ve: CD29, CD44, CD73, CD90 | - | 3D aggregate chondrogenic induction, followed by aggrecan histological staining and immunohistochemical staining for COLI, COLII and COLX | 28 days | - | - | [62] |
| Allogeneic human serum | hWJMSC | +ve: CD44, CD73, CD90, CD105 −ve: CD34, HLA-DR | - | 2D culture followed by alcian blue staining | 18–20 days | - | - | [63] |
| Medium | Donor | CD44 | CD90 | CD105 | CD146 | ||||
|---|---|---|---|---|---|---|---|---|---|
| %Positive | Intensity | %Positive | Intensity | %Positive | Intensity | %Positive | Intensity | ||
| FBS | Donor 1 | 100.00% | 426,771 | 99.89% | 136783 | 99.27% | 485,392 | 99.08% | 47,395 |
| Donor 2 | 100.00% | 515,385 | 99.98% | 55726 | 98.99% | 548,620 | 99.56% | 97,516 | |
| Donor 3 | 98.45% | 356,204 | 99.96% | 111981 | 99.13% | 540,016 | 99.58% | 75,128 | |
| ACF | Donor 1 | 100.00% | 386,393 | 99.97% | 28899 | 99.97% | 106,591 | 97.04% | 94,525 |
| Donor 2 | 100.00% | 341,008 | 100.00% | 32065 | 99.98% | 92,335 | 96.88% | 73,116 | |
| Donor 3 | 100.00% | 306,765 | 99.98% | 24265 | 99.99% | 123,255 | 99.96% | 108,784 | |
| XF | Donor 1 | 99.92% | 435,363 | 99.91% | 110798 | 99.98% | 227,249 | 68.30% | 21,207 |
| Donor 2 | 99.91% | 404,182 | 99.97% | 91289 | 99.96% | 94,691 | 89.73% | 35,109 | |
| Donor 3 | 99.97% | 440,183 | 100.00% | 121705 | 99.81% | 114,696 | 70.50% | 29,508 | |
| Age | Gender | Race | Company | |
|---|---|---|---|---|
| Donor 1 | 19 | F | C | Lonza |
| Donor 2 | 24 | M | B | Lonza |
| Donor 3 | 37 | M | C | Stem Cell Technologies |
| Primer | Forward Sequences | Reverse Sequences |
|---|---|---|
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
| P16 | GGGTTTTCGTGGTTCACATCC | CTAGACGCTGGCTCCTCAGTA |
| P21 | AGTCAGTTCCTTGTGGAGCC | GCATGGGTTCTGACGGACAT |
| P53 | ACAGCTTTGAGGTGCGTGTTT | CCCTTTCTTGCGGAGATTCTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Yang, Y.; Zhang, H.; Zhang, Y.; Wu, Y.; Denslin, V.; Othman, R.B.; Yang, Z.; Han, J. Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair. Int. J. Mol. Sci. 2024, 25, 10627. https://doi.org/10.3390/ijms251910627
Kang M, Yang Y, Zhang H, Zhang Y, Wu Y, Denslin V, Othman RB, Yang Z, Han J. Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair. International Journal of Molecular Sciences. 2024; 25(19):10627. https://doi.org/10.3390/ijms251910627
Chicago/Turabian StyleKang, Meiqi, Yanmeng Yang, Haifeng Zhang, Yuan Zhang, Yingnan Wu, Vinitha Denslin, Rashidah Binte Othman, Zheng Yang, and Jongyoon Han. 2024. "Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair" International Journal of Molecular Sciences 25, no. 19: 10627. https://doi.org/10.3390/ijms251910627
APA StyleKang, M., Yang, Y., Zhang, H., Zhang, Y., Wu, Y., Denslin, V., Othman, R. B., Yang, Z., & Han, J. (2024). Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair. International Journal of Molecular Sciences, 25(19), 10627. https://doi.org/10.3390/ijms251910627







