Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice
Abstract
1. Introduction
2. Results
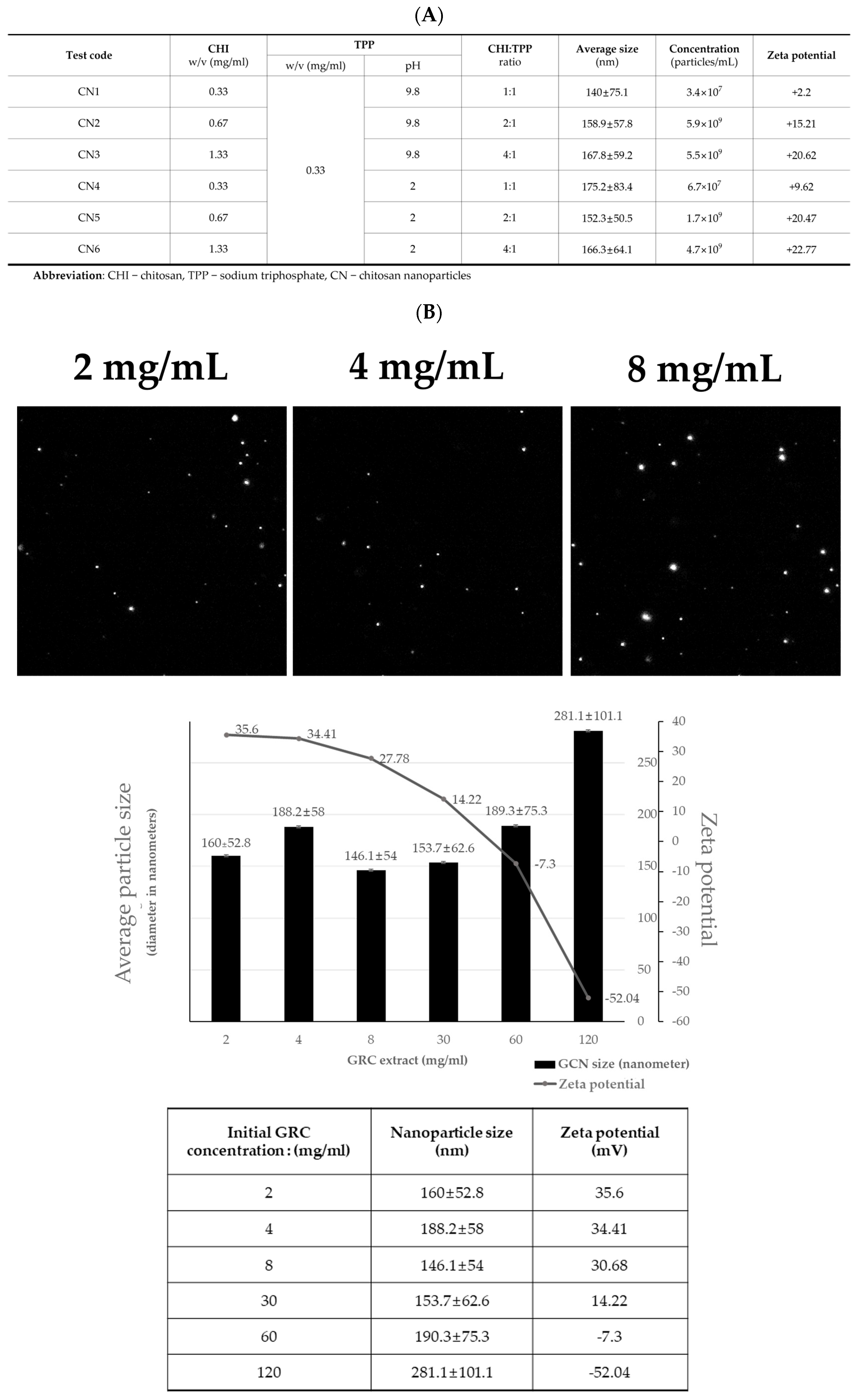
2.1. GCN Synthesis
2.2. CN Attenuates Inflammatory Cell Infiltration in PM-Treated Lung Tissue
2.3. GCN Alleviated PM-Induced Inflammatory Cell Infiltration in BALF
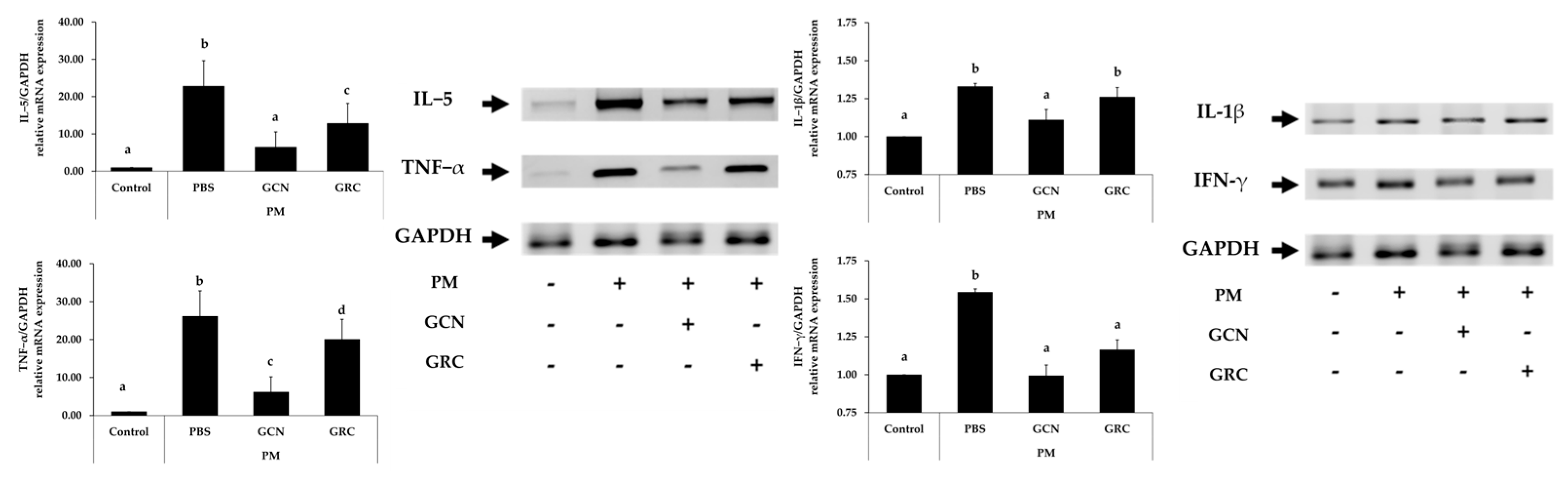
2.4. GCN Attenuates Cytokine Expression in PM-Treated Lung Tissue
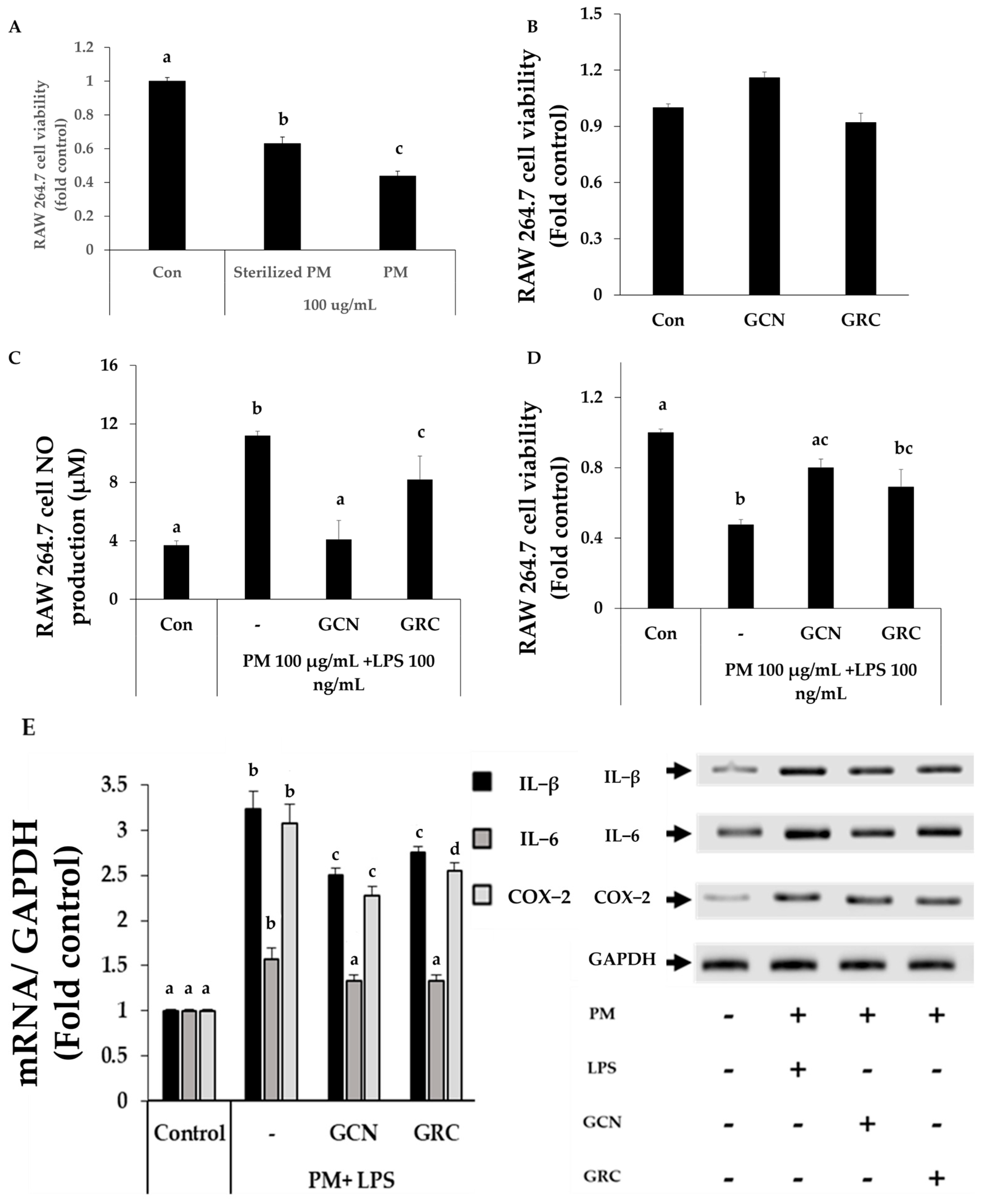
2.5. GCN Inhibits the Expression of Pro-Inflammatory Cytokines in PM-Stimulated RAW264.7 Cells
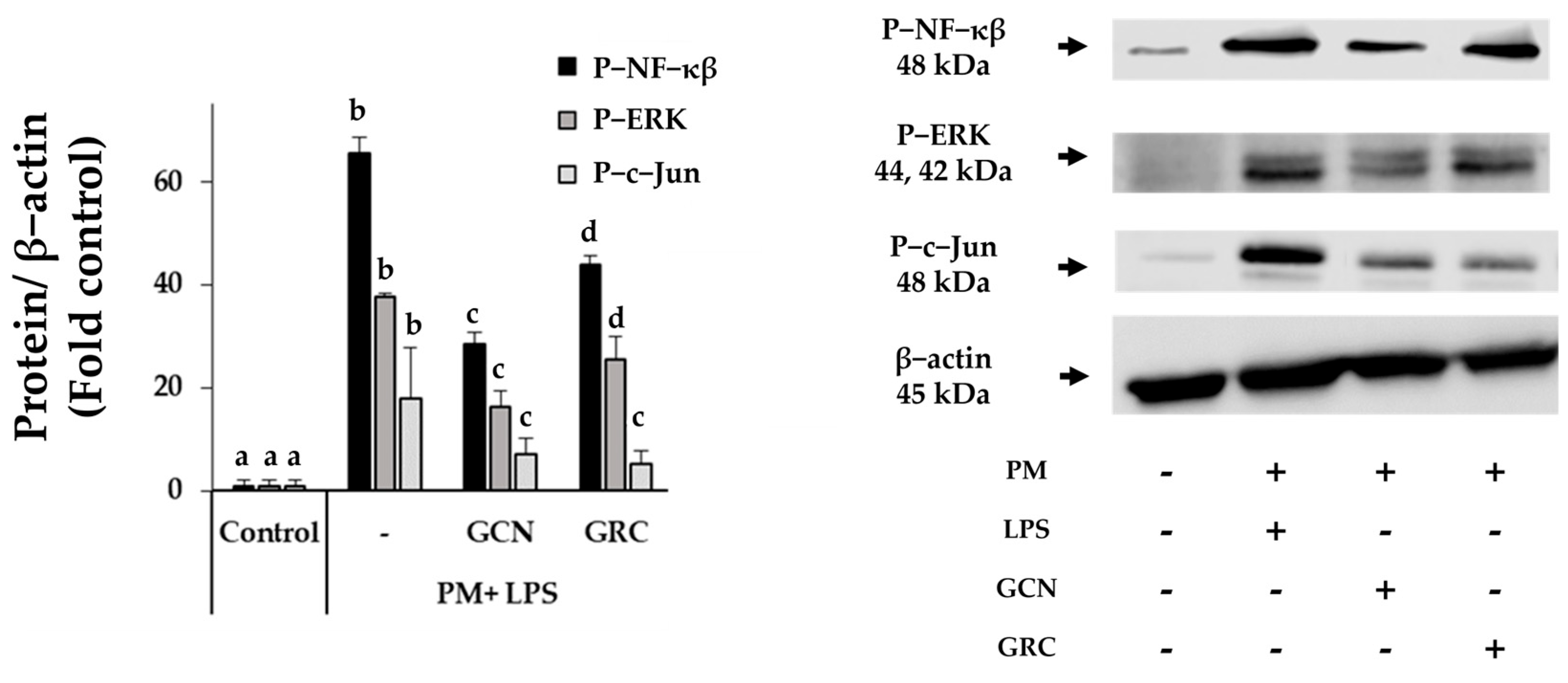
2.6. GCN Inhibits PM-Induced Activation of NF-κB/MAPK Signaling Pathways
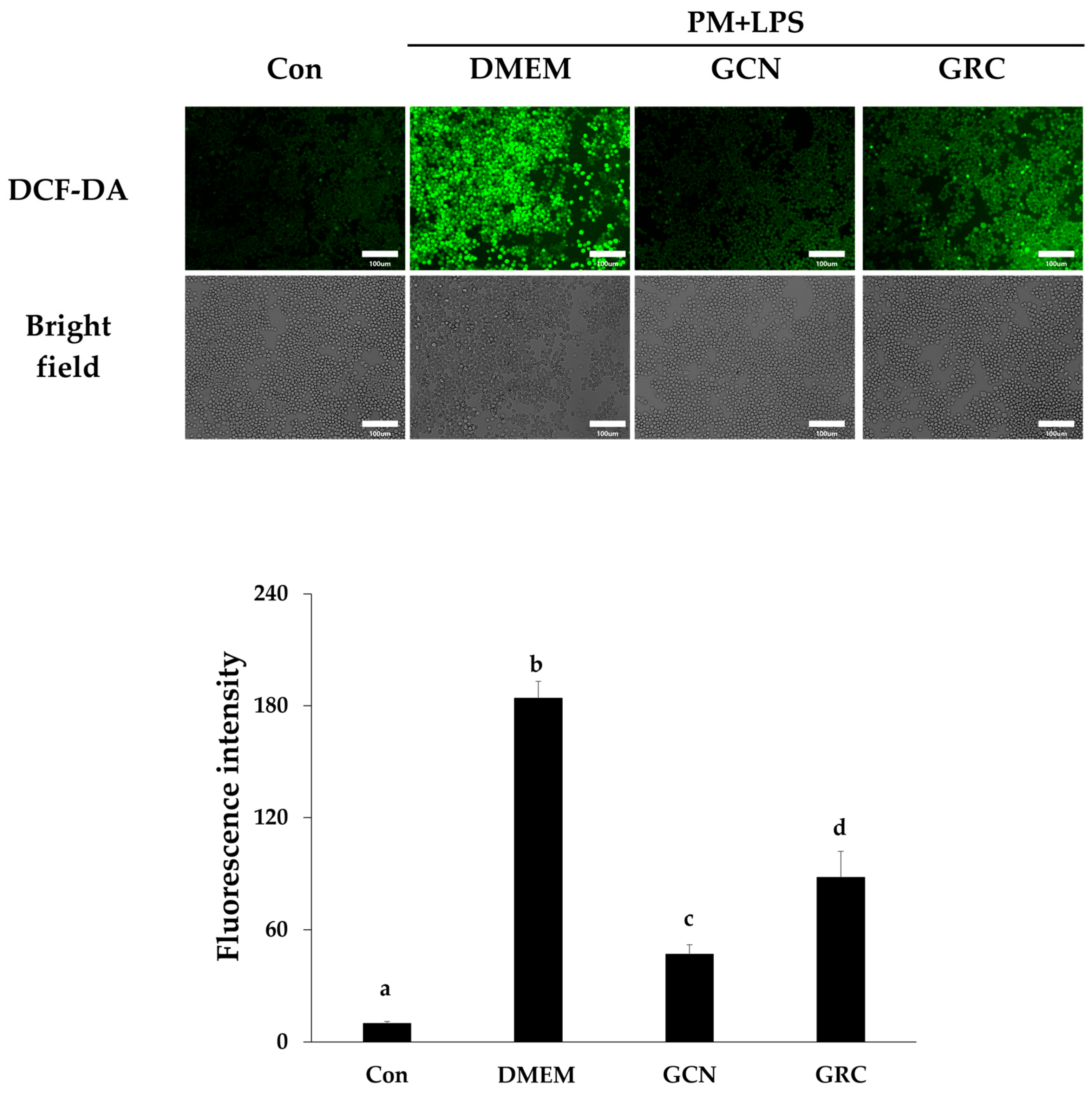
2.7. Enhanced Bioavailability of GCN Reduces ROS Production in RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Synthesis of Chitosan Nanoparticles (CN) and GRC Chitosan Nanoparticles (GCN)
4.2. NTA
4.3. PM Sample and Cell Culture Preparation
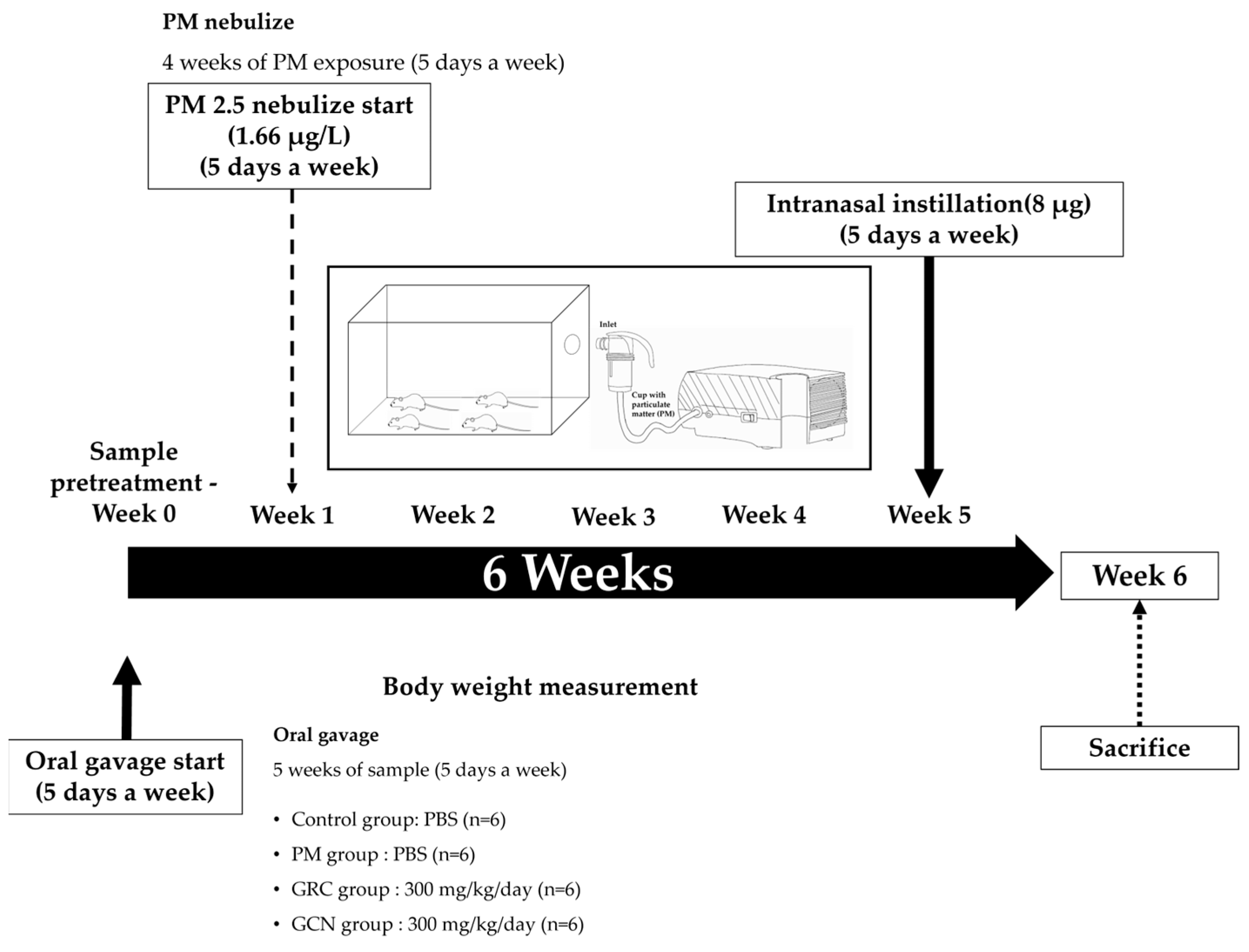
4.4. In Vivo PM Exposure
4.5. Immunohistochemistry
4.6. Hematoxylin and Eosin (H&E) Stain
4.7. Bronchoalveolar Lavage Fluid (BALF) Analysis
4.8. Cell Viability Assay
4.9. Measurement of Nitric Oxide (NO) Concentration
4.10. RNA Isolation and RT-PCR
4.11. Western Blot Analysis
4.12. Reactive Oxygen Species (ROS) Assay
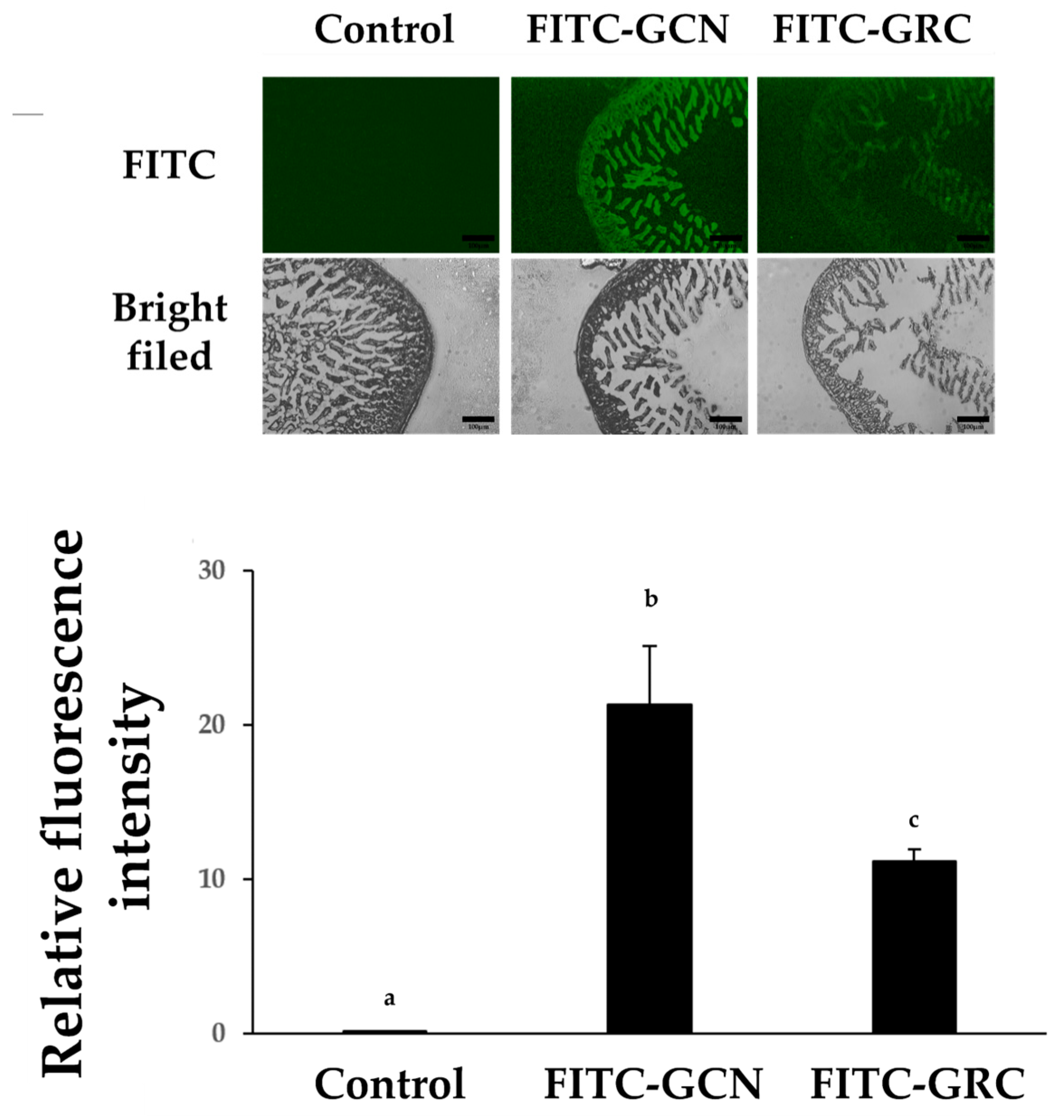
4.13. Ex Vivo Absorption Study in Mouse
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Moreno-Vinasco, L.; Lang, G.D.; Siegler, J.H.; Mathew, B.; Usatyuk, P.V.; Samet, J.M.; Geyh, A.S.; Breysse, P.N. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part. Fibre Toxicol. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, H.; Chen, D.; Zhang, J. Association between ambient particulate matter concentrations and hospitalization for ischemic heart disease (I20-I25, ICD-10) in China: A multicity case-crossover study. Atmos. Environ. 2018, 186, 129–135. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free. Radic. Biol. Med. 2001, 31, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.-C. Recent insights into particulate matter (PM2.5)-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, H.; Liu, Q.; Jiang, B.; Chen, J.; Zhang, T. Sulforaphane attenuates oxidative stress and inflammation induced by fine particulate matter in human bronchial epithelial cells. J. Funct. Foods 2021, 81, 104460. [Google Scholar] [CrossRef]
- Di, A.; Wu, Y.; Chen, M.; Nie, D.; Ge, X. Chemical characterization of seasonal PM2.5 samples and their cytotoxicity in human lung epithelial cells (A549). Int. J. Environ. Res. Public Health 2020, 17, 4599. [Google Scholar] [CrossRef]
- Dagher, Z.; Garçon, G.; Billet, S.; Gosset, P.; Ledoux, F.; Courcot, D.; Aboukais, A.; Shirali, P. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology 2006, 225, 12–24. [Google Scholar] [CrossRef]
- Kwon, H.-S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S. The effects and pathogenesis of PM2.5 and its components on chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Zhao, L.; Zhang, M.; Geng, H.; Dong, C.; Li, R. Effects of real-ambient PM2.5 exposure plus lipopolysaccharide on multiple organ damage in mice. Hum. Exp. Toxicol. 2022, 41, 09603271211061505. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Dou, M.; He, H.; Ju, M.; Ji, S.; Zhou, J.; Chen, C.; Zhang, D.; Miao, C. Particulate matter disrupts airway epithelial barrier via oxidative stress to promote Pseudomonas aeruginosa infection. J. Thorac. Dis. 2019, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Lee, S.M.; Park, K.C.; Park, G.N.; Cho, B.; Kim, I.; Kim, J.; Hong, S. Effects of fine particulate matter on Pseudomonas aeruginosa adhesion and biofilm formation in vitro. BioMed Res. Int. 2018, 2018, 6287932. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Zhou, J.; Wang, J.; Chen, C.; Song, Y.; Pan, J. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir. Res. 2018, 19, 5. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, H.; Yao, X.; Zhou, M.; Wang, J.; He, Z.; Zhang, H.; Lou, L.; Mao, W. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ. Pollut. 2018, 233, 483–493. [Google Scholar] [CrossRef]
- Zomuansangi, R.; Lalbiaktluangi, C.; Gupta, V.K.; Medders, A.A.; Vidal, J.E.; Singh, B.P.; Song, J.J.; Singh, P.K.; Singh, A.; Vellingiri, B. Interaction of bacteria and inhalable particulate matter in respiratory infectious diseases caused by bacteria. Atmos. Pollut. Res. 2023, 15, 102012. [Google Scholar] [CrossRef]
- Sarwar, F.; Alam, K.; Chow, C.W.; Saeed, M.; Malik, R.N. Pulmonary dysfunction augmenting bacterial aerosols in leather tanneries of Punjab, Pakistan. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2925–2937. [Google Scholar] [CrossRef]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free. Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef]
- Shen, M.; Liu, G.; Zhou, L.; Yin, H.; Arif, M.; Leung, K.M.Y. Spatial distribution, driving factors and health risks of fine particle-bound polycyclic aromatic hydrocarbons (PAHs) from indoors and outdoors in Hefei, China. Sci. Total Environ. 2022, 851, 158148. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahab, S.A. Indoor and outdoor relationships of atmospheric particulates in Oman. Indoor Built Environ. 2006, 15, 247–255. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chiang, E.T.; Moreno-Vinasco, L.; Lang, G.D.; Pendyala, S.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; Chillrud, S.N.; Natarajan, V. Particulate matter disrupts human lung endothelial barrier integrity via ROS-and p38 MAPK–dependent pathways. Am. J. Respir. Cell Mol. Biol. 2010, 42, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Dhong, K.-R.; Park, H.-J. Lactic Acid Bacteria Fermented Cordyceps militaris (GRC-SC11) Suppresses IgE Mediated Mast Cell Activation and Type I Hypersensitive Allergic Murine Model. Nutrients 2021, 13, 3849. [Google Scholar] [CrossRef]
- Phull, A.-R.; Ahmed, M.; Park, H.-J. Cordyceps militaris as a bio functional food source: Pharmacological potential, anti-inflammatory actions and related molecular mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Jo, W.-R.; Park, H.-J. Immune-enhancing activity of C. militaris fermented with Pediococcus pentosaceus (GRC-ON89A) in CY-induced immunosuppressed model. BMC Complement. Altern. Med. 2018, 18, 75. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Song, M.-J.; Lee, H.-J.; Park, T.-S.; Kim, M.I.; Park, H.-J. Pediococcus pentosaceus-fermented Cordyceps militaris inhibits inflammatory reactions and alleviates contact dermatitis. Int. J. Mol. Sci. 2018, 19, 3504. [Google Scholar] [CrossRef]
- Park, D.K.; Park, H.-J. Ethanol extract of Cordyceps militaris grown on germinated soybeans attenuates dextran-sodium-sulfate-(DSS-) induced colitis by suppressing the expression of matrix metalloproteinases and inflammatory mediators. BioMed Res. Int. 2013, 2013, 102918. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, H.K.; Park, H.J.; Park, Y.S. Solid–state fermentation of germinated black bean (Rhynchosia nulubilis) using Lactobacillus pentosus SC65 and its immunostimulatory effect. Food Biosci. 2018, 26, 57–64. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, H.-J. Germinated Rhynchosia nulubilis fermented with lactobacillus pentosus SC65 reduces particulate matter induced type II alveolar epithelial apoptotic cell death. Int. J. Mol. Sci. 2021, 22, 3660. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016, 81, 7–14. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Huang, Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013, 58, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Wang, B.-S.; Huang, S.C.; Duh, P.-D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.N.; Kim, J.; Lee, M.Y.; Park, D.K.; Hong, Y.-S.; Lee, C.H. Metabolomics revealed novel isoflavones and optimal cultivation time of Cordyceps militaris fermentation. J. Agric. Food Chem. 2010, 58, 4258–4267. [Google Scholar] [CrossRef]
- Marslin, G.; Khandelwal, V.; Franklin, G. Cordycepin nanoencapsulated in poly (lactic-co-glycolic acid) exhibits better cytotoxicity and lower hemotoxicity than free drug. Nanotechnol. Sci. Appl. 2020, 13, 37–45. [Google Scholar] [CrossRef]
- Soleymanfallah, S.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Preparation, physical properties, and evaluation of antioxidant capacity of aqueous grape extract loaded in chitosan-TPP nanoparticles. Food Sci. Nutr. 2022, 10, 3272–3281. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 1863–1873. [Google Scholar] [CrossRef]
- Acay, H.; Yildirim, A.; Erdem Güzel, E.; Kaya, N.; Baran, M.F. Evaluation and characterization of Pleurotus eryngii extract-loaded chitosan nanoparticles as antimicrobial agents against some human pathogens. Prep. Biochem. Biotechnol. 2020, 50, 897–906. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar]
- Singh, S.; Pandey, V.K.; Tewari, R.P.; Agarwal, V. Nanoparticle based drug delivery system: Advantages and applications. Indian J. Sci. Technol. 2011, 4, 177–180. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, G.-H.; Lee, H.G. Characteristics and antioxidant activity of Elsholtzia splendens extract-loaded nanoparticles. J. Agric. Food Chem. 2010, 58, 3316–3321. [Google Scholar] [CrossRef]
- El-Aziz, A.R.M.A.; Al-Othman, M.R.; Mahmoud, M.A.; Shehata, S.M.; Abdelazim, N.S. Chitosan nanoparticles as a carrier for Mentha longifolia extract: Synthesis, characterization and antifungal activity. Curr. Sci. 2018, 114, 2116–2122. [Google Scholar]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano-and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, A.; Castro, P.M.; Pintado, M. Production of antimicrobial chitosan nanoparticles against food pathogens. J. Food Eng. 2015, 167, 210–216. [Google Scholar] [CrossRef]
- He, F.; Liao, B.; Pu, J.; Li, C.; Zheng, M.; Huang, L.; Zhou, Y.; Zhao, D.; Li, B.; Ran, P. Exposure to ambient particulate matter induced COPD in a rat model and a description of the underlying mechanism. Sci. Rep. 2017, 7, 45666. [Google Scholar] [CrossRef]
- Silva, R.A.B.d.; Leonardo, M.R.; Faccioli, L.H.; Medeiros, A.I.; Nelson-Filho, P. Effect of different methods of sterilization on the inactivation of bacterial endotoxin (LPS) in endodontic files. Braz. J. Microbiol. 2007, 38, 270–272. [Google Scholar] [CrossRef][Green Version]
- Kaur, M.; Chandel, J.; Malik, J.; Naura, A.S. Particulate matter in COPD pathogenesis: An overview. Inflamm. Res. 2022, 71, 797–815. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.; Chen, H.; Fang, B.; Qiu, Y.; Chen, X.; Chen, L.; Shu, S.; Zhang, Y.; Zhao, Y. Design, synthesis, and structure–activity relationships of 2-benzylidene-1-indanone derivatives as anti-inflammatory agents for treatment of acute lung injury. Drug Des. Dev. Ther. 2018, 12, 887–899. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of NF-κB subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Han, H.; Oh, E.-Y.; Lee, J.-H.; Park, J.-W.; Park, H.J. Effects of particulate matter 10 inhalation on lung tissue RNA expression in a murine model. Tuberc. Respir. Dis. 2021, 84, 55. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic medicinal fungus Cordyceps with its nutraceutical and therapeutic potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhu, S. Antioxidant effects of soybean isoflavones. Antioxid. Hum. Health 1999, 123–130. [Google Scholar]
- Lee, H.-J.; Cho, H.-E.; Park, H.-J. Germinated black soybean fermented with Lactobacillus pentosus SC65 alleviates DNFB-induced delayed-type hypersensitivity in C57BL/6N mice. J. Ethnopharmacol. 2021, 265, 113236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.G.; Peng, W.B.; Liu, C.S. Uptake of oleoyl-chitosan nanoparticles by A549 cells. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 208–214. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Macedo, A.C.; Cardeira, M.; Moppert, X.; Guézennec, J.; Sevrin, C.; Grandfils, C.; Serra, A.T.; Freitas, F. Selenium Bio-Nanocomposite Based on Alteromonas macleodii Mo169 Exopolysaccharide: Synthesis, Characterization, and In Vitro Antioxidant Activity. Bioengineering 2023, 10, 193. [Google Scholar] [CrossRef]
- Cho, H.; Cho, Y.Y.; Bae, Y.H.; Kang, H.C. Nucleotides as nontoxic endogenous endosomolytic agents in drug delivery. Adv. Healthc. Mater. 2014, 3, 1007–1014. [Google Scholar] [CrossRef]
- Lu, Q.; Li, D.-C.; Jiang, J.-G. Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J. Agric. Food Chem. 2011, 59, 13004–13011. [Google Scholar] [CrossRef]
- De Grove, K.; Provoost, S.; Brusselle, G.; Joos, G.; Maes, T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin. Exp. Allergy 2018, 48, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kou, X.; Xie, L.; Cheng, F.; Geng, H. Effects of ambient PM 2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ. Sci. Pollut. Res. 2015, 22, 20167–20176. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jiang, S.; Xu, X. Particulate Air Pollution on Systemic Inflammatory Response: Roles of Monocytes and Neutrophils; European Respiratory Society: Lausanne, Switzerland, 2012. [Google Scholar]
- Park, D.K.; Choi, W.S.; Park, H.-J. Antiallergic activity of novel isoflavone methyl-glycosides from Cordyceps militaris grown on germinated soybeans in antigen-stimulated mast cells. J. Agric. Food Chem. 2012, 60, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef]
- Han, J.Y.; Im, J.; Choi, J.N.; Lee, C.H.; Park, H.J.; Park, D.K.; Yun, C.-H.; Han, S.H. Induction of IL-8 expression by Cordyceps militaris grown on germinated soybeans through lipid rafts formation and signaling pathways via ERK and JNK in A549 cells. J. Ethnopharmacol. 2010, 127, 55–61. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, K.-S.; Shen, G.; Grumezescu, A.M.; Holban, A.M.; Wang, Y.-T. Trends in the immunomodulatory effects of Cordyceps militaris: Total extracts, polysaccharides and cordycepin. Front. Pharmacol. 2020, 11, 575704. [Google Scholar] [CrossRef]
- Fonceca, A.M.; Zosky, G.R.; Bozanich, E.M.; Sutanto, E.N.; Kicic, A.; McNamara, P.S.; Knight, D.A.; Sly, P.D.; Turner, D.J.; Stick, S.M. Accumulation mode particles and LPS exposure induce TLR-4 dependent and independent inflammatory responses in the lung. Respir. Res. 2018, 19, 15. [Google Scholar] [CrossRef]
- Song, Y.; Ichinose, T.; He, M.; He, C.; Morita, K.; Yoshida, Y. Lipopolysaccharide attached to urban particulate matter 10 suppresses immune responses in splenocytes while particulate matter itself activates NF-κB. Toxicol. Res. 2016, 5, 1445–1452. [Google Scholar] [CrossRef]
- Wu, W.; Jin, Y.; Carlsten, C. Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol. 2018, 141, 833–844. [Google Scholar] [CrossRef]
- Ishii, H.; Fujii, T.; Hogg, J.C.; Hayashi, S.; Mukae, H.; Vincent, R.; van Eeden, S.F. Contribution of IL-1β and TNF-α to the initiation of the peripheral lung response to atmospheric particulates (PM10). Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L176–L183. [Google Scholar] [CrossRef]
- Malainou, C.; Abdin, S.M.; Lachmann, N.; Matt, U.; Herold, S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: Evolving concepts of therapeutic targeting. J. Clin. Investig. 2023, 133, e170501. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.; Ma, K.; Wang, Y.; Niu, B.; Zhang, L.; Li, F. lncRNA Gm16410 mediates PM2. 5-induced macrophage activation via PI3K/AKT pathway. Front. Cell Dev. Biol. 2021, 9, 618045. [Google Scholar]
- Su, R.; Jin, X.; Zhang, W.; Li, Z.; Liu, X.; Ren, J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere 2017, 167, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Mischler, S.E.; Cauda, E.G.; Di Giuseppe, M.; McWilliams, L.J.; St. Croix, C.; Sun, M.; Franks, J.; Ortiz, L.A. Differential activation of RAW 264.7 macrophages by size-segregated crystalline silica. J. Occup. Med. Toxicol. 2016, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef]
- Park, M.; Park, S.; Choi, Y.; Cho, Y.-L.; Kim, M.J.; Park, Y.-J.; Chung, S.W.; Lee, H.; Lee, S.-J. The mechanism underlying correlation of particulate matter-induced ferroptosis with inflammasome activation and iron accumulation in macrophages. Cell Death Discov. 2024, 10, 144. [Google Scholar] [CrossRef]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef]
- Stanca, L.; Geicu, O.I.; Serban, A.I.; Dinischiotu, A. Interplay of oxidative stress, inflammation, and autophagy in RAW 264.7 murine macrophage cell line challenged with Si/SiO2 quantum dots. Materials 2023, 16, 5083. [Google Scholar] [CrossRef]
- Connelly, L.; Jacobs, A.T.; Palacios-Callender, M.; Moncada, S.; Hobbs, A.J. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J. Biol. Chem. 2003, 278, 26480–26487. [Google Scholar] [CrossRef]
- Rosselli, M.; Keller, R.; Dubey, R.K. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update 1998, 4, 3–24. [Google Scholar] [CrossRef]
- Shen, J.; Shen, D.; Tang, Q.; Li, Z.; Jin, X.; Li, C. Mogroside V exerts anti-inflammatory effects on fine particulate matter-induced inflammation in porcine alveolar macrophages. Toxicol. Vitr. 2022, 80, 105326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Tang, M. Biological effects of airborne fine particulate matter (PM2. 5) exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 117833. [Google Scholar] [CrossRef]
- Veronesi, B.; Oortgiesen, M.; Carter, J.; Devlin, R. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol. Appl. Pharmacol. 1999, 154, 106–115. [Google Scholar] [CrossRef]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Carter, J.M.; Hassenbein, D.G.; Howard, B. Cytokines and particle-induced inflammatory cell recruitment. Environ. Health Perspect. 1997, 105, 1159–1164. [Google Scholar]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Bass, D.A. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J. Clin. Investig. 1975, 56, 870–879. [Google Scholar] [CrossRef]
- Varricchi, G.; Senna, G.; Loffredo, S.; Bagnasco, D.; Ferrando, M.; Canonica, G.W. Reslizumab and eosinophilic asthma: One step closer to precision medicine? Front. Immunol. 2017, 8, 247108. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Terracciano, R.; Savino, R.; Pelaia, G. Severe eosinophilic asthma: From the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des. Dev. Ther. 2017, 11, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Alam, R. The mechanism of IL-5 signal transduction. Am. J. Physiol. -Cell Physiol. 1998, 275, C623–C633. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Reuter, C.W.; Morgan, M.A.; Bergmann, L. Targeting the Ras signaling pathway: A rational, mechanism-based treatment for hematologic malignancies? Blood J. Am. Soc. Hematol. 2000, 96, 1655–1669. [Google Scholar]
- Corrigan, C.J.; Loke, T.-K. Clinical and molecular aspects of glucocorticoid resistant asthma. Ther. Clin. Risk Manag. 2007, 3, 771–787. [Google Scholar]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Ren, Y.; Ichinose, T.; He, M.; Youshida, S.; Nishikawa, M.; Sun, G. Co-exposure to lipopolysaccharide and desert dust causes exacerbation of ovalbumin-induced allergic lung inflammation in mice via TLR4/MyD88-dependent and-independent pathways. Allergy Asthma Clin. Immunol. 2019, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Soukup, J.M.; Sioutas, C.; Cassee, F.R. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp. Lung Res. 2003, 29, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Alexis, N.E.; Lay, J.C.; Zeman, K.; Bennett, W.E.; Peden, D.B.; Soukup, J.M.; Devlin, R.B.; Becker, S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J. Allergy Clin. Immunol. 2006, 117, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Lin, Z.; Wang, Y.; He, H.; Liu, T.; Kamp, D.W. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol. 2016, 31, 923–936. [Google Scholar] [CrossRef]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Shoenfelt, J.; Mitkus, R.J.; Zeisler, R.; Spatz, R.O.; Powell, J.; Fenton, M.J.; Squibb, K.A.; Medvedev, A.E. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 2009, 86, 303–312. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J. Thorac. Dis. 2017, 9, 4398. [Google Scholar] [CrossRef]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, 1–6. [Google Scholar] [CrossRef]
- Rundhaug, J.E.; Fischer, S.M. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem. Photobiol. 2008, 84, 322–329. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Yoshida, S.; Ito, T.; He, C.; Yoshida, Y.; Arashidani, K.; Takano, H.; Sun, G.; Shibamoto, T. PM2. 5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J. Appl. Toxicol. 2017, 37, 1203–1218. [Google Scholar] [CrossRef]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of proinflammatory responses in cells of the airway mucosa by particulate matter: Oxidant-and non-oxidant-mediated triggering mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [PubMed]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxidative Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Fernández, R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J. Hypertens. 2009, 27, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Cha, H.-J.; Choi, E.O.; Leem, S.-H.; Kim, G.-Y.; Moon, S.-K.; Chang, Y.-C.; Yun, S.-J.; Hwang, H.J.; Kim, B.W. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Song, J.; Kim, M.; Han, D.-W.; Park, H.-J.; Song, M. Cordyceps militaris grown on germinated soybean suppresses KRAS-driven colorectal cancer by inhibiting the RAS/ERK pathway. Nutrients 2018, 11, 20. [Google Scholar] [CrossRef]
- Tran, N.K.S.; Kim, G.-T.; Lee, D.Y.; Kim, Y.-J.; Park, H.-J.; Park, D.K.; Park, T.-S. Fermented Cordyceps militaris extract ameliorates hepatosteatosis via activation of fatty acid oxidation. J. Med. Food 2019, 22, 325–336. [Google Scholar] [CrossRef]
- Rodrigo, R.; Bosco, C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 317–327. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef]
- Radhi, M.; Ashraf, S.; Lawrence, S.; Tranholm, A.A.; Wellham, P.A.D.; Hafeez, A.; Khamis, A.S.; Thomas, R.; McWilliams, D.; De Moor, C.H. A systematic review of the biological effects of cordycepin. Molecules 2021, 26, 5886. [Google Scholar] [CrossRef]
- Sun, T.; Dong, W.; Jiang, G.; Yang, J.; Liu, J.; Zhao, L.; Ma, P. Cordyceps militaris improves chronic kidney disease by affecting TLR4/NF-κB redox signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7850863. [Google Scholar] [CrossRef]
- Pulok Mukherjee, K.; Harwansh, R.K.; Bhattacharyya, S. Chapter 10-Bioavailability of Herbal Products: Approach toward Improved Pharmacokinetics, Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Nagar, G. Phytosomes: A novel drug delivery for herbal extracts. Int. J. Pharm. Sci. Res. 2019, 4, 949–959. [Google Scholar]
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. Sci. Technol. (NUJST) 2003, 11, 51–66. [Google Scholar]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-based nanoparticles as effective drug delivery systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, K.; Gupta, R. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Cho, J.Y.; Shim, B.H.; Kim, D.K.; Lee, J. Bioavailability enhancing activities of natural compounds from medicinal plants. J. Med. Plants Res. 2009, 3, 1204–1211. [Google Scholar]
- Salatin, S.; Yari Khosroushahi, A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell. Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- El Leithy, E.S.; Abdel-Bar, H.M.; Ali, R.A.-M. Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. Int. J. Pharm. 2019, 571, 118708. [Google Scholar] [CrossRef]
- Tan, O.J.; Loo, H.L.; Thiagarajah, G.; Palanisamy, U.D.; Sundralingam, U. Improving oral bioavailability of medicinal herbal compounds through lipid-based formulations—A Scoping Review. Phytomedicine 2021, 90, 153651. [Google Scholar] [CrossRef]
- Warsito, M.F.; Agustiani, F. A review on factors affecting chitosan nanoparticles formation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012027. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (−)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2013, 61, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-J.; Yu, Y.-G.; Yin, S.-W.; Tang, C.-H.; Yang, X.-Q. Cellular uptake and intracellular antioxidant activity of zein/chitosan nanoparticles incorporated with quercetin. J. Agric. Food Chem. 2018, 66, 12783–12793. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Fathi, S. Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int. J. Pharm. 2012, 437, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.J.; Jeong, J.Y.; Joung, H.; Han, S.Y. Variations in Metabolite Profiles of Serum Coronas Produced around Pegylated Liposomal Drugs by Surface Property. Colloids Surf. B Biointerfaces 2023, 230, 113488. [Google Scholar] [CrossRef] [PubMed]
- Vignal, C.; Pichavant, M.; Alleman, L.Y.; Djouina, M.; Dingreville, F.; Perdrix, E.; Waxin, C.; Ouali Alami, A.; Gower-Rousseau, C.; Desreumaux, P. Effects of urban coarse particles inhalation on oxidative and inflammatory parameters in the mouse lung and colon. Part. Fibre Toxicol. 2017, 14, 46. [Google Scholar] [CrossRef]
- Kumar, N.; Park, R.J.; Jeong, J.I.; Woo, J.-H.; Kim, Y.; Johnson, J.; Yarwood, G.; Kang, S.; Chun, S.; Knipping, E. Contributions of international sources to PM2. 5 in South Korea. Atmos. Environ. 2021, 261, 118542. [Google Scholar] [CrossRef]
- Chan, Y.L.; Wang, B.; Chen, H.; Ho, K.F.; Cao, J.; Hai, G.; Jalaludin, B.; Herbert, C.; Thomas, P.S.; Saad, S. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2019, 317, L424–L430. [Google Scholar] [CrossRef]
- Dhong, K.-R.; Park, H.-J. Pediococcus Pentosaceus from the Sweet Potato Fermented Ger-Minated Brown Rice Can Inhibit Type I Hypersensitivity in RBL-2H3 Cell and BALB/c Mice Models. Microorganisms 2021, 9, 1855. [Google Scholar] [CrossRef]
- Dhong, K.-R.; Kwon, H.-K.; Park, H.-J. Immunostimulatory Activity of Cordyceps militaris Fermented with Pediococcus pentosaceus SC11 Isolated from a Salted Small Octopus in Cyclophosphamide-Induced Immunocompromised Mice and Its Inhibitory Activity against SARS-CoV 3CL Protease. Microorganisms 2022, 10, 2321. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kwon, H.-K.; Kim, H.S.; Kim, M.I.; Park, H.-J. Hair growth promoting effect of 4HGF encapsulated with PGA nanoparticles (PGA-4HGF) by β-catenin activation and its related cell cycle molecules. Int. J. Mol. Sci. 2019, 20, 3447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Back, S.-Y.; Song, J.G.; Han, H.-K. Enhanced oral delivery of insulin via the colon-targeted nanocomposite system of organoclay/glycol chitosan/Eudragit® S100. J. Nanobiotechnol. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.-J.; Dhong, K.-R.; Park, H.-J. Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice. Int. J. Mol. Sci. 2024, 25, 10642. https://doi.org/10.3390/ijms251910642
Park B-J, Dhong K-R, Park H-J. Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice. International Journal of Molecular Sciences. 2024; 25(19):10642. https://doi.org/10.3390/ijms251910642
Chicago/Turabian StylePark, Byung-Jin, Kyu-Ree Dhong, and Hye-Jin Park. 2024. "Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice" International Journal of Molecular Sciences 25, no. 19: 10642. https://doi.org/10.3390/ijms251910642
APA StylePark, B.-J., Dhong, K.-R., & Park, H.-J. (2024). Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice. International Journal of Molecular Sciences, 25(19), 10642. https://doi.org/10.3390/ijms251910642




