Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia
Abstract
:1. Introduction
2. Results
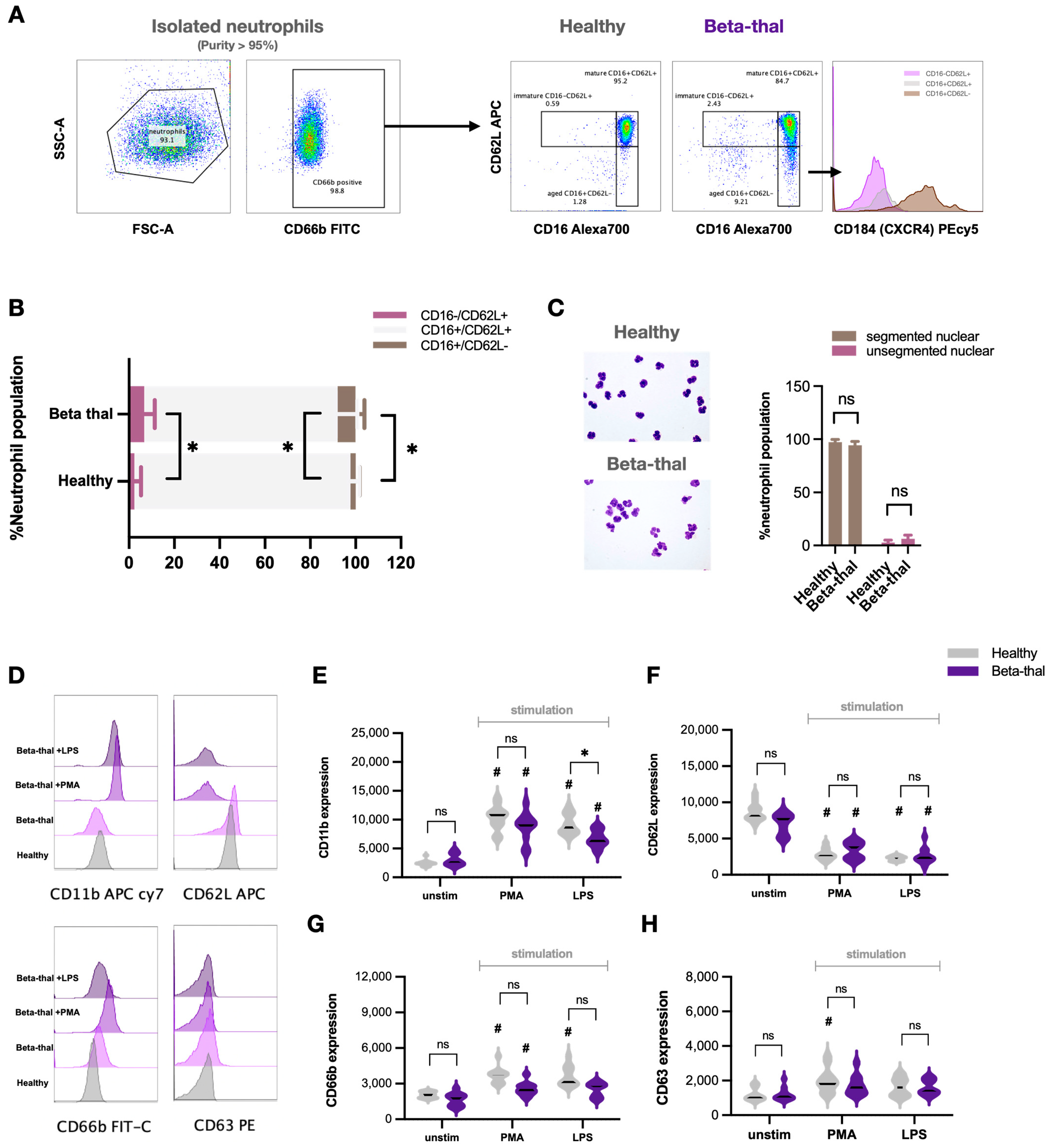
2.1. Neutrophil Diversity in β-Thalassemia
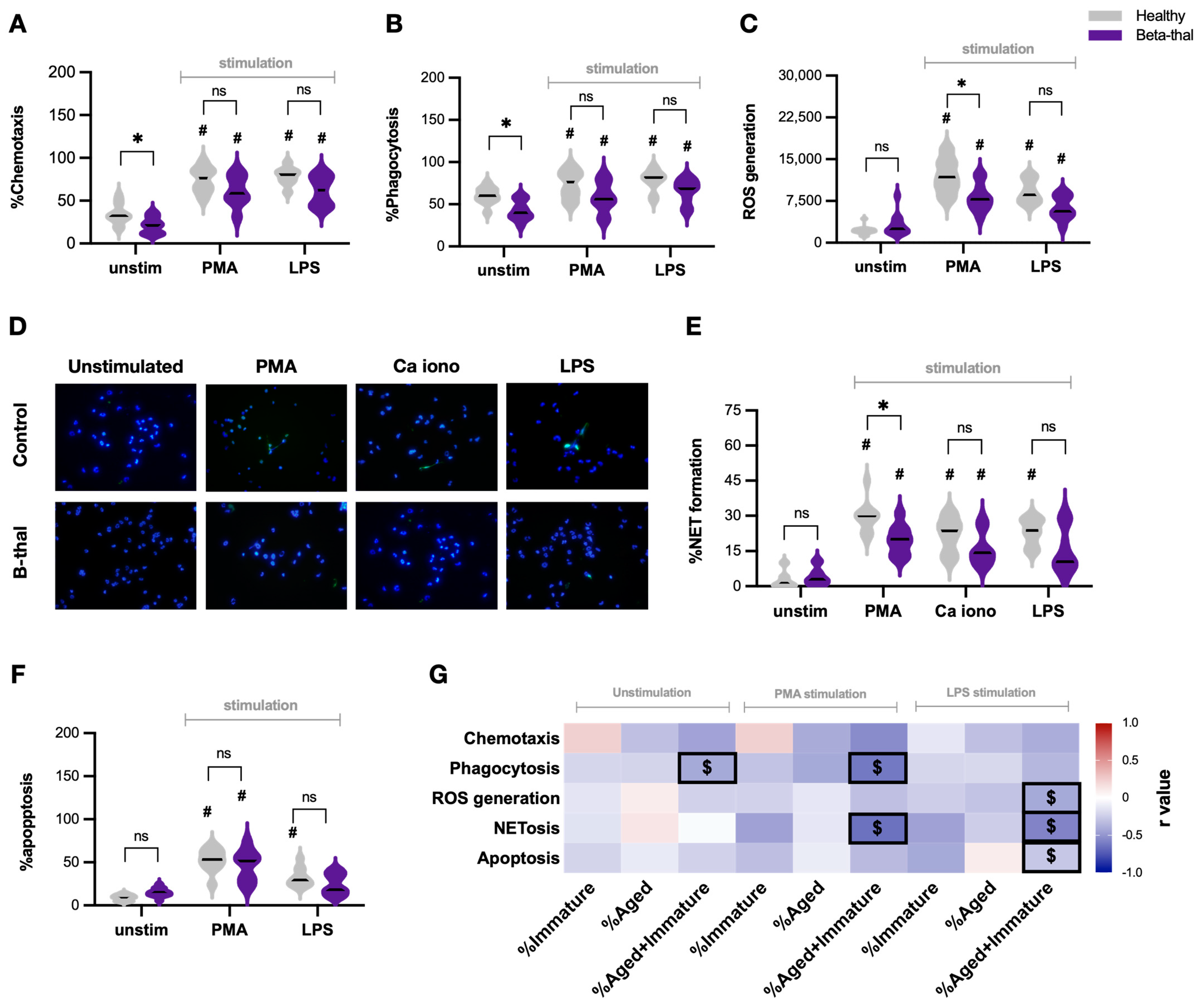
2.2. Defects of Neutrophil Function in β-Thalassemia
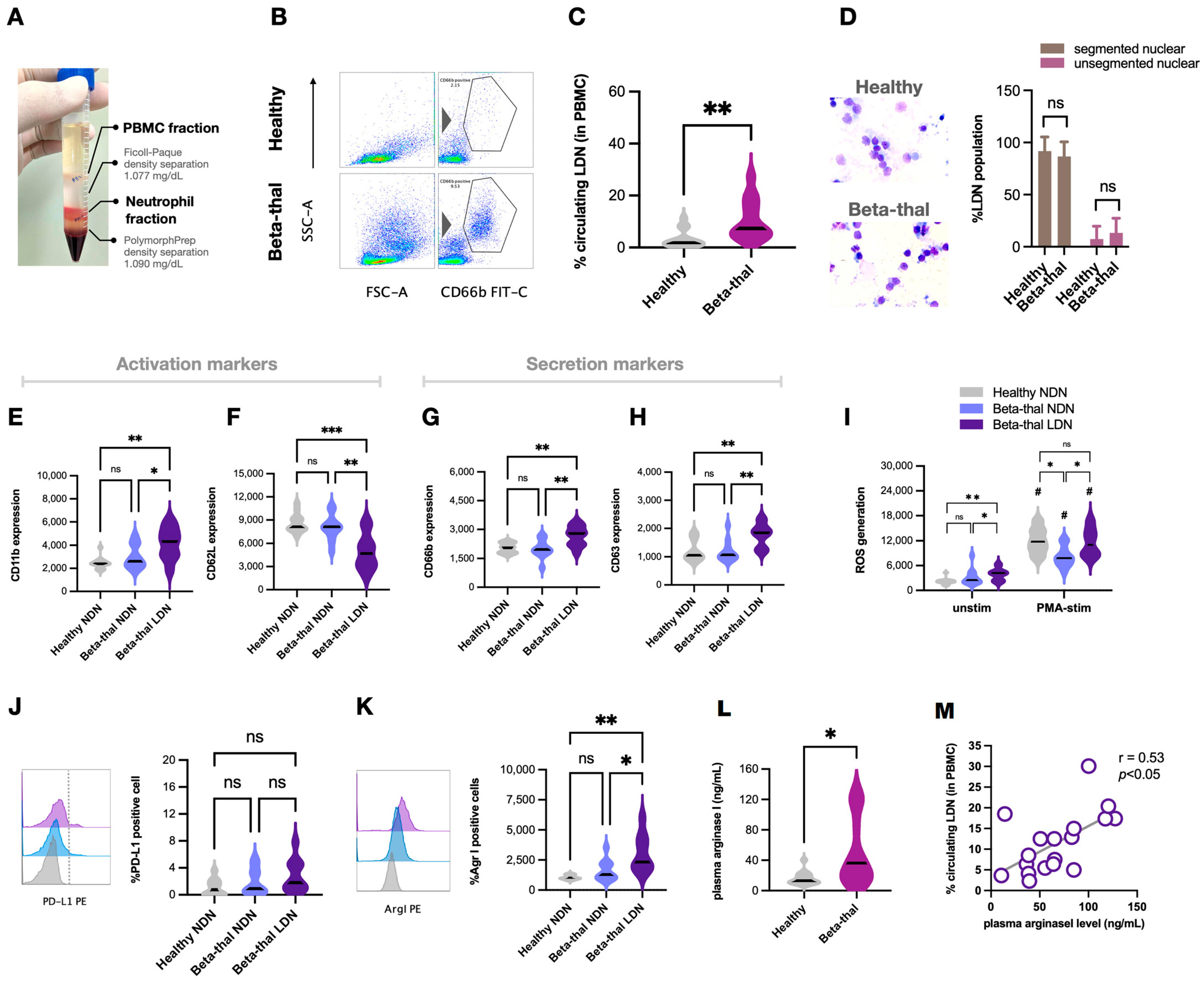
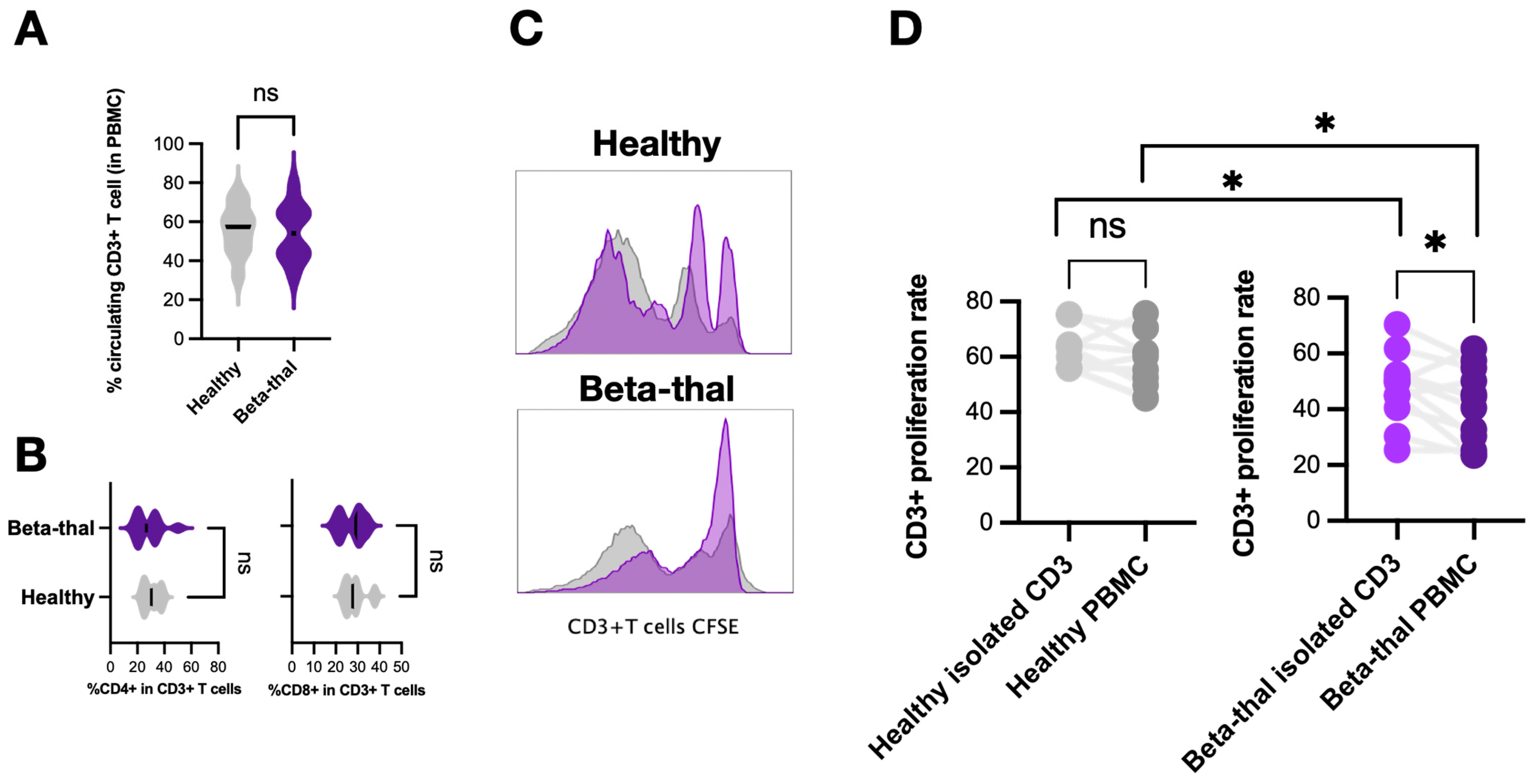
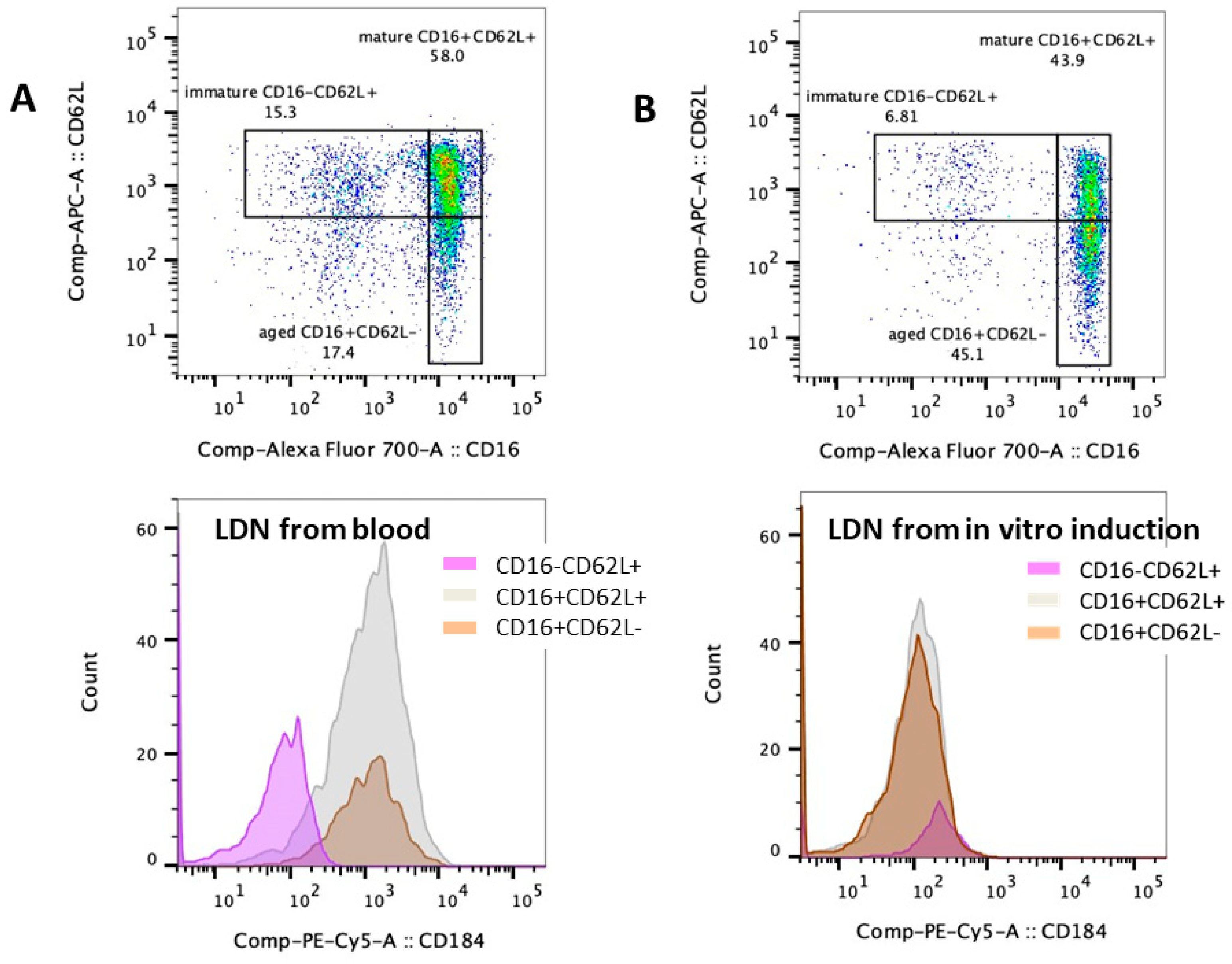
2.3. Elevation of Low-Density Neutrophils and the T Cells Suppression in β-Thalassemia
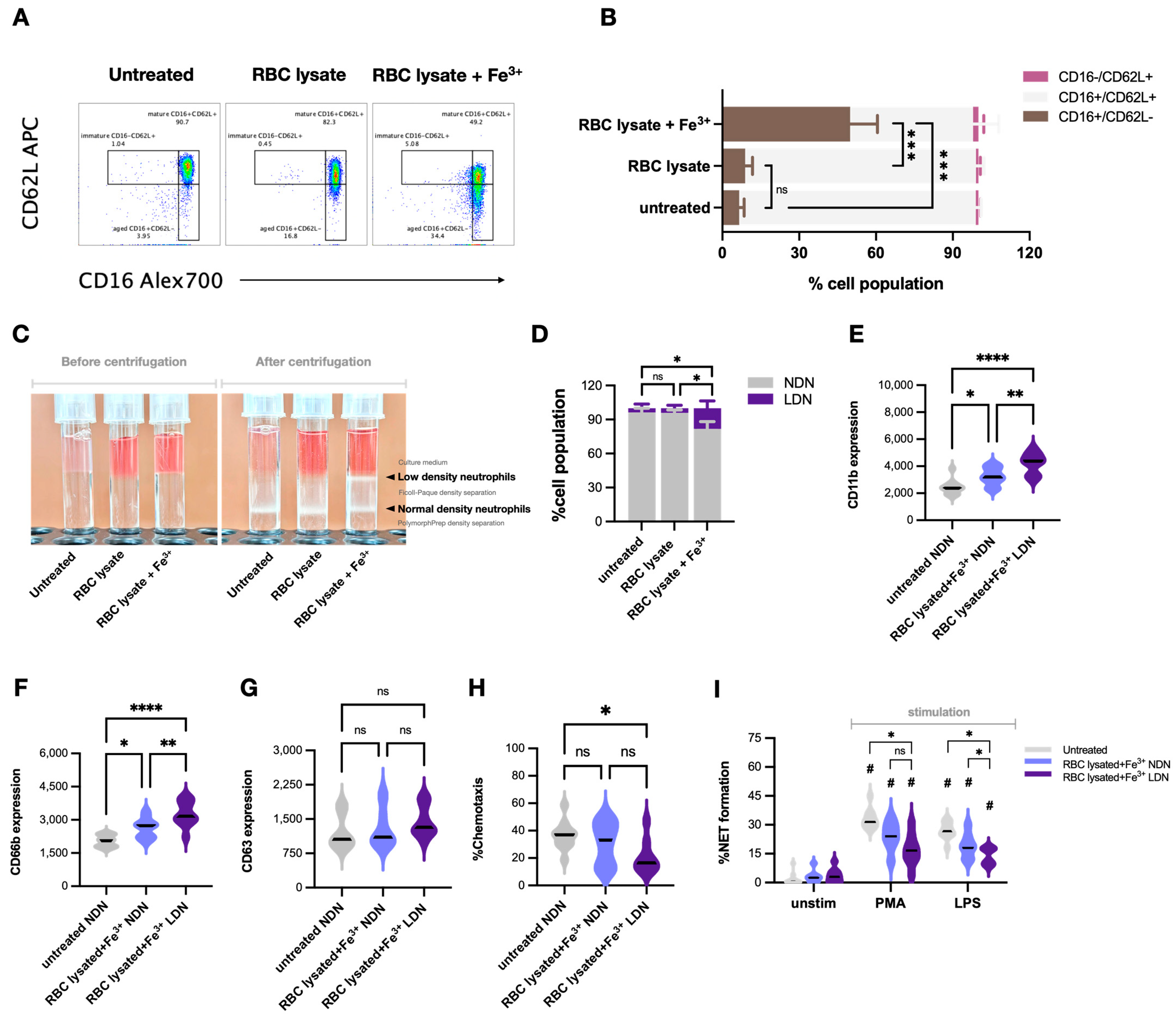
2.4. Impacts of Hemolysis and Iron Overload in Neutrophil Functions, the In Vitro Experiments
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Isolation of Peripheral Blood Mononuclear Cells (PBMC) and Neutrophils
4.3. Neutrophil Stimulation
4.4. Flow Cytometry Analysis
4.5. Human T Cell Isolation and Proliferation Assay
4.6. Wright Giemsa Stain Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, A.T.; Weatherall, D.J.; Cappellini, M.D. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Teawtrakul, N.; Jetsrisuparb, A.; Sirijerachai, C.; Chansung, K.; Wanitpongpun, C. Severe bacterial infections in patients with non-transfusion-dependent thalassemia: Prevalence and clinical risk factors. Int. J. Infect. Dis. 2015, 39, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Vento, S.; Cainelli, F.; Cesario, F. Infections and thalassaemia. Lancet Infect. Dis. 2006, 6, 226–233. [Google Scholar] [CrossRef]
- Sae-Khow, K.; Charoensappakit, A.; Visitchanakun, P.; Saisorn, W.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Pathogen-Associated Molecules from Gut Translocation Enhance Severity of Cecal Ligation and Puncture Sepsis in Iron-Overload β-Thalassemia Mice. J. Inflamm. Res. 2020, 13, 719–735. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Saisorn, W.; Wongphoom, J.; Chatthanathon, P.; Somboonna, N.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Gut leakage enhances sepsis susceptibility in iron-overloaded β-thalassemia mice through macrophage hyperinflammatory responses. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G966–G979. [Google Scholar] [CrossRef] [PubMed]
- Ghoti, H.; Zreid, H.; Ghoti, I.; Bourgonje, A.R.; Diepstra, A.; van Goor, H.; Avivi, I.; Jeadi, H.; van Eijk, L.E.; Weiss, G. Clinical outcome and humoral immune responses of β;-thalassemia major patients with severe iron overload to SARS-CoV-2 infection and vaccination: A prospective cohort study. eClinicalMedicine 2023, 62, 102096. [Google Scholar] [CrossRef]
- Farmakis, D.; Giakoumis, A.; Polymeropoulos, E.; Aessopos, A. Pathogenetic aspects of immune deficiency associated with beta-thalassemia. Med. Sci. Monit. 2003, 9, Ra19–Ra22. [Google Scholar]
- Nithichanon, A.; Tussakhon, I.; Samer, W.; Kewcharoenwong, C.; Ato, M.; Bancroft, G.J.; Lertmemongkolchai, G. Immune responses in beta-thalassaemia: Heme oxygenase 1 reduces cytokine production and bactericidal activity of human leucocytes. Sci. Rep. 2020, 10, 10297. [Google Scholar] [CrossRef]
- Ehsanipour, F.; Faranoush, P.; Foroughi-Gilvaee, M.R.; Sadighnia, N.; Fallahpour, M.; Motamedi, M.; Zandi, A.; Safaei, Z.; Zandi, A.; Faranoush, M. Evaluation of immune system in patients with transfusion-dependent beta-thalassemia in Rasoul-e-Akram Hospital in 2021: A descriptive cross-sectional study. Health Sci. Rep. 2022, 5, e871. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Palomino-Segura, M.; Sicilia, J.; Ballesteros, I.; Hidalgo, A. Strategies of neutrophil diversification. Nat. Immunol. 2023, 24, 575–584. [Google Scholar] [CrossRef]
- Adrover, J.M.; Nicolás-Ávila, J.A.; Hidalgo, A. Aging: A Temporal Dimension for Neutrophils. Trends Immunol. 2016, 37, 334–345. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.-W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Connelly, A.N.; Huijbregts, R.P.H.; Pal, H.C.; Kuznetsova, V.; Davis, M.D.; Ong, K.L.; Fay, C.X.; Greene, M.E.; Overton, E.T.; Hel, Z. Optimization of methods for the accurate characterization of whole blood neutrophils. Sci. Rep. 2022, 12, 3667. [Google Scholar] [CrossRef] [PubMed]
- Sae-khow, K.; Charoensappakit, A.; Chiewchengchol, D.; Leelahavanichkul, A. High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions. Biomedicines 2023, 11, 51. [Google Scholar] [CrossRef]
- Flörcken, A.; Takvorian, A.; Singh, A.; Gerhardt, A.; Ostendorf, B.N.; Dörken, B.; Pezzutto, A.; Westermann, J. Myeloid-derived suppressor cells in human peripheral blood: Optimized quantification in healthy donors and patients with metastatic renal cell carcinoma. Immunol. Lett. 2015, 168, 260–267. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Namazi Bayegi, S.; Ali Hamidieh, A.; Behfar, M.; Saghazadeh, A.; Bozorgmehr, M.; Tajik, N.; Delbandi, A.A.; Delavari, S.; Shekarabi, M.; Rezaei, N. Unbalanced T-cell subsets in pediatric patients with beta-thalassemia. Hum. Immunol. 2023, 84, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Ghatreh-Samani, M.; Esmaeili, N.; Soleimani, M.; Asadi-Samani, M.; Ghatreh-Samani, K.; Shirzad, H. Oxidative stress and age-related changes in T cells: Is thalassemia a model of accelerated immune system aging? Cent. Eur. J. Immunol. 2016, 41, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, S.; Devi, V.J.; Leo, S.R.; Barade, A.; Kulkarni, U.P.; George, B.; Sindhuvi, E. Dysregulated Neutrophil Iron Homeostasis in β-Thalassemia Impairs Phagocytosis and Reactive Oxygen Species Production. Blood 2021, 138, 942. [Google Scholar] [CrossRef]
- Siwaponanan, P.; Siegers, J.Y.; Ghazali, R.; Ng, T.; McColl, B.; Ng, G.Z.; Sutton, P.; Wang, N.; Ooi, I.; Thiengtavor, C.; et al. Reduced PU.1 expression underlies aberrant neutrophil maturation and function in β-thalassemia mice and patients. Blood 2017, 129, 3087–3099. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.-E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, C.; Zhu, Z.; Li, D.; Qu, L.; Xue, Q.; Wang, G.; Ji, T.; Wang, F. New findings on CD16brightCD62Ldim neutrophil subtypes in sepsis-associated ARDS: An observational clinical study. Front. Immunol. 2024, 15, 1331050. [Google Scholar] [CrossRef]
- Walker, E.M., Jr.; Walker, S.M. Effects of iron overload on the immune system. Ann. Clin. Lab. Sci. 2000, 30, 354–365. [Google Scholar]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzyńska, M.; Rokicki, R.; Koziarska-Rościszewska, M.; Rysz, J. Pathomechanisms of Immunological Disturbances in β-Thalassemia. Int. J. Mol. Sci. 2021, 22, 9677. [Google Scholar] [CrossRef]
- Rijneveld, A.W.; de Vos, A.F.; Florquin, S.; Verbeek, J.S.; van der Poll, T. CD11b limits bacterial outgrowth and dissemination during murine pneumococcal pneumonia. J. Infect. Dis. 2005, 191, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Nefedova, Y.; Lei, A.; Gabrilovich, D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin. Immunol. 2018, 35, 19–28. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, J. Relationship between Iron deposition and T lymphocytes in children with β-thalassemia with haematopoietic stem cell transplantation. Front. Pediatr. 2022, 10, 939157. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.S.; Maged, Z.; Khalil, R.; Sabri, F.; Hassan, O.; El-Alfy, M.E. T Cell Functions in Infants and Children with Beta-Thalassemia. Acta Haematol. 2009, 79, 153–156. [Google Scholar] [CrossRef]
- Blanco-Camarillo, C.; Alemán, O.R.; Rosales, C. Low-Density Neutrophils in Healthy Individuals Display a Mature Primed Phenotype. Front. Immunol. 2021, 12, 672520. [Google Scholar] [CrossRef]
- Hassani, M.; Hellebrekers, P.; Chen, N.; van Aalst, C.; Bongers, S.; Hietbrink, F.; Koenderman, L.; Vrisekoop, N. On the origin of low-density neutrophils. J. Leukoc. Biol. 2020, 107, 809–818. [Google Scholar] [CrossRef]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Ozbay Kurt, F.G.; Lasser, S.; Arkhypov, I.; Utikal, J.; Umansky, V. Enhancing immunotherapy response in melanoma: Myeloid-derived suppressor cells as a therapeutic target. J. Clin. Investig. 2023, 133, e170762. [Google Scholar] [CrossRef]
- Bronte, V.; Serafini, P.; Mazzoni, A.; Segal, D.M.; Zanovello, P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003, 24, 301–305. [Google Scholar] [CrossRef]
- Ma, Z.; Lian, J.; Yang, M.; Wuyang, J.; Zhao, C.; Chen, W.; Liu, C.; Zhao, Q.; Lou, C.; Han, J.; et al. Overexpression of Arginase-1 is an indicator of poor prognosis in patients with colorectal cancer. Pathol. Res. Pract. 2019, 215, 152383. [Google Scholar] [CrossRef]
- Larkin, S.K.; Morris, C.R.; Styles, L.A.; Kuypers, F.A. Elevated Plasma Arginase Levels in Hemoglobinopathies. Blood 2005, 106, 2346. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Martinenaite, E.; Kjeldsen, J.W.; Holmstroem, R.B.; Mørk, S.K.; Pedersen, A.W.; Ehrnrooth, E.; Andersen, M.H.; Svane, I.M. Arginase-1 targeting peptide vaccine in patients with metastatic solid tumors—A phase I trial. Front. Immunol. 2022, 13, 1023023. [Google Scholar] [CrossRef]
- Niu, F.; Yu, Y.; Li, Z.; Ren, Y.; Li, Z.; Ye, Q.; Liu, P.; Ji, C.; Qian, L.; Xiong, Y. Arginase: An emerging and promising therapeutic target for cancer treatment. Biomed. Pharmacother. 2022, 149, 112840. [Google Scholar] [CrossRef]
- Yarosz, E.L.; Ye, C.; Kumar, A.; Black, C.; Choi, E.-K.; Seo, Y.-A.; Chang, C.-H. Cutting Edge: Activation-Induced Iron Flux Controls CD4 T Cell Proliferation by Promoting Proper IL-2R Signaling and Mitochondrial Function. J. Immunol. 2020, 204, 1708–1713. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, B.; Basiorka, A.A.; Yang, J.; Cao, L.; Zhang, J.; List, A.; Ji, P. Age-related inflammatory bone marrow microenvironment induces ineffective erythropoiesis mimicking del(5q) MDS. Leukemia 2018, 32, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, X.; Zhang, J.; Yang, J.; Ni, J.; Wang, Y. Exploring the bone marrow micro environment in thalassemia patients: Potential therapeutic alternatives. Front. Immunol. 2024, 15, 1403458. [Google Scholar] [CrossRef] [PubMed]
- Calzetti, F.; Finotti, G.; Tamassia, N.; Bianchetto-Aguilera, F.; Castellucci, M.; Canè, S.; Lonardi, S.; Cavallini, C.; Matte, A.; Gasperini, S.; et al. CD66b−CD64dimCD115− cells in the human bone marrow represent neutrophil-committed progenitors. Nat. Immunol. 2022, 23, 679–691. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp. Cell Res. 2016, 342, 200–209. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Edberg, J.C.; Moon, J.J.; Chang, D.J.; Kimberly, R.P. Differential regulation of human neutrophil FcgammaRIIa (CD32) and FcgammaRIIIb (CD16)-induced Ca2+ transients. J. Biol. Chem. 1998, 273, 8071–8079. [Google Scholar] [CrossRef]
- Saisorn, W.; Saithong, S.; Phuengmaung, P.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Visitchanakun, P.; Chareonsappakit, A.; Pisitkun, P.; Chiewchengchol, D.; Leelahavanichkul, A. Acute Kidney Injury Induced Lupus Exacerbation Through the Enhanced Neutrophil Extracellular Traps (and Apoptosis) in Fcgr2b Deficient Lupus Mice with Renal Ischemia Reperfusion Injury. Front. Immunol. 2021, 12, 669162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ahn, M.H.; Jung, J.Y.; Suh, C.H.; Kim, H.A. An Update on the Pathogenic Role of Neutrophils in Systemic Juvenile Idiopathic Arthritis and Adult-Onset Still’s Disease. Int. J. Mol. Sci. 2021, 22, 13038. [Google Scholar] [CrossRef] [PubMed]
- Douda, D.; Khan, M.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Saithong, S.; Worasilchai, N.; Saisorn, W.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Chindamporn, A.; Tovichayathamrong, P.; Torvorapanit, P.; Chiewchengchol, D.; Chancharoenthana, W.; et al. Neutrophil Extracellular Traps in Severe SARS-CoV-2 Infection: A Possible Impact of LPS and (1→3)-β-D-glucan in Blood from Gut Translocation. Cells 2022, 11, 1103. [Google Scholar] [CrossRef]
- Suksawad, N.; Udompornpitak, K.; Thawinpipat, N.; Korwattanamongkol, P.; Visitchanakun, P.; Phuengmaung, P.; Saisorn, W.; Kueanjinda, P.; Leelahavanichkul, A. Cyclic GMP–AMP Synthase (cGAS) Deletion Reduces Severity in Bilateral Nephrectomy Mice through Changes in Neutrophil Extracellular Traps and Mitochondrial Respiration. Biomedicines 2023, 11, 1208. [Google Scholar] [CrossRef]
- Saithong, S.; Saisorn, W.; Visitchanakun, P.; Sae-Khow, K.; Chiewchengchol, D.; Leelahavanichkul, A. A Synergy Between Endotoxin and (1→3)-Beta-D-Glucan Enhanced Neutrophil Extracellular Traps in Candida Administered Dextran Sulfate Solution Induced Colitis in FcGRIIB-/- Lupus Mice, an Impact of Intestinal Fungi in Lupus. J. Inflamm. Res. 2021, 14, 2333–2352. [Google Scholar] [CrossRef]
- Sae-Khow, K.; Tachaboon, S.; Wright, H.L.; Edwards, S.W.; Srisawat, N.; Leelahavanichkul, A.; Chiewchengchol, D. Defective Neutrophil Function in Patients with Sepsis Is Mostly Restored by ex vivo Ascorbate Incubation. J. Inflamm. Res. 2020, 13, 263–274. [Google Scholar] [CrossRef]
| Patients (23) * | Control (20) * | |
|---|---|---|
| Age (Year) | 22 ± 3 | 22 ± 7 |
| Gender (Female/Male) | 14/18 | 12/8 |
| Serum Endotoxin (EU/mL) # | 0.07 ± 0.05 | 0 |
| Splenectomy (%) | 6/23 (26%) | None |
| Hemoglobin (g/dL) # | 8.5 ± 0.6 | 13 ± 3 |
| No. of Transfusions (6 Months before Recruitment) | 4.5 ± 1.3 | None |
| Lymphocyte Count (×103/mL) | 11.3 ± 6.5 | 13.1 ± 4.5 |
| Platelet (×105/mL) | 2.2 ± 1.1 | 1.9 ± 1.5 |
| Total Bilirubin (mg/dL) # | 1.7 ± 0.5 | 0.6 ± 0.2 |
| Direct Bilirubin (mg/dL) | 0.6 ± 0.3 | 0.3 ± 0.1 |
| Indirect Bilirubin (mg/dL) | 1.1 ± 0.5 | 0.2 ± 0.1 |
| Fasting Plasma Glucose (mg/dL) | 92 ± 11 | 82 ± 17 |
| Serum Calcium (mg/dL) | 8.9 ± 0.5 | 9.2 ± 0.9 |
| Serum Phosphate (mg/dL) | 4.3 ± 0.3 | 4.2 ± 0.8 |
| Serum Albumin (g/dL) | 4.2 ± 0.5 | 4.5 ± 0.3 |
| Serum Alanine Transaminase (ALT) (U/L) # | 117 ± 25 | 22 ± 15 |
| Serum Aspartate Transaminase (AST) (U/L) # | 102 ± 35 | 30 ± 7 |
| Blood Urea Nitrogen (BUN) (mg/dL) | 22 ± 5 | 21 ± 4 |
| Serum Creatinine (mg/dL) | 1.2 ± 0.4 | 1.1 ± 0.3 |
| Serum Ferritin (ng/mL) | 3172 ± 1244 | 97 ± 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sae-Khow, K.; Charoensappakit, A.; Leelahavanichkul, A. Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia. Int. J. Mol. Sci. 2024, 25, 10651. https://doi.org/10.3390/ijms251910651
Sae-Khow K, Charoensappakit A, Leelahavanichkul A. Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia. International Journal of Molecular Sciences. 2024; 25(19):10651. https://doi.org/10.3390/ijms251910651
Chicago/Turabian StyleSae-Khow, Kritsanawan, Awirut Charoensappakit, and Asada Leelahavanichkul. 2024. "Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia" International Journal of Molecular Sciences 25, no. 19: 10651. https://doi.org/10.3390/ijms251910651






