Novel Chimeric Peptides Based on the Enolase Peptide Antigen (CEP-1) Bearing Three Post-Translational Modifications (Citrullination, Homocitrullination and Acetylation) for Determining the Diagnosis and Severity of Rheumatoid Arthritis
Abstract
:1. Introduction
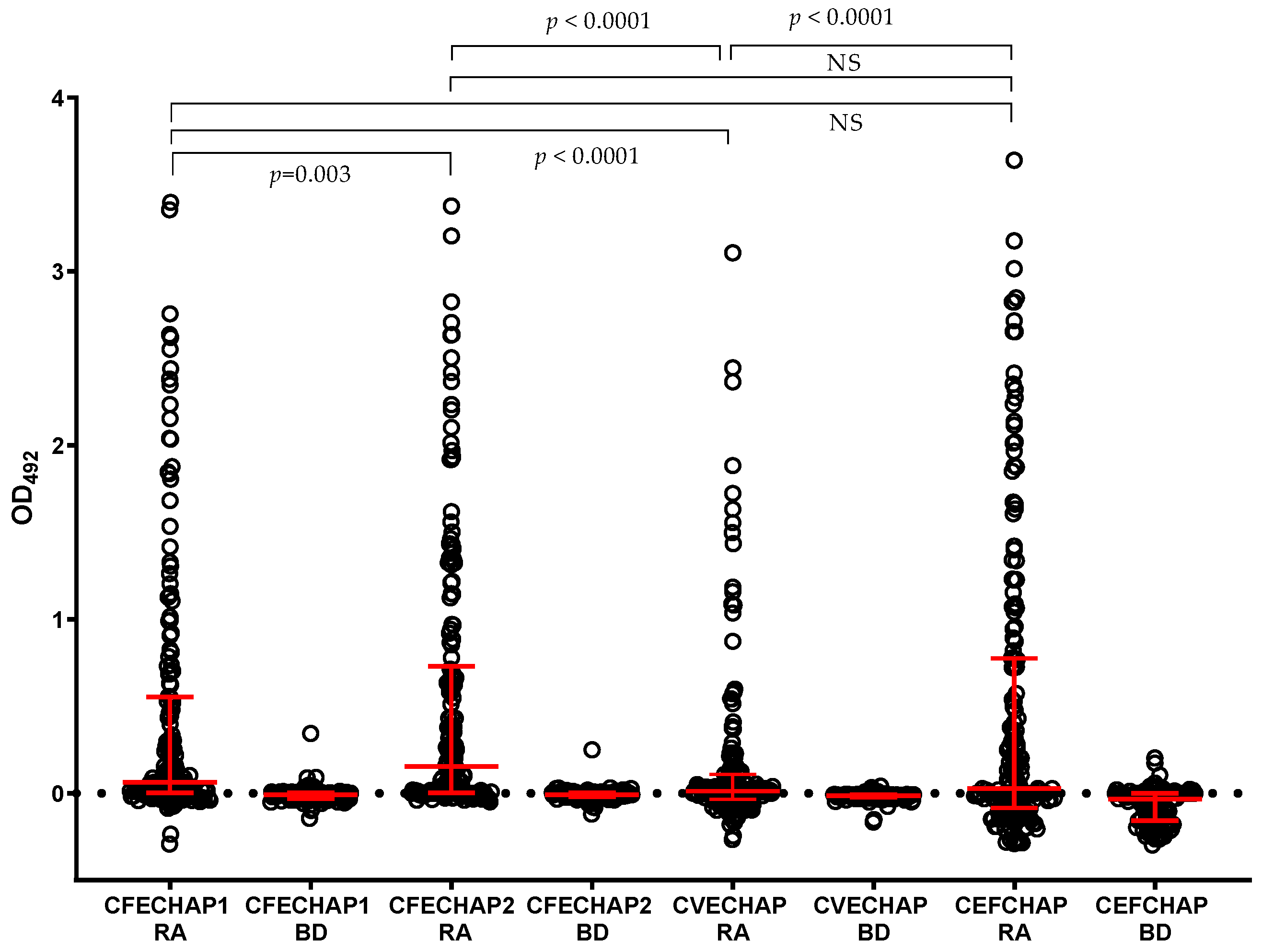
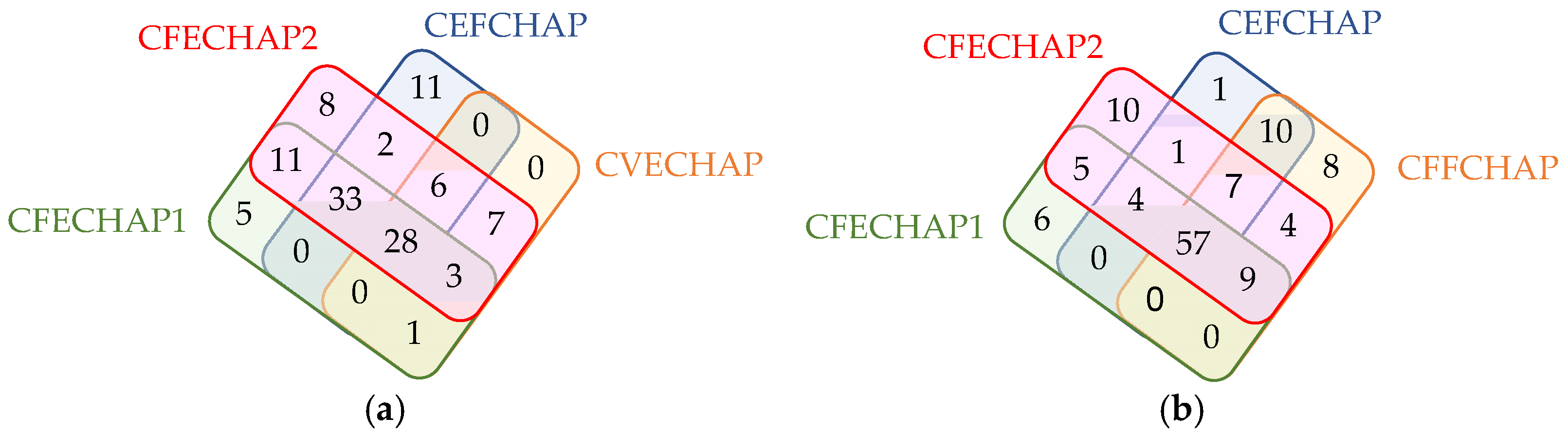
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of Chimeric Peptides
4.2. Serum Samples
4.3. ELISAs
4.4. Statistical Analysis
4.5. Ethics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sangha, O. Epidemiology of rheumatic diseases. Rheumatology 2000, 39, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Costantino, M.; De Bellis, E.; Manzo, V.; Sellitto, C.; Stefanelli, B.; Colucci, F.; Filippelli, A. Biomarkers to Personalize the Treatment of Rheumatoid Arthritis: Focus on Autoantibodies and Pharmacogenetics. Biomolecules 2020, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Haro, I.; Sanmartí, R.; Gómara, M.J. Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 15803. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, W.J.; Zendman, A.J. Anti-CCP2 antibodies: An overview and perspective of the diagnostic abilities of this serological marker for early rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2008, 34, 36–39. [Google Scholar] [CrossRef]
- Vos, I.; Van Mol, C.; Trouw, L.A.; Mahler, M.; Bakker, J.A.; Van Offel, J.; De Clerck, L.; Huizinga, T.W. Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): Diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays. Clin. Rheumatol. 2017, 36, 1487–1492. [Google Scholar] [CrossRef]
- Pérez, M.L.; Gómara, M.J.; Ercilla, G.; Sanmartí, R.; Haro, I. Antibodies to citrullinated human fibrinogen synthetic peptides in diagnosing rheumatoid arthritis. J. Med. Chem. 2007, 50, 3573–3584. [Google Scholar] [CrossRef]
- Sanmartí, R.; Graell, E.; Pérez, M.L.; Ercilla, G.; Viñas, O.; Gómez-Puerta, J.A.; Gratacós, J.; Balsa, A.; Gómara, M.J.; Larrosa, M.; et al. Antibodies against chimeric fibrin/filaggrin citrullinated synthetic peptides in rheumatoid arthritis. Diagnostic and prognostic value. Arthritis Res. Ther. 2009, 11, R135. [Google Scholar] [CrossRef]
- Malakoutihak, M.; Gómara, M.J.; Gómez-Puerta, J.A.; Sanmartí, R.; Haro, I. The use of chimeric vimentin citrullinated peptides for the diagnosis of rheumatoid arthritis. J. Med. Chem 2011, 54, 7486–7492. [Google Scholar] [CrossRef]
- García-Moreno, C.; Gómara, M.J.; Bleda, M.J.; Sanmartí, R.; Haro, I. A multiplex assay based on chimeric citrullinated peptides for the diagnosis of rheumatoid arthritis. PLoS ONE 2019, 14, e0215927. [Google Scholar] [CrossRef]
- van der Woude, D.; Toes, R.E.M. Immune response to post-translationally modified proteins in rheumatoid arthritis: What makes it special? Ann. Rheum. Dis. 2024, 83, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-J.; Ju, J.H. Impact of Posttranslational Modification in Pathogenesis of Rheumatoid Arthritis: Focusing on Citrullination, Carbamylation, and Acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, C.; Gómara, M.J.; Castellanos-Moreira, R.; Sanmartí, R.; Haro, I. Peptides bearing multiple post-translational modifications as antigenic targets for severe rheumatoid arthritis patients. Int. J. Mol. Sci. 2021, 22, 13290. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, C.; Hilberg, O.; Pedersen, A.B.; Ulrichsen, S.P.; Løkke, A.; Bendstrup, E.; Ellingsen, T. A population-based cohort study of rheuma-toid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann. Rheum. Dis. 2017, 76, 1700. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.; Tatzer, V.; Wait, R.; Peston, D.; Lundberg, K.; Donatien, P.; Moyes, D.; Taylor, P.C.; Venables, P.J. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R1421-9. [Google Scholar] [CrossRef]
- Lundberg, K.; Kinloch, A.; Fisher, B.A.; Wegner, N.; Wait, R.; Charles, P.; Mikuls, T.R.; Venables, P.J. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008, 58, 3009–3019. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Liu, C.; Cheng, L.; Yan, S.; Chen, H.; Li, Y. Diagnostic value of anti-citrullinated α-enolase peptide 1 antibody in patients with rheumatoid arthritis: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2021, 24, 633–646. [Google Scholar] [CrossRef]
- Solomon, J.J.; Matson, S.; Kelmenson, L.B.; Chung, J.H.; Hobbs, S.B.; Rosas, I.O.; Dellaripa, P.F.; Doyle, T.J.; Poli, S.; Esposito, A.J.; et al. IgA antibodies directed against citrullinated protein antigens are elevated in patients with idiopathic pulmonary fibrosis. Chest 2020, 157, 1513–1521. [Google Scholar] [CrossRef]
- Harlow, L.; Rosas, I.O.; Gochuico, B.R.; Mikuls, T.R.; Dellaripa, P.F.; Oddis, C.V.; Ascherman, D.P. Identification of citrullinated Hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013, 65, 869–879. [Google Scholar] [CrossRef]
- Alunno, A.; Bistoni, O.; Pratesi, F.; La Paglia, G.M.C.; Puxeddu, I.; Migliorini, P.; Gerli, R. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology 2018, 57, 850–855. [Google Scholar] [CrossRef]
- Castellanos-Moreira, R.; Rodriguez-Garcia, S.C.; Gomara, M.J.; Ruiz-Esquide, V.; Cuervo, A.; Casafont-Sole, I.; Ramirez, J.; Holgado, S.; Gomez-puerta, J.A.; Cañete, J.D.; et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: Evidence of a new autoantibody linked to interstitial lung disease. Ann. Rheum. Dis. 2020, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- England, B.R.; Duryee, M.J.; Roul, P.; Mahajan, T.D.; Singh, N.; Poole, J.A.; Ascherman, D.P.; Caplan, L.; Demoruelle, M.K.; Deane, K.D.; et al. Malondialdehyde–Acetaldehyde adducts and antibody responses in rheumatoid arthritis–associated interstitial lung disease. Arthritis Rheumatol. 2019, 71, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.R. Using the Larsen index to assess radiographic progression in rheumatoid arthritis. J. Rheumatol. 2000, 27, 264–268. [Google Scholar] [PubMed]
- Stainer, A.; Tonutti, A.; De Santis, M.; Amati, F.; Ceribelli, A.; Bongiovanni, G.; Torrisi, C.; Iacopino, A.; Mangiameli, G.; Aliberti, S.; et al. Unmet needs and perspectives in rheumatoid arthritis-associated interstitial lung disease: A critical review. Front. Med. 2023, 10, 1129939. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Li, L.; Zhang, F.; Li, Y.; Zhang, S. High levels of antibodies to citrullinated α-enolase peptide-1 (CEP-1) identify erosions and interstitial lung disease (ILD) in a Chinese rheumatoid arthritis cohort. Clin. Immunol. 2019, 200, 10–15. [Google Scholar] [CrossRef]


| Biotinyl-PEG2-Peptide | 1 RP-HPLC (k’) | 2 Mass Calcd. | 2 Mass Found |
|---|---|---|---|
| CFEP | 4.4 | 4369.2 | 4368.6 |
| CFECHAP-1 | 5.1 | 4513.2 | 4513.9 |
| CFECHAP-2 | 4.9 | 4513.2 | 4514.2 |
| CVEP | 5.0 | 5411.7 | 4512.1 |
| CVECHAP | 6.7 | 5474.1 | 5474.2 |
| CEFP | 5.5 | 4833.3 | 4832.8 |
| CEFCHAP | 5.6 | 4892.3 | 4891.5 |
| Peptides | Sensitivity | 95% CI | Specificity | 95% CI | LR | AUC |
|---|---|---|---|---|---|---|
| CFECHAP-1 | 44.94 | 37.82–52.28 | 99.10 | 95.07–99.95 | 49.89 | 0.792 (0.740–0.844) |
| CFECHAP-2 | 53.93 | 46.60–61.09 | 99.10 | 95.07–99.95 | 59.87 | 0.852 (0.809–0.896) |
| CVECHAP | 25.28 | 19.46–32.14 | 100.0 | 96.65–100.0 | – | 0.687 (0.623–0.751) |
| CEFCHAP | 44.94 | 37.82–52.28 | 97.30 | 92.35–99.26 | 16.63 | 0.763 (0.710–0.816) |
| AMPA IgG Specificity | RA Sera (n = 178) | ACPA-Positive (n = 127) | ACPA-Negative (n = 51) |
|---|---|---|---|
| CFECHAP-1 | 81 (45.5%) | 78 (61.4%) | 3 (5.9%) |
| CFECHAP-2 | 97 (54.5%) | 94 (74%) | 3 (5.9%) |
| CVECHAP | 45 (25.3%) | 44 (34.6%) | 1 (2.0%) |
| CEFCHAP | 80 (44.9%) | 79 (62.2%) | 1 (2.0%) |
| CFFCHAP | 95 (53.4%) | 91 (71.7%) | 4 (7.8%) |
| Any peptide | 122 (68.5%) | 113 (89.0%) | 9 (17.6%) |
| RA-ILD (n = 37) | RA-non-ILD (n = 141) | p-Value | |
|---|---|---|---|
| anti-CFECHAP-1-positive (%) | 19 (51.3) | 62 (44.0) | NS |
| median titer anti-CFECHAP-1 (IQR) | 0.131 (0.861) | 0.055 (0.522) | 0.015 |
| anti-CFECHAP-2-positive (%) | 28 (75.7) | 69 (48.9) | 0.0036 |
| median titer anti-CFECHAP-2 (IQR) | 0.352 (0.987) | 0.075 (0.649) | NS |
| anti-CVECHAP-positive (%) | 10 (27.0) | 35 (24.8) | NS |
| median titer anti-CVECHAP (IQR) | 0.017 (0.133) | 0.009 (0.104) | NS |
| anti-CEFCHAP-positive (%) | 21 (56.8) | 59 (41.8) | NS |
| median titer anti-CEFCHAP (IQR) | 0.268 (1.880) | 0.001 (0.534) | NS |
| anti-CFFCHAP-positive (%) | 24 (64.9) | 71 (50.4) | NS |
| median titer anti-CFFCHAP (IQR) | 0.425 (2.811) | 0.108 (1.013) | 0.026 |
| anti-CCP3-positive (%) | 29 (78.4) | 98 (69.5) | NS |
| median titer anti-CCP3 AU/mL (IQR) | 674 (2215) | 143 (1139) | NS |
| Median Larsen Score 18 | Larsen < 18 (n = 88) | Larsen ≥ 18 (n = 90) | p-Value |
|---|---|---|---|
| anti-CFECHAP-1-positive (%) | 37 (42.0) | 44 (48.9) | NS |
| median titer anti-CFECHAP-1 (IQR) | 0.048 (0.326) | 0.080 (0.687) | NS |
| anti-CFECHAP-2-positive (%) | 42 (47.7) | 55 (61.1) | NS |
| median titer anti-CFECHAP-2 (IQR) | 0.071 (0.631) | 0.274 (0.767) | NS |
| anti-CVECHAP-positive (%) | 21 (23.9) | 24 (26.7) | NS |
| median titer anti-CVECHAP (IQR) | 0.009 (0.084) | 0.012 (0.109) | NS |
| anti-CEFCHAP-positive (%) | 33 (37.5) | 47 (52.2) | 0.048 |
| median titer anti-CEFCHAP (IQR) | 0.000 (0.424) | 0.162 (1.105) | 0.0274 |
| anti-CFFCHAP-positive (%) | 40 (45.4) | 55 (61.1) | 0.036 |
| median titer anti-CFFCHAP (IQR) | 0.058 (0.527) | 0.285 (2.014) | 0.0073 |
| anti-CCP3-positive (%) | 60 (68.2) | 67 (74.4) | NS |
| median titer anti-CCP3 AU/mL (IQR) | 104 (1011) | 454 (2015) | 0.025 |
| Female (%) | 141 (79) |
| Age mean (±SD) | 59.7 (13.0) |
| Mean disease duration (±SD) | 6.6 (5.0) |
| Ever smokers (%) | 83 (46) |
| Caucasian (%) | 151 (84) |
| Rheumatoid factor-positive | 111 (62) |
| Extra-articular manifestations | |
| Sicca syndrome | 33 (18) |
| Rheumatoid nodules | 21 (12) |
| Serositis | 3 (2) |
| Treatment | |
| Glucocorticoids (%) | 108 (60) |
| csDMARDs (%) | 155 (87) |
| MTX (%) | 115 (64) |
| bDMARDs (%) | 47 (26) |
| Mean DAS 28 (±SD) | 2.94 (1.18) |
| Radiographic erosive disease (%) | 89 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómara, M.J.; Sarmiento-Monroy, J.C.; Castellanos-Moreira, R.; Gómez-Puerta, J.A.; Sanmartí, R.; Haro, I. Novel Chimeric Peptides Based on the Enolase Peptide Antigen (CEP-1) Bearing Three Post-Translational Modifications (Citrullination, Homocitrullination and Acetylation) for Determining the Diagnosis and Severity of Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 10654. https://doi.org/10.3390/ijms251910654
Gómara MJ, Sarmiento-Monroy JC, Castellanos-Moreira R, Gómez-Puerta JA, Sanmartí R, Haro I. Novel Chimeric Peptides Based on the Enolase Peptide Antigen (CEP-1) Bearing Three Post-Translational Modifications (Citrullination, Homocitrullination and Acetylation) for Determining the Diagnosis and Severity of Rheumatoid Arthritis. International Journal of Molecular Sciences. 2024; 25(19):10654. https://doi.org/10.3390/ijms251910654
Chicago/Turabian StyleGómara, María José, Juan C. Sarmiento-Monroy, Raul Castellanos-Moreira, José A Gómez-Puerta, Raimon Sanmartí, and Isabel Haro. 2024. "Novel Chimeric Peptides Based on the Enolase Peptide Antigen (CEP-1) Bearing Three Post-Translational Modifications (Citrullination, Homocitrullination and Acetylation) for Determining the Diagnosis and Severity of Rheumatoid Arthritis" International Journal of Molecular Sciences 25, no. 19: 10654. https://doi.org/10.3390/ijms251910654






