Syntaxin 3B: A SNARE Protein Required for Vision
Abstract
:1. Syntaxin 3B Overview
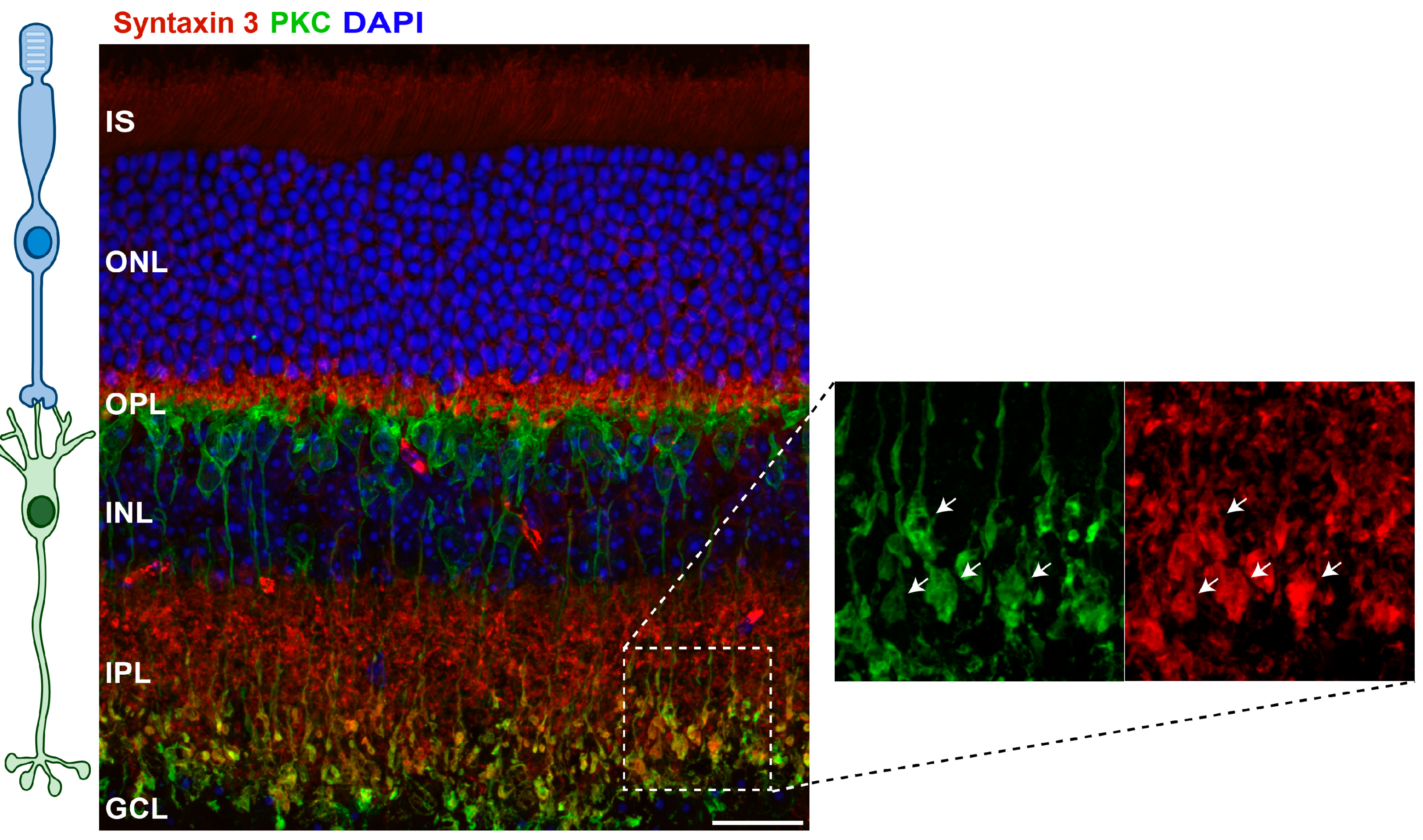
2. Syntaxin 3B and Retinal Ribbon-Style Synapses
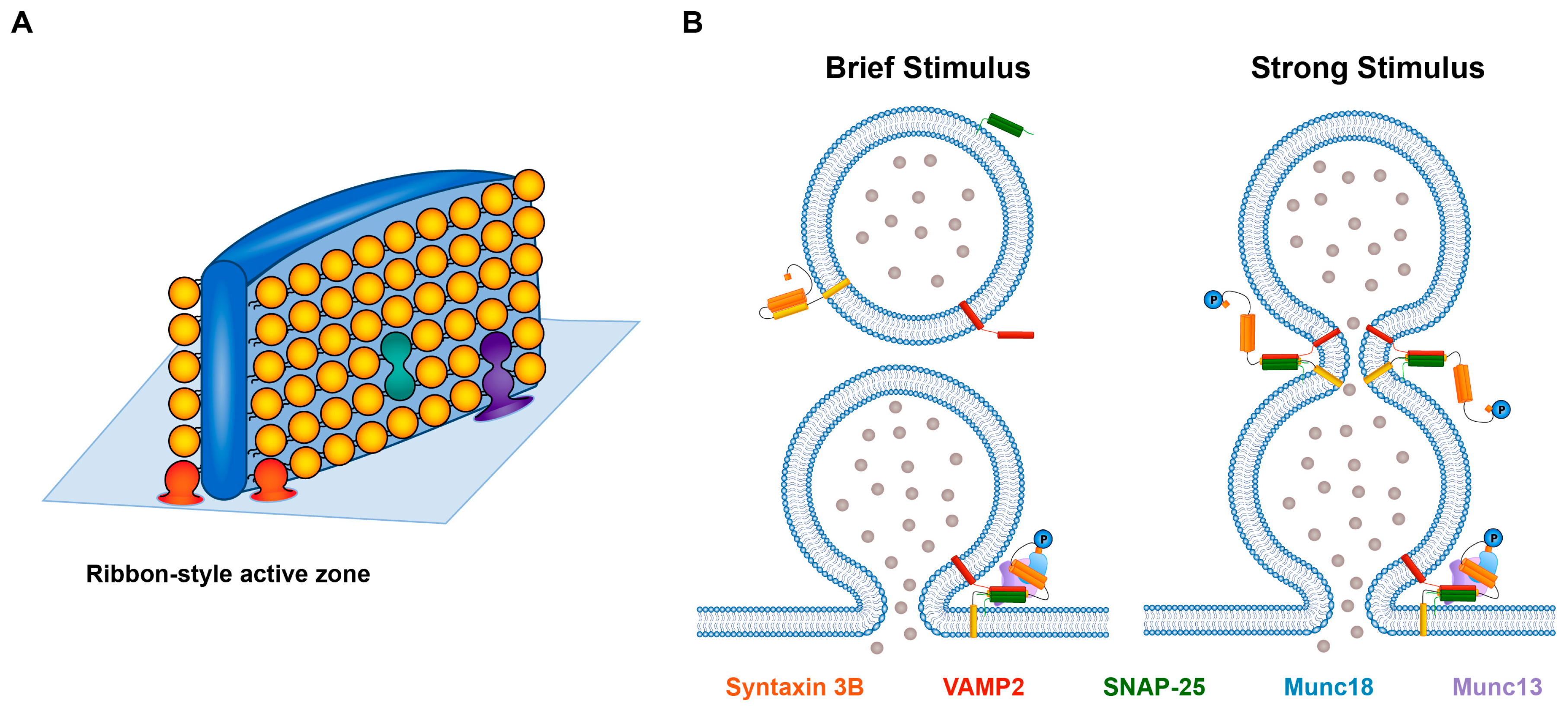
3. Non-Synaptic Roles of Syntaxin 3B
4. Inactivation of the Syntaxin 3 Gene in Mice
5. Retinal Dystrophy and Mutations in the Human Syntaxin 3 Gene
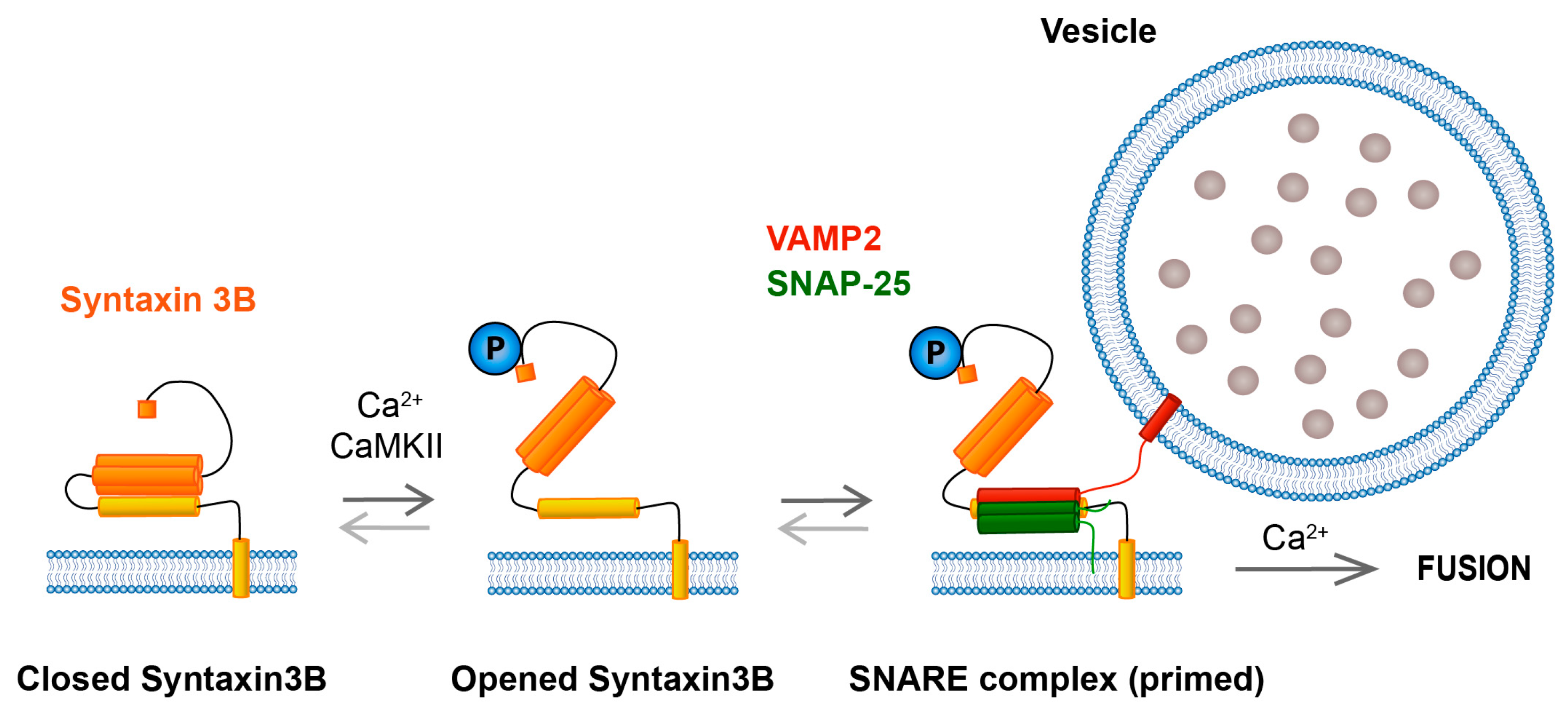
6. Syntaxin 3B Functionality Is Regulated by Phosphorylation at T14
7. Role of the Juxtamembrane Domain (JMD) of Syntaxin 3B in Exocytosis
8. Regulation of Syntaxin 3B Function by Munc18
9. Regulation of Syntaxin 3B Function by Complexin
10. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, M.K.; Garcia-Arraras, J.E.; Elferink, L.A.; Peterson, K.; Fleming, A.M.; Hazuka, C.D.; Scheller, R.H. The syntaxin family of vesicular transport receptors. Cell 1993, 74, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Xu, J. The Synaptic Vesicle Release Machinery. Annu. Rev. Biophys. 2015, 44, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Sudhof, T.C. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices—Guilty as charged? Annu. Rev. Cell Dev. Biol. 2012, 28, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Scheller, R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Weber, T.; Zemelman, B.V.; McNew, J.A.; Westermann, B.; Gmachl, M.; Parlati, F.; Sollner, T.H.; Rothman, J.E. SNAREpins: Minimal machinery for membrane fusion. Cell 1998, 92, 759–772. [Google Scholar] [CrossRef]
- Vardar, G.; Chang, S.; Arancillo, M.; Wu, Y.J.; Trimbuch, T.; Rosenmund, C. Distinct Functions of Syntaxin-1 in Neuronal Maintenance, Synaptic Vesicle Docking, and Fusion in Mouse Neurons. J. Neurosci. 2016, 36, 7911–7924. [Google Scholar] [CrossRef]
- Detrait, E.; Eddleman, C.S.; Yoo, S.; Fukuda, M.; Nguyen, M.P.; Bittner, G.D.; Fishman, H.M. Axolemmal repair requires proteins that mediate synaptic vesicle fusion. J. Neurobiol. 2000, 44, 382–391. [Google Scholar] [CrossRef]
- Bloom, O.E.; Morgan, J.R. Membrane trafficking events underlying axon repair, growth, and regeneration. Mol. Cell Neurosci. 2011, 48, 339–348. [Google Scholar] [CrossRef]
- Soo Hoo, L.; Banna, C.D.; Radeke, C.M.; Sharma, N.; Albertolle, M.E.; Low, S.H.; Weimbs, T.; Vandenberg, C.A. The SNARE Protein Syntaxin 3 Confers Specificity for Polarized Axonal Trafficking in Neurons. PLoS ONE 2016, 11, e0163671. [Google Scholar] [CrossRef]
- Curtis, L.B.; Doneske, B.; Liu, X.; Thaller, C.; McNew, J.A.; Janz, R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J. Comp. Neurol. 2008, 510, 550–559. [Google Scholar] [CrossRef]
- Ibaraki, K.; Horikawa, H.P.; Morita, T.; Mori, H.; Sakimura, K.; Mishina, M.; Saisu, H.; Abe, T. Identification of four different forms of syntaxin 3. Biochem. Biophys. Res. Commun. 1995, 211, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.; Datta, P.; Liu, X.; Bogdanova, N.; Heidelberger, R.; Janz, R. Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina. Neuroscience 2010, 166, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Janecke, A.R.; Liu, X.; Adam, R.; Punuru, S.; Viestenz, A.; Strauss, V.; Laass, M.; Sanchez, E.; Adachi, R.; Schatz, M.P.; et al. Pathogenic STX3 variants affecting the retinal and intestinal transcripts cause an early-onset severe retinal dystrophy in microvillus inclusion disease subjects. Hum. Genet. 2021, 140, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Betancourt-Solis, M.; Dey, H.; Heidelberger, R.; McNew, J.A. Regulation of Syntaxin3B-Mediated Membrane Fusion by T14, Munc18, and Complexin. Biomolecules 2023, 13, 1463. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Yao, X.; Chow, D.C.; Forte, J.G.; Bennett, M.K. Association of syntaxin 3 and vesicle-associated membrane protein (VAMP) with H+/K(+)-ATPase-containing tubulovesicles in gastric parietal cells. Mol. Biol. Cell 1997, 8, 399–407. [Google Scholar] [CrossRef]
- Gaisano, H.Y.; Ghai, M.; Malkus, P.N.; Sheu, L.; Bouquillon, A.; Bennett, M.K.; Trimble, W.S. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol. Biol. Cell 1996, 7, 2019–2027. [Google Scholar] [CrossRef]
- Delgrossi, M.H.; Breuza, L.; Mirre, C.; Chavrier, P.; Le Bivic, A. Human syntaxin 3 is localized apically in human intestinal cells. J. Cell Sci. 1997, 110 Pt 18, 2207–2214. [Google Scholar] [CrossRef]
- Low, S.H.; Chapin, S.J.; Weimbs, T.; Komuves, L.G.; Bennett, M.K.; Mostov, K.E. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 1996, 7, 2007–2018. [Google Scholar] [CrossRef]
- Jurado, S.; Goswami, D.; Zhang, Y.; Molina, A.J.; Sudhof, T.C.; Malenka, R.C. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron 2013, 77, 542–558. [Google Scholar] [CrossRef]
- Plitt, M.H.; Kaganovsky, K.; Sudhof, T.C.; Giocomo, L.M. Hippocampal place code plasticity in CA1 requires postsynaptic membrane fusion. bioRxiv 2023, Preprint. [Google Scholar] [CrossRef]
- Shi, S.; Ma, K.; Bin, N.R.; Harada, H.; Xie, X.; Huang, M.; Liu, H.; Lee, S.; Wang, X.F.; Adachi, R.; et al. Syntaxin-3 is dispensable for basal neurotransmission and synaptic plasticity in postsynaptic hippocampal CA1 neurons. Sci. Rep. 2020, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Arendt, K.L.; Zhang, Y.; Jurado, S.; Malenka, R.C.; Sudhof, T.C.; Chen, L. Retinoic Acid and LTP Recruit Postsynaptic AMPA Receptors Using Distinct SNARE-Dependent Mechanisms. Neuron 2015, 86, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Heidelberger, R.; Janz, R. Phosphorylation of syntaxin 3B by CaMKII regulates the formation of t-SNARE complexes. Mol. Cell Neurosci. 2014, 60, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gething, C.; Ferrar, J.; Misra, B.; Howells, G.; Andrzejewski, A.L.; Bowen, M.E.; Choi, U.B. Conformational change of Syntaxin-3b in regulating SNARE complex assembly in the ribbon synapses. Sci. Rep. 2022, 12, 9261. [Google Scholar] [CrossRef] [PubMed]
- Wiegerinck, C.L.; Janecke, A.R.; Schneeberger, K.; Vogel, G.F.; van Haaften-Visser, D.Y.; Escher, J.C.; Adam, R.; Thoni, C.E.; Pfaller, K.; Jordan, A.J.; et al. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 2014, 147, 65–68. [Google Scholar] [CrossRef]
- Julia, J.; Shui, V.; Mittal, N.; Heim-Hall, J.; Blanco, C.L. Microvillus inclusion disease, a diagnosis to consider when abnormal stools and neurological impairments run together due to a rare syntaxin 3 gene mutation. J. Neonatal Perinat. Med. 2019, 12, 313–319. [Google Scholar] [CrossRef]
- Alsaleem, B.M.R.; Ahmed, A.B.M.; Fageeh, M.A. Microvillus Inclusion Disease Variant in an Infant with Intractable Diarrhea. Case Rep. Gastroenterol. 2017, 11, 647–651. [Google Scholar] [CrossRef]
- Giovannone, A.J.; Winterstein, C.; Bhattaram, P.; Reales, E.; Low, S.H.; Baggs, J.E.; Xu, M.; Lalli, M.A.; Hogenesch, J.B.; Weimbs, T. Soluble syntaxin 3 functions as a transcriptional regulator. J. Biol. Chem. 2018, 293, 5478–5491. [Google Scholar] [CrossRef]
- Campbell, J.R.; Li, H.; Wang, Y.; Kozhemyakin, M.; Hunt, A.J., Jr.; Liu, X.; Janz, R.; Heidelberger, R. Phosphorylation of the Retinal Ribbon Synapse Specific t-SNARE Protein Syntaxin3B Is Regulated by Light via a Ca2+-Dependent Pathway. Front. Cell Neurosci. 2020, 14, 587072. [Google Scholar] [CrossRef]
- Hays, C.L.; Grassmeyer, J.J.; Wen, X.; Janz, R.; Heidelberger, R.; Thoreson, W.B. Simultaneous Release of Multiple Vesicles from Rods Involves Synaptic Ribbons and Syntaxin 3B. Biophys. J. 2020, 118, 967–979. [Google Scholar] [CrossRef]
- Datta, P.; Gilliam, J.; Thoreson, W.B.; Janz, R.; Heidelberger, R. Two Pools of Vesicles Associated with Synaptic Ribbons Are Molecularly Prepared for Release. Biophys. J. 2017, 113, 2281–2298. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B.; Rabl, K.; Townes-Anderson, E.; Heidelberger, R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron 2004, 42, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, R. Mechanisms of tonic, graded release: Lessons from the vertebrate photoreceptor. J. Physiol. 2007, 585, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Rouze, N.C.; Schwartz, E.A. Continuous and transient vesicle cycling at a ribbon synapse. J. Neurosci. 1998, 18, 8614–8624. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.F.; Vila, A.; Zhou, Z.Y.; Heidelberger, R. Synaptic vesicle dynamics in mouse rod bipolar cells. Vis. Neurosci. 2008, 25, 523–533. [Google Scholar] [CrossRef]
- Wan, Q.F.; Zhou, Z.Y.; Thakur, P.; Vila, A.; Sherry, D.M.; Janz, R.; Heidelberger, R. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 2010, 66, 884–895. [Google Scholar] [CrossRef]
- Lagnado, L.; Schmitz, F. Ribbon Synapses and Visual Processing in the Retina. Annu. Rev. Vis. Sci. 2015, 1, 235–262. [Google Scholar] [CrossRef]
- Heidelberger, R.; Thoreson, W.B.; Witkovsky, P. Synaptic transmission at retinal ribbon synapses. Prog. Retin. Eye Res. 2005, 24, 682–720. [Google Scholar] [CrossRef]
- Sterling, P.; Matthews, G. Structure and function of ribbon synapses. Trends Neurosci. 2005, 28, 20–29. [Google Scholar] [CrossRef]
- Schmitz, F. The making of synaptic ribbons: How they are built and what they do. Neuroscientist 2009, 15, 611–624. [Google Scholar] [CrossRef]
- Morgans, C.W. Presynaptic proteins of ribbon synapses in the retina. Microsc. Res. Tech. 2000, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B.; Zenisek, D. Presynaptic Proteins and Their Roles in Visual Processing by the Retina. Annu. Rev. Vis. Sci. 2024, 10, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Morgans, C.W.; Brandstatter, J.H.; Kellerman, J.; Betz, H.; Wassle, H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J. Neurosci. 1996, 16, 6713–6721. [Google Scholar] [CrossRef] [PubMed]
- Kakakhel, M.; Tebbe, L.; Makia, M.S.; Conley, S.M.; Sherry, D.M.; Al-Ubaidi, M.R.; Naash, M.I. Syntaxin 3 is essential for photoreceptor outer segment protein trafficking and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 20615–20624. [Google Scholar] [CrossRef] [PubMed]
- Sherry, D.M.; Mitchell, R.; Standifer, K.M.; du Plessis, B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006, 7, 54. [Google Scholar] [CrossRef]
- Brandstatter, J.H.; Wassle, H.; Betz, H.; Morgans, C.W. The plasma membrane protein SNAP-25, but not syntaxin, is present at photoreceptor and bipolar cell synapses in the rat retina. Eur. J. Neurosci. 1996, 8, 823–828. [Google Scholar] [CrossRef]
- Ullrich, B.; Sudhof, T.C. Distribution of synaptic markers in the retina: Implications for synaptic vesicle traffic in ribbon synapses. J. Physiol.-Paris 1994, 88, 249–257. [Google Scholar] [CrossRef]
- Shekhar, K.; Lapan, S.W.; Whitney, I.E.; Tran, N.M.; Macosko, E.Z.; Kowalczyk, M.; Adiconis, X.; Levin, J.Z.; Nemesh, J.; Goldman, M.; et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 2016, 166, 1308–1323. [Google Scholar] [CrossRef]
- Zulliger, R.; Conley, S.M.; Mwoyosvi, M.L.; Stuck, M.W.; Azadi, S.; Naash, M.I. SNAREs Interact with Retinal Degeneration Slow and Rod Outer Segment Membrane Protein-1 during Conventional and Unconventional Outer Segment Targeting. PLoS ONE 2015, 10, e0138508. [Google Scholar] [CrossRef]
- Heidelberger, R. Adenosine triphosphate and the late steps in calcium-dependent exocytosis at a ribbon synapse. J. Gen. Physiol. 1998, 111, 225–241. [Google Scholar] [CrossRef]
- Heidelberger, R.; Sterling, P.; Matthews, G. Roles of ATP in synaptic vesicle pool depletion and replenishment. J. Neurophysiol. 2001, 88, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, S.; Tsai, M.C.; von Gersdorff, H.; Wadiche, J.I. The ubiquitous nature of multivesicular release. Trends Neurosci. 2015, 38, 428–438. [Google Scholar] [CrossRef]
- James, B.; Piekarz, P.; Moya-Diaz, J.; Lagnado, L. The Impact of Multivesicular Release on the Transmission of Sensory Information by Ribbon Synapses. J. Neurosci. 2022, 42, 9401–9414. [Google Scholar] [CrossRef] [PubMed]
- James, B.; Darnet, L.; Moya-Diaz, J.; Seibel, S.H.; Lagnado, L. An amplitude code transmits information at a visual synapse. Nat. Neurosci. 2019, 22, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.H.; Lassova, L.; Vardi, N.; Diamond, J.S. Coordinated multivesicular release at a mammalian ribbon synapse. Nat. Neurosci. 2004, 7, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.; Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010, 11, 812–822. [Google Scholar] [CrossRef]
- Parsons, T.D.; Sterling, P. Synaptic ribbon. Conveyor belt or safety belt? Neuron 2003, 37, 379–382. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Henry, D.; Akmentin, W.; Matthews, G. Nanoscale dynamics of synaptic vesicle trafficking and fusion at the presynaptic active zone. Elife 2016, 5, e13245. [Google Scholar] [CrossRef]
- Alvarez de Toledo, G.; Fernandez, J.M. Compound versus multigranular exocytosis in peritoneal mast cells. J. Gen. Physiol. 1990, 95, 397–409. [Google Scholar] [CrossRef]
- Sanchez, E.; Gonzalez, E.A.; Moreno, D.S.; Cardenas, R.A.; Ramos, M.A.; Davalos, A.J.; Manllo, J.; Rodarte, A.I.; Petrova, Y.; Moreira, D.C.; et al. Syntaxin 3, but not syntaxin 4, is required for mast cell-regulated exocytosis, where it plays a primary role mediating compound exocytosis. J. Biol. Chem. 2019, 294, 3012–3023. [Google Scholar] [CrossRef]
- Brochetta, C.; Suzuki, R.; Vita, F.; Soranzo, M.R.; Claver, J.; Madjene, L.C.; Attout, T.; Vitte, J.; Varin-Blank, N.; Zabucchi, G.; et al. Munc18-2 and syntaxin 3 control distinct essential steps in mast cell degranulation. J. Immunol. 2014, 192, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Scepek, S.; Lindau, M. Focal exocytosis by eosinophils—Compound exocytosis and cumulative fusion. Embo J. 1993, 12, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Berglund, L.; Nielsen, L.P.; Nielsen, S.; Hoffmann, H.J.; Dahl, R. Regulated exocytosis in immune function: Are SNARE-proteins involved? Respir. Med. 2000, 94, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Koo, E.; Kwan, E.; Kang, Y.; Park, S.; Xie, H.; Sugita, S.; Gaisano, H.Y. Syntaxin-3 regulates newcomer insulin granule exocytosis and compound fusion in pancreatic beta cells. Diabetologia 2013, 56, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.B.; Sheu, L.; Ghai, M.; Bouquillon, A.; Grondin, G.; Weller, U.; Beaudoin, A.R.; Bennett, M.K.; Trimble, W.S.; Gaisano, H.Y. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology 1996, 137, 1340–1348. [Google Scholar] [CrossRef]
- Borisovska, M. Syntaxins on granules promote docking of granules via interactions with munc18. Sci. Rep. 2018, 8, 193. [Google Scholar] [CrossRef]
- Von Gersdorff, H.; Matthews, G. Inhibition of Endocytosis by Elevated Internal Calcium In a Synaptic Terminal. Nature 1994, 370, 652–655. [Google Scholar] [CrossRef]
- Heidelberger, R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J. Neurosci. 2001, 21, 6467–6474. [Google Scholar] [CrossRef]
- Neves, G.; Lagnado, L. The kinetics of exocytosis and endocytosis in the synaptic terminal of goldfish retinal bipolar cells. J. Physiol. 1999, 515, 181–202. [Google Scholar] [CrossRef]
- Matthews, G. Synaptic exocytosis and endocytosis: Capacitance measurements. Curr. Opin. Neurobiol. 1996, 6, 358–364. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Gronborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brugger, B.; Ringler, P.; et al. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Ahmed, S.; Jahn, R.; Klingauf, J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc. Natl. Acad. Sci. USA 2011, 108, 14318–14323. [Google Scholar] [CrossRef] [PubMed]
- Mohrmann, R.; de Wit, H.; Verhage, M.; Neher, E.; Sorensen, J.B. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science 2010, 330, 502–505. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaart, G.; Holt, M.G.; Bunt, G.; Riedel, D.; Wouters, F.S.; Jahn, R. One SNARE complex is sufficient for membrane fusion. Nat. Struct. Mol. Biol. 2010, 17, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Z.; Zhao, Y.; Sung, C.H. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 2007, 130, 535–547. [Google Scholar] [CrossRef]
- Kwok, M.C.; Holopainen, J.M.; Molday, L.L.; Foster, L.J.; Molday, R.S. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol. Cell Proteom. 2008, 7, 1053–1066. [Google Scholar] [CrossRef]
- Mazelova, J.; Ransom, N.; Astuto-Gribble, L.; Wilson, M.C.; Deretic, D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J. Cell Sci. 2009, 122, 2003–2013. [Google Scholar] [CrossRef]
- Kandachar, V.; Tam, B.M.; Moritz, O.L.; Deretic, D. An interaction network between the SNARE VAMP7 and Rab GTPases within a ciliary membrane-targeting complex. J. Cell Sci. 2018, 131, jcs222034. [Google Scholar] [CrossRef]
- Huang, M.; Chow, C.H.; Gurdita, A.; Harada, H.; Pham Truong, V.Q.B.; Eide, S.; Sun, H.S.; Feng, Z.P.; Monnier, P.P.; Wallace, V.A.; et al. SNAP-25, but not SNAP-23, is essential for photoreceptor development, survival, and function in mice. Commun. Biol. 2024, 7, 34. [Google Scholar] [CrossRef]
- Veleri, S.; Nellissery, J.; Mishra, B.; Manjunath, S.H.; Brooks, M.J.; Dong, L.; Nagashima, K.; Qian, H.; Gao, C.; Sergeev, Y.V.; et al. REEP6 mediates trafficking of a subset of Clathrin-coated vesicles and is critical for rod photoreceptor function and survival. Hum. Mol. Genet. 2017, 26, 2218–2230. [Google Scholar] [CrossRef]
- Tebbe, L.; Kakakhel, M.; Al-Ubaidi, M.R.; Naash, M.I. The role of syntaxins in retinal function and health. Front. Cell Neurosci. 2024, 18, 1380064. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hurtado, M.; Dao, C.; Saenz, A.E.; Heidelberger, R. Syntaxin 3 is haplosufficient for long-term photoreceptor survival in the mouse retina. Front. Ophthalmol. 2023, 3, 1208805. [Google Scholar] [CrossRef] [PubMed]
- Ratnapriya, R.; Sosina, O.A.; Starostik, M.R.; Kwicklis, M.; Kapphahn, R.J.; Fritsche, L.G.; Walton, A.; Arvanitis, M.; Gieser, L.; Pietraszkiewicz, A.; et al. Retinal transcriptome and eQTL analyses identify genes associated with age-related macular degeneration. Nat. Genet. 2019, 51, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Giovannone, A.J.; Reales, E.; Bhattaram, P.; Nackeeran, S.; Monahan, A.B.; Syed, R.; Weimbs, T. The H(abc) domain of syntaxin 3 is a ubiquitin binding domain. Sci. Rep. 2020, 10, 21350. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.B.; Fasshauer, D.; Jahn, R.; Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 1998, 395, 347–353. [Google Scholar] [CrossRef]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Sheng, Y.; Zhang, M.; Zou, W.; Wu, L.; Kang, L.; Rizo, J.; Zhang, R.; Xu, T.; et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat. Struct. Mol. Biol. 2015, 22, 547–554. [Google Scholar] [CrossRef]
- Gerber, S.H.; Rah, J.C.; Min, S.W.; Liu, X.; de Wit, H.; Dulubova, I.; Meyer, A.C.; Rizo, J.; Arancillo, M.; Hammer, R.E.; et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science 2008, 321, 1507–1510. [Google Scholar] [CrossRef]
- Cooper, B.; Hemmerlein, M.; Ammermuller, J.; Imig, C.; Reim, K.; Lipstein, N.; Kalla, S.; Kawabe, H.; Brose, N.; Brandstatter, J.H.; et al. Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J. Neurosci. 2012, 32, 8040–8052. [Google Scholar] [CrossRef]
- Tadokoro, S.; Shibata, T.; Inoh, Y.; Amano, T.; Nakanishi, M.; Hirashima, N.; Utsunomiya-Tate, N. Phosphorylation of syntaxin-3 at Thr 14 negatively regulates exocytosis in RBL-2H3 mast cells. Cell Biol. Int. 2016, 40, 589–596. [Google Scholar] [CrossRef]
- Risinger, C.; Bennett, M.K. Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J. Neurochem. 1999, 72, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Hirling, H.; Scheller, R.H. Phosphorylation of synaptic vesicle proteins: Modulation of the alpha SNAP interaction with the core complex. Proc. Natl. Acad. Sci. USA 1996, 93, 11945–11949. [Google Scholar] [CrossRef] [PubMed]
- Foletti, D.L.; Lin, R.; Finley, M.A.; Scheller, R.H. Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J. Neurosci. 2000, 20, 4535–4544. [Google Scholar] [CrossRef] [PubMed]
- Kohansal-Nodehi, M.; Chua, J.J.; Urlaub, H.; Jahn, R.; Czernik, D. Analysis of protein phosphorylation in nerve terminal reveals extensive changes in active zone proteins upon exocytosis. Elife 2016, 5, e14530. [Google Scholar] [CrossRef] [PubMed]
- Dubois, T.; Kerai, P.; Learmonth, M.; Cronshaw, A.; Aitken, A. Identification of syntaxin-1A sites of phosphorylation by casein kinase I and casein kinase II. Eur. J. Biochem. 2002, 269, 909–914. [Google Scholar] [CrossRef]
- Vardar, G.; Salazar-Lazaro, A.; Brockmann, M.; Weber-Boyvat, M.; Zobel, S.; Kumbol, V.W.; Trimbuch, T.; Rosenmund, C. Reexamination of N-terminal domains of syntaxin-1 in vesicle fusion from central murine synapses. Elife 2021, 10, e69498. [Google Scholar] [CrossRef]
- Mennerick, S.; Matthews, G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 1996, 17, 1241–1249. [Google Scholar] [CrossRef]
- Gomis, A.; Burrone, J.; Lagnado, L. Two actions of calcium regulate the supply of releasable vesicles at the ribbon synapse of retinal bipolar cells. J. Neurosci. 1999, 19, 6309–6317. [Google Scholar] [CrossRef]
- Van Hook, M.J.; Parmelee, C.M.; Chen, M.; Cork, K.M.; Curto, C.; Thoreson, W.B. Calmodulin enhances ribbon replenishment and shapes filtering of synaptic transmission by cone photoreceptors. J. Gen. Physiol. 2014, 144, 357–378. [Google Scholar] [CrossRef]
- Heidelberger, R. Electrophysiological approaches to the study of neuronal exocytosis and synaptic vesicle dynamics. Rev. Physiol. Biochem. Pharmacol. 2001, 143, 1–80. [Google Scholar]
- Vardar, G.; Salazar-Lazaro, A.; Zobel, S.; Trimbuch, T.; Rosenmund, C. Syntaxin-1A modulates vesicle fusion in mammalian neurons via juxtamembrane domain dependent palmitoylation of its transmembrane domain. Elife 2022, 11, e78182. [Google Scholar] [CrossRef] [PubMed]
- Singer-Lahat, D.; Barak-Broner, N.; Sheinin, A.; Greitzer-Antes, D.; Michaelevski, I.; Lotan, I. The Dual Function of the Polybasic Juxtamembrane Region of Syntaxin 1A in Clamping Spontaneous Release and Stimulating Ca2+-Triggered Release in Neuroendocrine Cells. J. Neurosci. 2018, 38, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.D.; Tryoen-Toth, P.; Tsai, B.; Vitale, N.; Stuenkel, E.L. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol. Biol. Cell 2008, 19, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kweon, D.H.; Shin, Y.K. Membrane topologies of neuronal SNARE folding intermediates. Biochemistry 2002, 41, 10928–10933. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Vicogne, J.; Zaitseva, I.; McLaughlin, S.; Pessin, J.E. Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane. Mol. Biol. Cell 2009, 20, 4910–4919. [Google Scholar] [CrossRef]
- Greitzer-Antes, D.; Barak-Broner, N.; Berlin, S.; Oron, Y.; Chikvashvili, D.; Lotan, I. Tracking Ca2+-dependent and Ca2+-independent conformational transitions in syntaxin 1A during exocytosis in neuroendocrine cells. J. Cell Sci. 2013, 126, 2914–2923. [Google Scholar] [CrossRef]
- Khelashvili, G.; Galli, A.; Weinstein, H. Phosphatidylinositol 4,5-biphosphate (PIP(2)) lipids regulate the phosphorylation of syntaxin N-terminus by modulating both its position and local structure. Biochemistry 2012, 51, 7685–7698. [Google Scholar] [CrossRef]
- Barak-Broner, N.; Singer-Lahat, D.; Chikvashvili, D.; Lotan, I. CK2 Phosphorylation Is Required for Regulation of Syntaxin 1A Activity in Ca2+-Triggered Release in Neuroendocrine Cells. Int. J. Mol. Sci. 2021, 22, 13556. [Google Scholar] [CrossRef]
- Han, G.A.; Malintan, N.T.; Collins, B.M.; Meunier, F.A.; Sugita, S. Munc18-1 as a key regulator of neurosecretion. J. Neurochem. 2010, 115, 1–10. [Google Scholar] [CrossRef]
- Gulyás-Kovács, A.; de Wit, H.; Milosevic, I.; Kochubey, O.; Toonen, R.; Klingauf, J.; Verhage, M.; Sørensen, J.B. Munc18-1: Sequential Interactions with the Fusion Machinery Stimulate Vesicle Docking and Priming. J. Neurosci. 2007, 27, 8676. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Wang, L.; Wang, C.; Liu, C.; Guo, A.; Liu, M.; Zhang, L.; Ma, C.; Zhang, X.; et al. p.His16Arg of STXBP1 (MUNC18-1) Associated With Syntaxin 3B Causes Autosomal Dominant Congenital Nystagmus. Front. Cell Dev. Biol. 2020, 8, 591781. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Missler, M.; Li, C.; Sudhof, T.C. Complexins: Cytosolic proteins that regulate SNAP receptor function. Cell 1995, 83, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mohrmann, R.; Dhara, M.; Bruns, D. Complexins: Small but capable. Cell Mol. Life Sci. 2015, 72, 4221–4235. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef]
- Reim, K.; Wegmeyer, H.; Brandstatter, J.H.; Xue, M.; Rosenmund, C.; Dresbach, T.; Hofmann, K.; Brose, N. Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol. 2005, 169, 669–680. [Google Scholar] [CrossRef]
- Landgraf, I.; Muhlhans, J.; Dedek, K.; Reim, K.; Brandstatter, J.H.; Ammermuller, J. The absence of Complexin 3 and Complexin 4 differentially impacts the ON and OFF pathways in mouse retina. Eur. J. Neurosci. 2012, 36, 2470–2481. [Google Scholar] [CrossRef]
- Schaub, J.R.; Lu, X.; Doneske, B.; Shin, Y.K.; McNew, J.A. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat. Struct. Mol. Biol. 2006, 13, 748–750. [Google Scholar] [CrossRef]
- Babai, N.; Sendelbeck, A.; Regus-Leidig, H.; Fuchs, M.; Mertins, J.; Reim, K.; Brose, N.; Feigenspan, A.; Brandstatter, J.H. Functional Roles of Complexin 3 and Complexin 4 at Mouse Photoreceptor Ribbon Synapses. J. Neurosci. 2016, 36, 6651–6667. [Google Scholar] [CrossRef]
- Vaithianathan, T.; Henry, D.; Akmentin, W.; Matthews, G. Functional roles of complexin in neurotransmitter release at ribbon synapses of mouse retinal bipolar neurons. J. Neurosci. 2015, 35, 4065–4070. [Google Scholar] [CrossRef]
- Lux, U.T.; Meyer, J.; Jahn, O.; Davison, A.; Babai, N.; Giessl, A.; Wartenberg, A.; Sticht, H.; Brose, N.; Reim, K.; et al. Light-dependent regulation of neurotransmitter release from rod photoreceptor ribbon synapses involves an interplay of Complexin 4 and Transducin with the SNARE complex. Front. Mol. Neurosci. 2024, 17, 1308466. [Google Scholar] [CrossRef]
- Bhoi, J.D.; Zhang, Z.; Janz, R.; You, Y.; Wei, H.; Wu, J.; Ribelayga, C.P. The SNARE regulator Complexin3 is a target of the cone circadian clock. J. Comp. Neurol. 2021, 529, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Reim, K.; Regus-Leidig, H.; Ammermuller, J.; El-Kordi, A.; Radyushkin, K.; Ehrenreich, H.; Brandstatter, J.H.; Brose, N. Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4. J. Cell Sci. 2009, 122, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Dick, O.; tom Dieck, S.; Altrock, W.D.; Ammermuller, J.; Weiler, R.; Garner, C.C.; Gundelfinger, E.D.; Brandstatter, J.H. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 2003, 37, 775–786. [Google Scholar] [CrossRef] [PubMed]
- tom Dieck, S.; Altrock, W.D.; Kessels, M.M.; Qualmann, B.; Regus, H.; Brauner, D.; Fejtova, A.; Bracko, O.; Gundelfinger, E.D.; Brandstatter, J.H. Molecular dissection of the photoreceptor ribbon synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J. Cell Biol. 2005, 168, 825–836. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, H.; Perez-Hurtado, M.; Heidelberger, R. Syntaxin 3B: A SNARE Protein Required for Vision. Int. J. Mol. Sci. 2024, 25, 10665. https://doi.org/10.3390/ijms251910665
Dey H, Perez-Hurtado M, Heidelberger R. Syntaxin 3B: A SNARE Protein Required for Vision. International Journal of Molecular Sciences. 2024; 25(19):10665. https://doi.org/10.3390/ijms251910665
Chicago/Turabian StyleDey, Himani, Mariajose Perez-Hurtado, and Ruth Heidelberger. 2024. "Syntaxin 3B: A SNARE Protein Required for Vision" International Journal of Molecular Sciences 25, no. 19: 10665. https://doi.org/10.3390/ijms251910665





