C5a Induces Inflammatory Signaling and Apoptosis in PC12 Cells through C5aR-Dependent Signaling: A Potential Mechanism for Adrenal Damage in Sepsis
Abstract
1. Introduction
1.1. Sepsis
1.2. The Complement System
1.3. Complement C5a
1.4. Primary Adrenal Dysfunction
1.5. Research Objectives and Hypothesis
2. Results
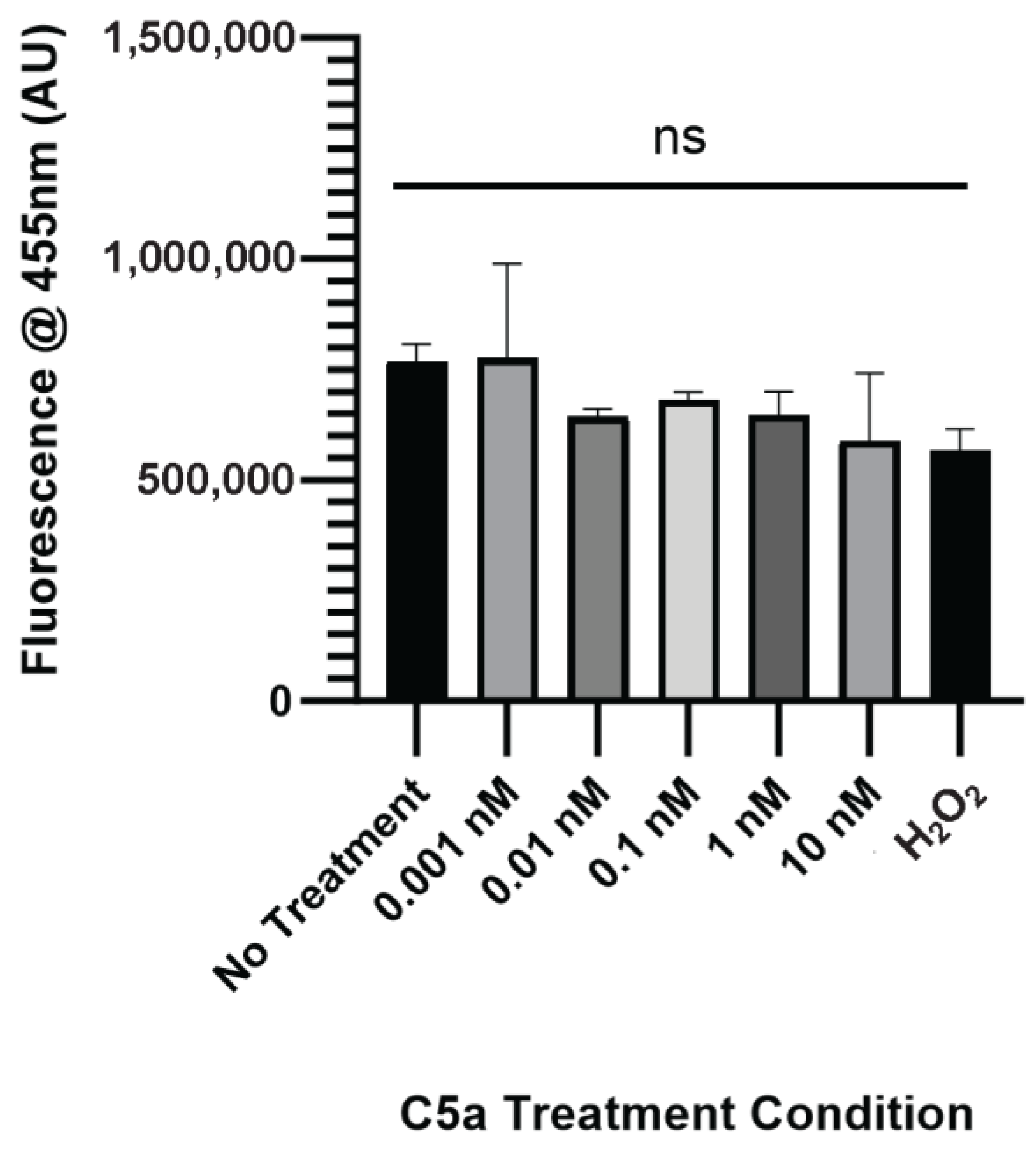
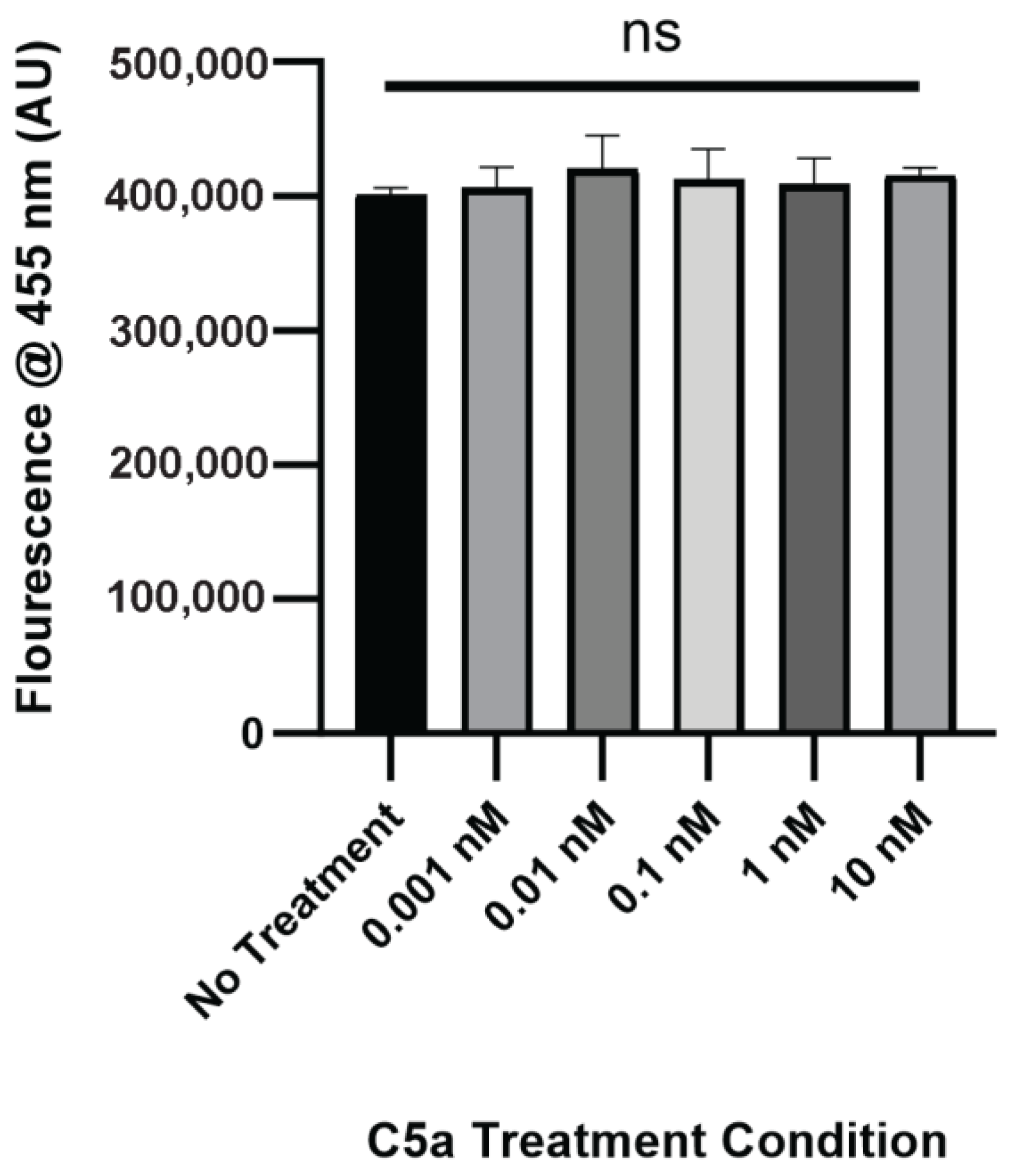
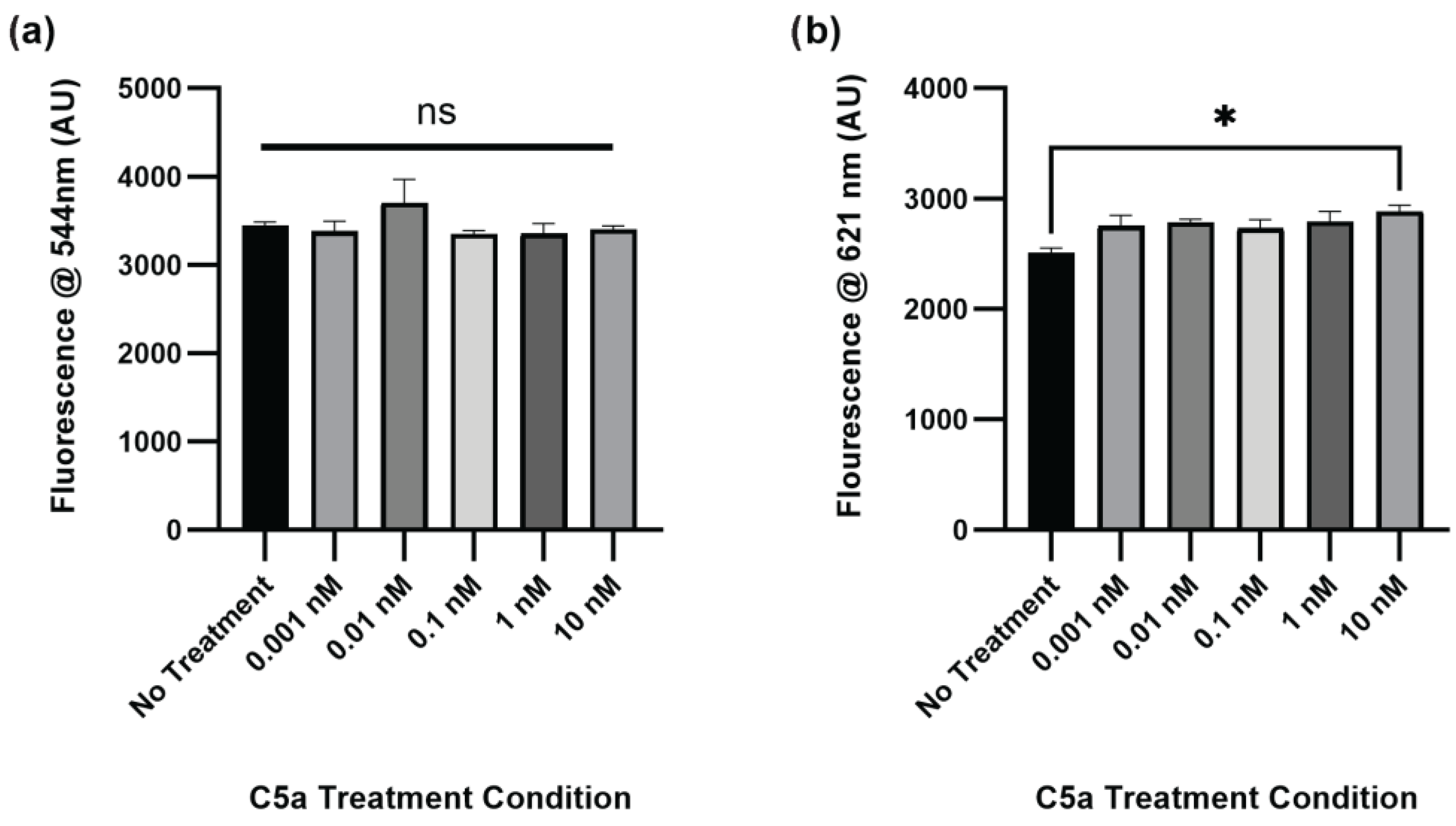
2.1. Complement C5a Induces Caspase Activation and Apoptosis in PC12 Cells
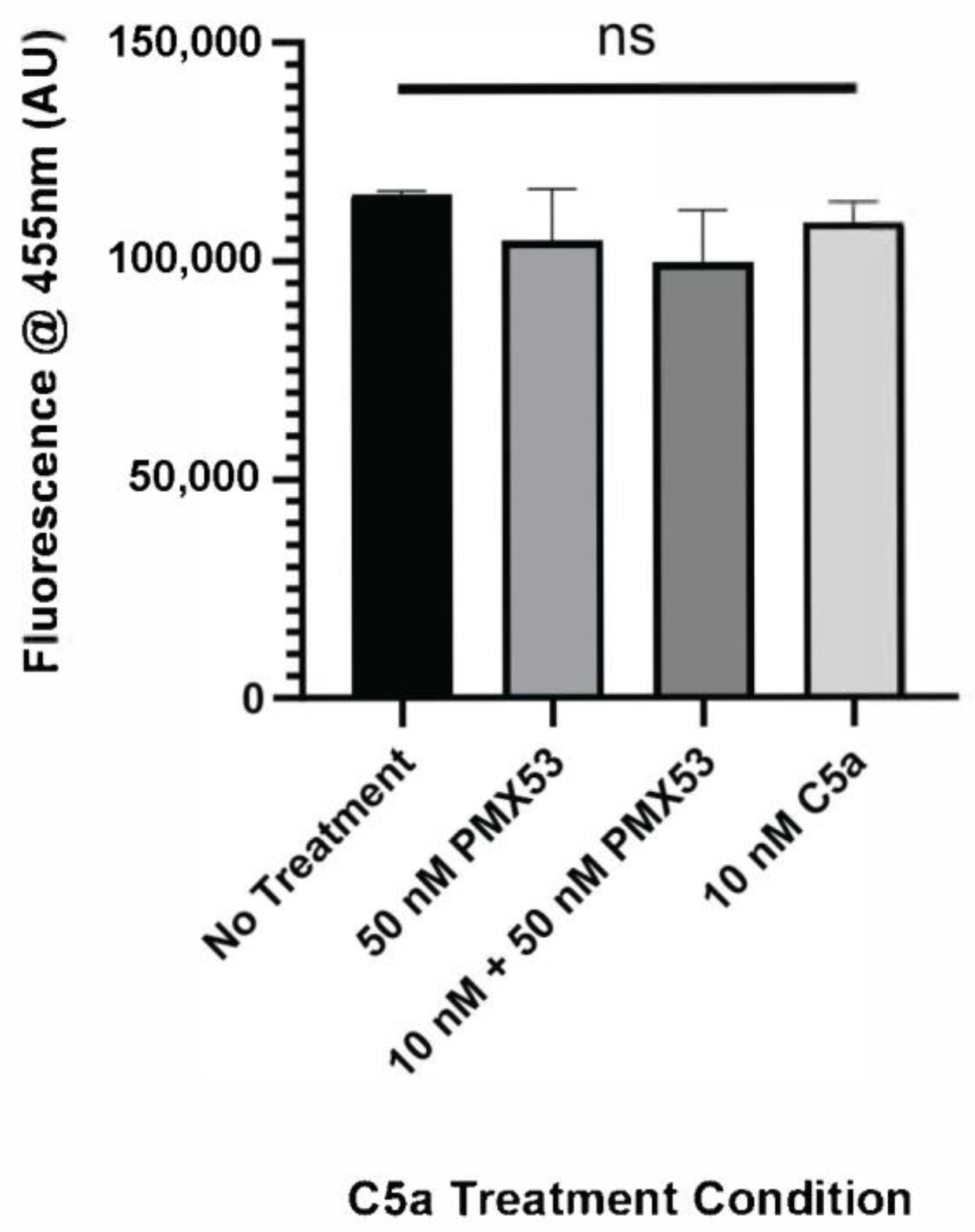
2.2. PMX-53 Inhibits C5a-Induced Caspase Upregulation
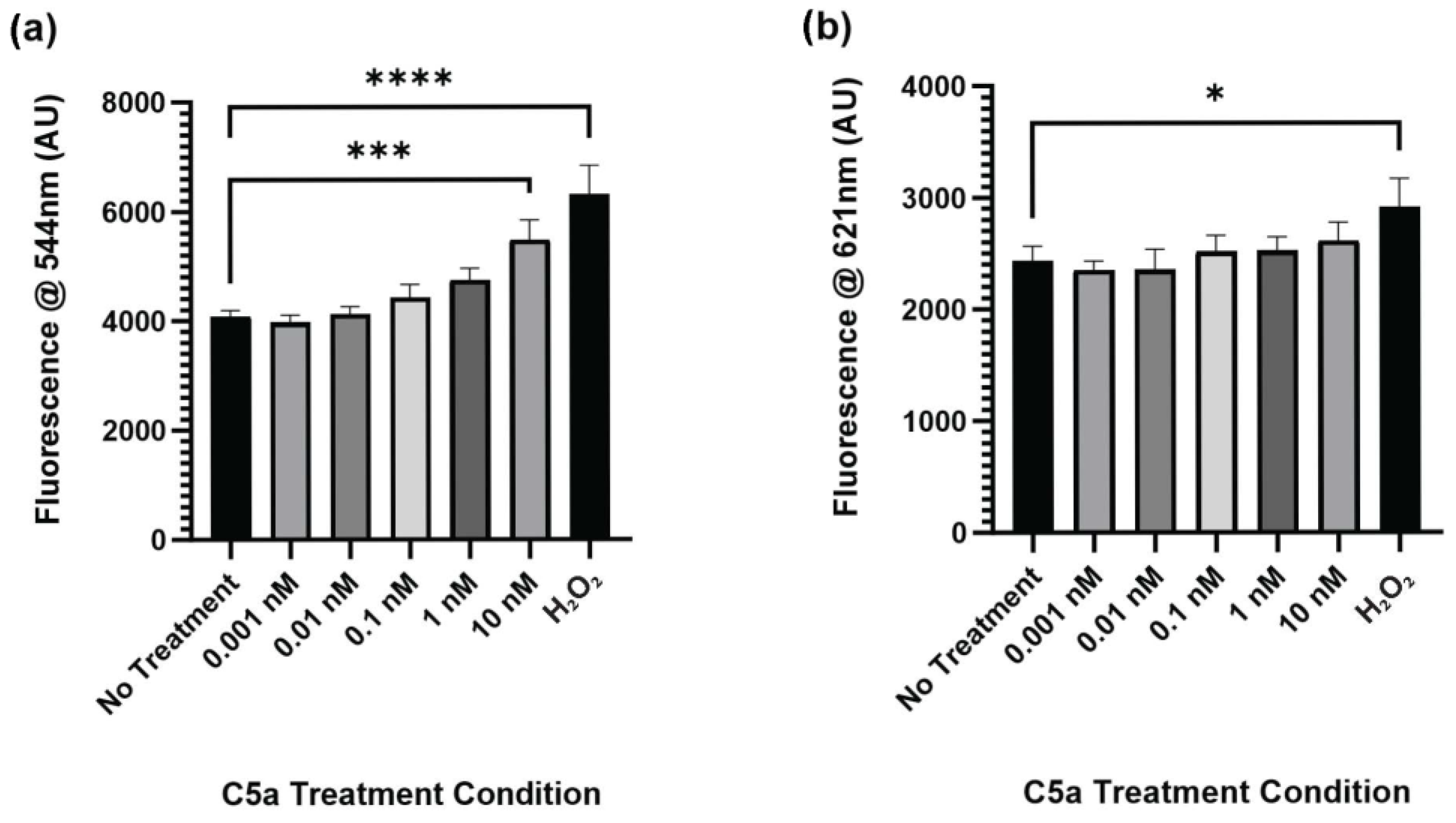
2.3. C5a Induces Inflammatory Signaling in PC12 Cells
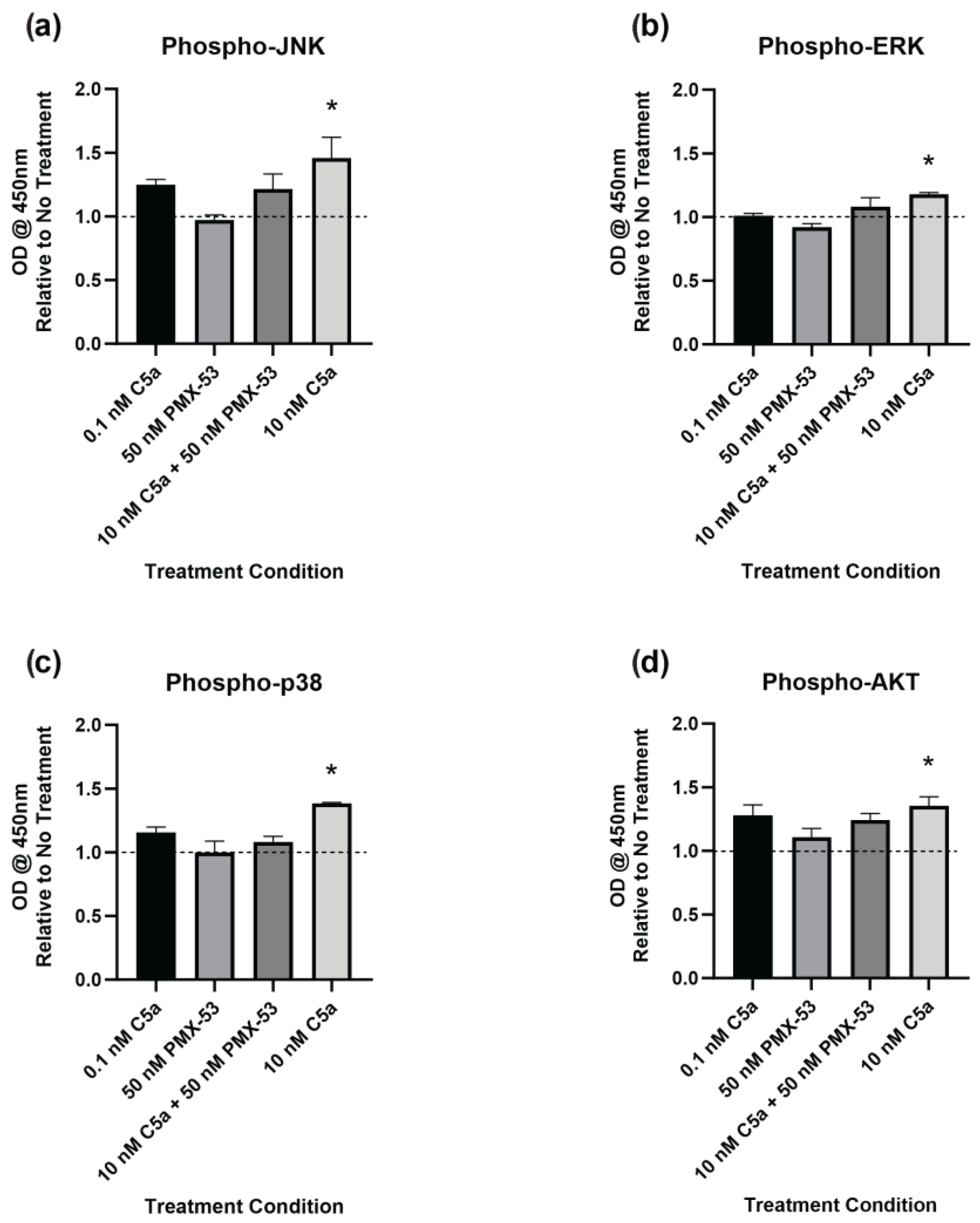
2.4. Complement C5a Induces Protein Kinase Signaling in PC12 Cells
3. Discussion
3.1. C5a and Apoptosis
3.2. C5a and Cellular Signaling
3.2.1. JNK
3.2.2. ERK
3.2.3. p38
3.2.4. AKT
3.2.5. Intercellular and Inflammatory Signaling
3.2.6. Chemotaxis
3.3. Clinical Relevance and Future Directions
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. C5a Treatment
4.4. Caspase Activation and Cell Death Assay
4.5. Antibody Array
4.6. Phosphorylation ELISA
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Singer, M. Mechanisms of Sepsis-Induced Organ Dysfunction. Crit. Care Med. 2007, 35, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. New Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.J.; Bersani, L.; Mantovani, A. Tumor Necrosis Factor Is Chemotactic for Monocytes and Polymorphonuclear Leukocytes. J. Immunol. 1987, 138, 1469–1474. [Google Scholar] [CrossRef]
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like Receptors: Significance, Ligands, Signaling Pathways, and Functions in Mammals. Int. Rev. Immunol. 2018, 37, 20–36. [Google Scholar] [CrossRef]
- Munoz, C.; Carlet, J.; Fitting, C.; Misset, B.; Blériot, J.P.; Cavaillon, J.M. Dysregulation of In Vitro Cytokine Production by Monocytes During Sepsis. J. Clin. Investig. 1991, 88, 1747. [Google Scholar] [CrossRef]
- Walport, M.J. Complement. New Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The Complement System. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Halstensen, T.S.; Brandtzaeg, P. Local Complement Activation in Inflammatory Bowel Disease. Immunol. Res. 1991, 10, 485–492. [Google Scholar] [CrossRef]
- Mannes, M.; Schmidt, C.Q.; Nilsson, B.; Ekdahl, K.N.; Huber-Lang, M. Complement as Driver of Systemic Inflammation and Organ Failure in Trauma, Burn, and Sepsis. Semin. Immunopathol. 2021, 43, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Korb, L.C.; Ahearn, J.M. C1q Binds Directly and Specifically to Surface Blebs of Apoptotic Human Keratinocytes: Complement Deficiency and Systemic Lupus Erythematosus Revisited. J. Immunol. 1997, 158, 4525–4528. [Google Scholar] [CrossRef]
- El-Lati, S.G.; Dahinden, C.A.; Church, M.K. Complement Peptides C3a- and C5a-Induced Mediator Release from Dissociated Human Skin Mast Cells. J. Investig. Dermatol. 1994, 102, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Hirata, T.; Enomoto, M.; Araki, T.; Ishimaru, H.; Takahashi, T.A. A Putative Chemoattractant Receptor, C5L2, Is Expressed in Granulocyte and Immature Dendritic Cells, but Not in Mature Dendritic Cells. Mol. Immunol. 2000, 37, 407–412. [Google Scholar] [CrossRef]
- Mollnes, T.E.; Brekke, O.-L.; Fung, M.; Fure, H.; Christiansen, D.; Bergseth, G.; Videm, V.; Lappegård, K.T.; Köhl, J.; Lambris, J.D. Essential Role of the C5a Receptor in E Coli–Induced Oxidative Burst and Phagocytosis Revealed by a Novel Lepirudin-Based Human Whole Blood Model of Inflammation. Blood 2002, 100, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Zha, W.s.; Ye, L.p.; Wang, F.; Wang, H.; Shen, T.; Wu, C.h.; Zhu, Q.x. Complement C5a-C5aR Interaction Enhances MAPK Signaling Pathway Activities to Mediate Renal Injury in Trichloroethylene Sensitized BALB/c Mice. J. Appl. Toxicol. 2016, 36, 271–284. [Google Scholar] [CrossRef]
- Klos, A.; Wende, E.; Wareham, K.J.; Monk, P.N. International Union of Basic and Clinical Pharmacology. LXXXVII. Complement Peptide C5a, C4a, and C3a Receptors. Pharmacol. Rev. 2013, 65, 500–543. [Google Scholar] [CrossRef]
- Li, X.X.; Lee, J.D.; Kemper, C.; Woodruff, T.M. The Complement Receptor C5aR2: A Powerful Modulator of Innate and Adaptive Immunity. J. Immunol. 2019, 202, 3339–3348. [Google Scholar] [CrossRef]
- Scola, A.M.; Johswich, K.O.; Morgan, B.P.; Klos, A.; Monk, P.N. The Human Complement Fragment Receptor, C5L2, Is a Recycling Decoy Receptor. Mol. Immunol. 2009, 46, 1149–1162. [Google Scholar] [CrossRef]
- Bengtson, A.; Holmberg, P.; Heideman, M. The Ischaemic Leg as a Source of Complement Activation. Br. J. Surg. 2005, 74, 697–700. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Jenkins, M.K.; Nelson, R.D.; Chenoweth, D.; Simmons, R.L. Neutrophil Dysfunction in Sepsis. II. Evidence for the Role of Complement Activation Products in Cellular Deactivation. Surgery 1981, 90, 319–327. [Google Scholar] [PubMed]
- Nakae, H.; Endo, S.; Inada, K.; Yoshida, M. Chronological Changes in the Complement System in Sepsis. Surg. Today 1996, 26, 225–229. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Chen, A.J.; Nadeau, B.A.; Day, D.E.; Sarma, J.V.; Huber-Lang, M.S.; Ward, P.A. The Complement Anaphylatoxin C5a Induces Apoptosis in Adrenomedullary Cells during Experimental Sepsis. PLoS ONE 2008, 3, e2560. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chen, Z.F.; Yan, J.; Li, Q.F.; Huang, Y.; Xu, H.; Zhang, X.; Jiang, H. Complement C5a Exacerbates Acute Lung Injury Induced through Autophagy-Mediated Alveolar Macrophage Apoptosis. Cell Death Dis. 2014, 5, e1330. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Tutino, V.M.; Redae, Y.; Meng, H.; Siddiqui, A.; Woodruff, T.M.; Jarvis, J.N.; Hennon, T.; Schwartz, S.; Quigg, R.J.; et al. C5a Induces Caspase-dependent Apoptosis in Brain Vascular Endothelial Cells in Experimental Lupus. Immunology 2016, 148, 407. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Nonaka, H.; Revollo, I.S.; Semba, U.; Li, Y.; Ota, Y.; Irie, A.; Harada, K.; Kehrl, J.H.; Yamamoto, T. Pro- and Anti-Apoptotic Dual Functions of the C5a Receptor: Involvement of Regulator of G Protein Signaling 3 and Extracellular Signal-Regulated Kinase. Lab. Investig. 2009, 89, 676–694. [Google Scholar] [CrossRef][Green Version]
- Martinus, R.D.; Cook, C.J. The Effect of Complement C5a on Mitochondrial Functions of PC12 Cells. Neuroreport 2011, 22, 581–585. [Google Scholar] [CrossRef]
- Francis, K.; Lewis, B.; Monk, P.; Scanlon, M.; Ham, J. Complement C5a Receptors Are Expressed Throughout the Anterior Pituitary Gland. Endocr. Abstr. 2005, 9, 126. [Google Scholar]
- Chiou, W.F.; Tsai, H.R.; Yang, L.M.; Tsai, W.J. C5a Differentially Stimulates the ERK1/2 and P38 MAPK Phosphorylation Through Independent Signaling Pathways to Induced Chemotactic Migration in RAW264.7 Macrophages. Int. Immunopharmacol. 2004, 4, 1329–1341. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Albuszies, G.; Baumgart, K.; Matejovic, M.; Wachter, U.; Vogt, J.; Radermacher, P.; Calzia, E. Glucose Metabolism and Catecholamines. Crit. Care Med. 2007, 35, S518. [Google Scholar] [CrossRef] [PubMed]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic Receptors and Cardiovascular Effects of Catecholamines. Ann. Endocrinol. 2021, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Prigent, H.; Maxime, V.; Annane, D. Clinical Review: Corticotherapy in Sepsis. Crit. Care 2004, 8, 122–129. [Google Scholar] [CrossRef]
- Hahn, P.Y.; Wang, P.; Tait, S.M.; Ba, Z.F.; Reich, S.S.; Chaudry, I.H. Sustained Elevation in Circulating Catecholamine Levels During Polymicrobial Sepsis. Shock. 1995, 4, 269–273. [Google Scholar] [CrossRef]
- Epstein, F.H.; Parrillo, J.E. Pathogenetic Mechanisms of Septic Shock. New Engl. J. Med. 2010, 328, 1471–1477. [Google Scholar] [CrossRef]
- Dalegrave, D.; Silva, R.L.; Becker, M.; Gehrke, L.V.; Friedman, G. Relative Adrenal Insufficiency as a Predictor of Disease Severity and Mortality in Severe Septic Shock. Rev. Bras. Ter. Intensiv. 2012, 24, 362. [Google Scholar] [CrossRef]
- McKechnie, K.; Dean, H.G.; Furman, B.L.; Parratt, J.R. Plasma Catecholamines during Endotoxin Infusion in Conscious Unrestrained Rats: Effects of Adrenal Demedullation and/or Guanethidine Treatment. Circ. Shock. 1985, 17, 85–94. [Google Scholar]
- Prigent, H.; Maxime, V.; Annane, D. Science Review: Mechanisms of Impaired Adrenal Function in Sepsis and Molecular Actions of Glucocorticoids. Crit. Care 2004, 8, 243–252. [Google Scholar] [CrossRef]
- Hoffmann, R. Adrenal Lesions in Calves Dying from Endotoxin Shock, with Special Reference to the Waterhouse-Friderichsen Syndrome. J. Comp. Pathol. 1977, 87, 231–239. [Google Scholar] [CrossRef]
- Cherkasova, M.N.; Borovaya, T.G.; Zhukhovitskii, V.G.; Pukhalskaia, V.G. Results of Structural and Bacteriological Analysis of the Mouse Adrenal Glands in a Sepsis Model. Bull. Exp. Biol. Med. 2022, 173, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Jung, B.; Aufort, S.; Chanques, G.; Jaber, S.; Gallix, B. Emergency Radiology—Adrenal Gland Volume Measurement in Septic Shock and Control Patients: A Pilot Study. Eur. Radiol. 2010, 20, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Nougaret, S.; Chanques, G.; Mercier, G.; Cisse, M.; Aufort, S.; Gallix, B.; Annane, D.; Jaber, S. The Absence of Adrenal Gland Enlargement during Septic Shock Predicts MortalityA Computed Tomography Study of 239 Patients. Anesthesiology 2011, 115, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a Noradrenergic Clonal Line of Rat Adrenal Pheochromocytoma Cells Which Respond to Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Sangaran, P.G.; Ibrahim, Z.A.; Chik, Z.; Mohamed, Z.; Ahmadiani, A. Lipopolysaccharide Pre-Conditioning Attenuates Pro-Inflammatory Responses and Promotes Cytoprotective Effect in Differentiated PC12 Cell Lines via Pre-Activation of Toll-Like Receptor-4 Signaling Pathway Leading to the Inhibition of Caspase-3/Nuclear Factor-Κappa B Pathway. Front. Cell. Neurosci. 2021, 14, 469. [Google Scholar] [CrossRef]
- Hui, W.; Xu, Y.S.; Miao Lin, W.; Chao, C.; Bian, R.; Yuan, H.; Yi, W.; Guo, T.; Zhu, L.L.; Zhou, H. Protective Effect of Naringin Against the LPS-Induced Apoptosis of PC12 Cells: Implications for the Treatment of Neurodegenerative Disorders. Int. J. Mol. Med. 2017, 39, 819–830. [Google Scholar] [CrossRef]
- Bloise, E.; Ciarmela, P.; Dela Cruz, C.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. The Fas/FasL Signaling Pathway. In Signaling Pathways in Liver Diseases; Dufour, J.-F., Clavien, P.-A., Eds.; Springer: Berlin, Germany, 2005; pp. 129–138. ISBN 978-3-540-27194-9. [Google Scholar]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A Small Protein with Major Functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Function and Regulation of IL-1α in Inflammatory Diseases and Cancer. Immunol. Rev. 2018, 281, 124. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef]
- Lindemann, A.; Mertelsmann, R. Interleukin-3: Structure and Function. Cancer Investig. 1993, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Hural, J. Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 1997, 17, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 Alpha)/CCL3: As a Biomarker. General. Methods Biomark. Res. Their Appl. 2015, 1–2, 223. [Google Scholar] [CrossRef]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF Family: Four Gene Products Form Five Dimeric Isoforms. Cytokine Growth Factor. Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC Chemokine CCL20 and Its Receptor CCR6. Cytokine Growth Factor. Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Campbell, S.J.; Hughes, P.M.; Iredale, J.P.; Wilcockson, D.C.; Waters, S.; Docagne, F.; Perry, V.H.; Anthony, D.C. CINC-1 Is an Acute-Phase Protein Induced by Focal Brain Injury Causing Leukocyte Mobilization and Liver Injury. FASEB J. 2003, 17, 1168–1170. [Google Scholar] [CrossRef]
- Takano, K.; Nakagawa, H. Contribution of Cytokine-Induced Neutrophil Chemoattractant CINC-2 and CINC-3 to Neutrophil Recruitment in Lipopolysaccharide-Induced Inflammation in Rats. Inflamm. Res. 2001, 50, 503–508. [Google Scholar] [CrossRef]
- Van De Stolpe, A.; Van Der Saag, P.T. Intercellular Adhesion Molecule-1. J. Mol. Med. 1996, 74, 13–33. [Google Scholar] [CrossRef]
- Wynn, T.A. IL-13 Effector Functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef]
- Kanczkowski, W.; Zacharowski, K.; Wirth, M.P.; Ehrhart-Bornstein, M.; Bornstein, S.R. Differential Expression and Action of Toll-like Receptors in Human Adrenocortical Cells. Mol. Cell. Endocrinol. 2009, 300, 57–65. [Google Scholar] [CrossRef]
- Jennewein, C.; Tran, N.; Kanczkowski, W.; Heerdegen, L.; Kantharajah, A.; Dröse, S.; Bornstein, S.; Scheller, B.; Zacharowski, K. Mortality of Septic Mice Strongly Correlates with Adrenal Gland Inflammation. Crit. Care Med. 2016, 44, e190–e199. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A. The Dark Side of C5a in Sepsis. Nat. Rev. Immunol. 2004, 4, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dermitzaki, E.; Tsatsanis, C.; Gravanis, A.; Margioris, A.N. Corticotropin-Releasing Hormone Induces Fas Ligand Production and Apoptosis in PC12 Cells via Activation of P38 Mitogen-Activated Protein Kinase. J. Biol. Chem. 2002, 277, 12280–12287. [Google Scholar] [CrossRef]
- Zheng, Y.; You, F.; Li, Q.; Chen, J.; Yang, H. The Effect of Geniste on Aβ25–35-Induced PC12 Cell Apoptosis through the JNK-Dependent Fas Pathway. Food Funct. 2016, 7, 4702–4708. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Z.; Zhang, F.; Liu, Y.; Zhang, Y.; Sun, X.; Sang, M.; Luo, H. Ropivacaine Mesylate Exerts Neurotoxicity via Up-Regulation of Fas/FasL Expression in Rat Pheochromocytoma PC12 Cells. Am. J. Transl. Res. 2019, 11, 1626. [Google Scholar]
- Shivshankar, P.; Li, Y.D.; Mueller-Ortiz, S.L.; Wetsel, R.A. In Response to Complement Anaphylatoxin Peptides C3a and C5a, Human Vascular Endothelial Cells Migrate and Mediate the Activation of B-Cells and Polarization of T-Cells. FASEB J. 2020, 34, 7540–7560. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Woodruff, T.K.; Mayo, K.E. Activin A-Induced HepG2 Liver Cell Apoptosis: Involvement of Activin Receptors and Smad Proteins. Endocrinology 2000, 141, 1263–1272. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, Y.; Li, J.; Liu, B.; Liu, Z.; Cui, X. Activin A Induces Apoptosis of Human Lung Adenocarcinoma A549 Cells Through Endoplasmic Reticulum Stress Pathway. Oncol. Rep. 2024, 51, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Zhao, Y.; Sun, H.; Ge, J.; Liu, Z. Activin A Induces Apoptosis of Mouse Myeloma Cells Via the Mitochondrial Pathway. Oncol. Lett. 2018, 15, 2590. [Google Scholar] [CrossRef]
- Xue, L.X.; Liu, H.Y.; Cui, Y.; Dong, Y.; Wang, J.Q.; Ji, Q.Y.; He, J.T.; Yao, M.; Wang, Y.Y.; Shao, Y.K.; et al. Neuroprotective Effects of Activin A on Endoplasmic Reticulum Stress-Mediated Apoptotic and Autophagic PC12 Cell Death. Neural Regen. Res. 2017, 12, 779. [Google Scholar] [CrossRef]
- Xu, G.H.; He, J.T.; Guo, H.L.; Mei, C.L.; Wang, J.Q.; Li, Z.S.; Chen, H.; Mang, J.; Yang, H.; Xu, Z.X. Activin A Prevents Neuron-like PC12 Cell Apoptosis after Oxygen-Glucose Deprivation. Neural Regen. Res. 2013, 8, 1016. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Iguchi, M.; Watanabe, K.; Hoshino, R.; Tsujimoto, M.; Kohno, M. Specific Activation of the P38 Mitogen-Activated Protein Kinase Signaling Pathway and Induction of Neurite Outgrowth in PC12 Cells by Bone Morphogenetic Protein-2. J. Biol. Chem. 1999, 274, 26503–26510. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.J.; Lin, W.C.; Yang, Y.H.; Tseng, Y.L.; Lin, Y.H.; Chou, C.H.; Tsau, Y.K. High Concentration of C5a-Induced Mitochondria-Dependent Apoptosis in Murine Kidney Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4465. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP Kinase Signalling Cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The Crucial Role of Protein Phosphorylation in Cell Signaling and Its Use as Targeted Therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- An, L.L.; Gorman, J.V.; Stephens, G.; Swerdlow, B.; Warrener, P.; Bonnell, J.; Mustelin, T.; Fung, M.; Kolbeck, R. Complement C5a Induces PD-L1 Expression and Acts in Synergy with LPS Through Erk1/2 and JNK Signaling Pathways. Sci. Rep. 2016, 6, 33346. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.C.; Lu, C.L.; Chen, L.; Ge, K.; Yang, L.L.; Li, W.; Wu, Y.H. C5a/C5aR Pathway Is Essential for Up-Regulating SphK1 Expression Through P38-MAPK Activation in Acute Liver Failure. World J. Gastroenterol. 2016, 22, 10148–10157. [Google Scholar] [CrossRef]
- Zhang, Z. Association of C5a/C5aR Pathway to Activate ERK1/2 and P38 MAPK in Acute Kidney Injury—A Mouse Model. Rev. Rom. Med. Lab. 2022, 30, 31–40. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Yang, F.; Li, G.; Zheng, L.; Wu, Y. C5a/C5aR Pathway Is Essential for the Pathogenesis of Murine Viral Fulminant Hepatitis by Way of Potentiating Fgl2/Fibroleukin Expression. Hepatology 2014, 60, 114–124. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.q.; Zhang, L.; Tang, M.; Cao, X.; Xu, G.l.; Wu, Y.Z. Complement C5a/C5aR Pathway Potentiates the Pathogenesis of Gastric Cancer by Down-Regulating P21 Expression. Cancer Lett. 2018, 412, 30–36. [Google Scholar] [CrossRef]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.T. Nuclear and Cytosolic JNK Signalling in Neurons. Nat. Rev. Neurosci. 2014, 15, 285–299. [Google Scholar] [CrossRef]
- Hirata, Y.; Adachi, K.; Kiuchi, K. Activation of JNK Pathway and Induction of Apoptosis by Manganese in PC12 Cells. J. Neurochem. 1998, 71, 1607–1615. [Google Scholar] [CrossRef]
- Zheng, G.; Luo, W.; Zhang, X.; Zhao, F.; Wang, J.; Chen, Y.; Chen, J. Effect of JNK Pathway in Apoptosis of PC12 Cell by MPP+. J. Hyg. Res. 2011, 40, 109–111. [Google Scholar]
- Zhang, H.; Wang, S.; Wang, Y.; Lu, A.; Hu, C.; Yan, C. DHA Ameliorates MeHg-induced PC12 Cell Apoptosis by Inhibiting the ROS/JNK Signaling Pathway. Mol. Med. Rep. 2021, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Shacka, J.J.; Sahawneh, M.A.; Gonzalez, J.D.; Ye, Y.Z.; D’Alessandro, T.L.; Estévez, A.G. Two Distinct Signaling Pathways Regulate Peroxynitrite-Induced Apoptosis in PC12 Cells. Cell Death Differ. 2006, 13, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Bitto, A.; Pallio, G.; Irrera, N.; Galfo, F.; Interdonato, M.; Mecchio, A.; De Luca, F.; Minutoli, L.; Squadrito, F.; et al. Blockade of the JNK Signalling as a Rational Therapeutic Approach to Modulate the Early and Late Steps of the Inflammatory Cascade in Polymicrobial Sepsis. Mediat. Inflamm. 2015, 2015, 591572. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Hu, D.; Chen, S.; Wang, S.; Xu, Y.; Huang, Y.; Shi, Y.; Zhang, H. Protective Role of JNK Inhibitor SP600125 in Sepsis-Induced Acute Lung Injury. Int. J. Clin. Exp. Pathol. 2019, 12, 528. [Google Scholar]
- Schramek, H. MAP Kinases: From Intracellular Signals to Physiology and Disease. News Physiol. Sci. 2002, 17, 62–67. [Google Scholar] [CrossRef]
- Zhang, L.; Jope, R.S. Oxidative Stress Differentially Modulates Phosphorylation of ERK, P38 and CREB Induced by NGF or EGF in PC12 Cells. Neurobiol. Aging 1999, 20, 271–278. [Google Scholar] [CrossRef]
- Goillot, E.; Raingeaud, J.; Ranger, A.; Tepper, R.I.; Davis, R.J.; Harlow, E.D.; Sanchez, I. Mitogen-Activated Protein Kinase-Mediated Fas Apoptotic Signaling Pathway. Proc. Natl. Acad. Sci. USA 1997, 94, 3302–3307. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Ko, W.C.; Lin, C.M.; Lin, J.W.; Wu, J.C.; Lin, C.J.; Cheng, H.H.; Shih, C.M. Antioxidant N-Acetylcysteine Blocks Nerve Growth Factor-Induced H2O2/ERK Signaling in PC12 Cells. Ann. N. Y. Acad. Sci. 2005, 1042, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, K.H.; Shin, K.S.; Lee, M.K. The Roles of Cyclic AMP–ERK–Bad Signaling Pathways on 6-Hydroxydopamine-Induced Cell Survival and Death in PC12 Cells. Toxicol. Vitr. 2013, 27, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 Activation Controls Cell Proliferation and Cell Death Is Subcellular Localization the Answer? Cell Cycle 2009, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Meng, L.Q.; Xu, P.C.; Chen, M.; Zhao, M.H. P38MAPK, ERK and PI3K Signaling Pathways Are Involved in C5a-Primed Neutrophils for ANCA-Mediated Activation. PLoS ONE 2012, 7, e38317. [Google Scholar] [CrossRef][Green Version]
- Ono, K.; Han, J. The P38 Signal Transduction Pathway Activation and Function. Cell Signal 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yuyama, K.; Yamamoto, H. Low Concentrations of Nitric Oxide (NO) Induced Cell Death in PC12 Cells through Activation of P38 Mitogen-Activated Protein Kinase (P38 MAPK) but Not via Extracellular Signal-Regulated Kinases (ERK1/2) or c-Jun N-Terminal Protein Kinase (JNK). Neurosci. Lett. 2006, 392, 170–173. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Chen, S.; Shen, B.; Yu, F.; Zhang, Y.; Shen, R. Apocynin Attenuates Cobalt Chloride-Induced Pheochromocytoma Cell Apoptosis by Inhibiting P38-MAPK/Caspase-3 Pathway. Cell. Physiol. Biochem. 2018, 48, 208–214. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, E.; Sun, Z.; Song, Z.; Ye, Z.; Zhang, Z. P38 MAPK Inhibitor SB203580 Attenuates the Toxicity of Ropivacaine on PC12 Cells. J. Cent. S. Univ. Med. Sci. 2019, 44, 985–989. [Google Scholar] [CrossRef]
- Zhuang, S.; Demirs, J.T.; Kochevar, I.E. P38 Mitogen-Activated Protein Kinase Mediates Bid Cleavage, Mitochondrial Dysfunction, and Caspase-3 Activation during Apoptosis Induced by Singlet Oxygen but Not by Hydrogen Peroxide. J. Biol. Chem. 2000, 275, 25939–25948. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M.; Melander, F.; Leandersson, K.; Rönnstrand, L.; Wernstedt, C.; Andersson, T. P38-MAPK Signals Survival by Phosphorylation of Caspase-8 and Caspase-3 in Human Neutrophils. J. Exp. Med. 2004, 199, 449. [Google Scholar] [CrossRef] [PubMed]
- Speidel, D. Transcription-Independent P53 Apoptosis: An Alternative Route to Death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.W.; Wang, J.H.; Redmond, H.P. NF-ΚB and P38 MAPK Inhibition Improve Survival in Endotoxin Shock and in a Cecal Ligation and Puncture Model of Sepsis in Combination With Antibiotic Therapy. J. Surg. Res. 2009, 152, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional Roles of P38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Fattahi, F.; Zetoune, F.S.; Ward, P.A. Complement as a Major Inducer of Harmful Events in Infectious Sepsis. Shock 2020, 54, 595–605. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Weihl, C.C.; Ghadge, G.D.; Kennedy, S.G.; Hay, N.; Miller, R.J.; Roos, R.P. Mutant Presenilin-1 Induces Apoptosis and Downregulates Akt/PKB. J. Neurosci. 1999, 19, 5360–5369. [Google Scholar] [CrossRef]
- Alvarez-Tejado, M.; Naranjo-Suárez, S.; Jiménez, C.; Carrera, A.C.; Landázuri, M.O.; Del Peso, L. Hypoxia Induces the Activation of the Phosphatidylinositol 3-Kinase/Akt Cell Survival Pathway in PC12 Cells: Protective Role in Apoptosis. J. Biol. Chem. 2001, 276, 22368–22374. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, W.; Sun, H.; Zhou, Q.; Huang, J.Q.; Li, X.; Xie, Y.; Chen, J. Salidroside Protects PC12 Cells from MPP+-Induced Apoptosis via Activation of the PI3K/Akt Pathway. Food Chem. Toxicol. 2012, 50, 2591–2597. [Google Scholar] [CrossRef]
- Toledo-Pereyra, L.H.; Lopez-Neblina, F.; Reuben, J.S.; Toledo, A.H.; Ward, P.A. Selectin Inhibition Modulates Akt/MAPK Signaling and Chemokine Expression After Liver Ischemia–Reperfusion. J. Investig. Surg. 2004, 17, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ba, L.; Huang, W.; Liu, Y.; Pan, H.; Mingyao, E.; Shi, P.; Wang, Y.; Li, S.; Qi, H.; et al. Role of Carvacrol in Cardioprotection against Myocardial Ischemia/Reperfusion Injury in Rats through Activation of MAPK/ERK and Akt/ENOS Signaling Pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, F.; Kalbitz, M.; Malan, E.A.; Abe, E.; Jajou, L.; Huber-Lang, M.S.; Bosmann, M.; Russell, M.W.; Zetoune, F.S.; Ward, P.A. Complement-Induced Activation of MAPKs and Akt during Sepsis: Role in Cardiac Dysfunction. FASEB J. 2017, 31, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Mullany, L.K.; Nelsen, C.J.; Hanse, E.A.; Goggin, M.M.; Anttila, C.K.; Peterson, M.; Bitterman, P.B.; Raghavan, A.; Crary, G.S.; Albrecht, J.H. Akt-Mediated Liver Growth Promotes Induction of Cyclin E through a Novel Translational Mechanism and a P21-Mediated Cell Cycle Arrest. J. Biol. Chem. 2007, 282, 21244–21252. [Google Scholar] [CrossRef] [PubMed]
- Eglite, S.; Plüss, K.; Dahinden, C.A. Requirements for C5a Receptor-Mediated IL-4 and IL-13 Production and Leukotriene C4 Generation in Human Basophils. J. Immunol. 2000, 165, 2183–2189. [Google Scholar] [CrossRef]
- Snyderman, R.; Phillips, J.; Mergenhagen, S.E. Polymorphonuclear Leukocyte Chemotactic Activity in Rabbit Serum and Guinea Pig Serum Treated with Immune Complexes: Evidence for C5a as the Major Chemotactic Factor. Infect. Immun. 1970, 1, 521. [Google Scholar] [CrossRef]
- Becker, E.L. The Relationship of the Chemotactic Behavior of the Complement-Derived Factors, C3a, C5a, C567, and a Bacterial Chemotactic Factor to Their Ability to Activate the Proesterase 1 of Rabbit Polymorphonuclear Leukocytes. J. Exp. Med. 1972, 135, 376. [Google Scholar] [CrossRef]
- Snyderman, R.; Pike, M.C.; McCarley, D.; Lang, L. Quantification of Mouse Macrophage Chemotaxis in Vitro: Role of C5 for the Production of Chemotactic Activity. Infect. Immun. 1975, 11, 488. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, F.; Wang, F.; Jiang, Y.; Tang, J.; Hu, X.; Luo, L.; Jin, Y.; Huang, L.; Sun, D.; et al. C5a Enhances Vδ1 T Cells Recruitment via the CCL2-CCR2 Axis in IgA Nephropathy. Int. Immunopharmacol. 2023, 125, 111065. [Google Scholar] [CrossRef]
- Skei, J.M.; Fingert, J.H.; Russell, S.R.; Stone, E.M.; Mullins, R.F. Complement Component C5a Activates ICAM-1 Expression on Human Choroidal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5336–5342. [Google Scholar] [CrossRef]
- Lawrence, I.D.; Warner, J.A.; Cohan, V.L.; Hubbard, W.C.; Kagey-Sobotka, A.; Lichtenstein, L.M. Purification and Characterization of Human Skin Mast Cells. Evidence for Human Mast Cell Heterogeneity. J. Immunol. 1987, 139, 3062–3069. [Google Scholar] [CrossRef] [PubMed]
- Oskeritzian, C.A.; Zhao, W.; Min, H.K.; Xia, H.Z.; Pozez, A.; Kiev, J.; Schwartz, L.B. Surface CD88 Functionally Distinguishes the MCTC from the MCT Type of Human Lung Mast Cell. J. Allergy Clin. Immunol. 2005, 115, 1162. [Google Scholar] [CrossRef] [PubMed]
- Thorley, J. FDA Approves Avacopan for ANCA-Associated Vasculitis. Lancet Rheumatol. 2022, 4, e21. [Google Scholar] [CrossRef]
- Dubois, E.A.; Cohen, A.F. Eculizumab. Br. J. Clin. Pharmacol. 2009, 68, 318. [Google Scholar] [CrossRef]


| Protein | Percentage Change | SEM (%) | Significant (p < 0.05) | Function |
|---|---|---|---|---|
| Activin A | 17.59 | 4.868 | Yes | Cell Cycle Progression, Cell Proliferation, and Apoptosis [47] |
| FAS | 91.74 | 23.7 | Yes | Induction of Apoptosis [48] |
| FASL | 99.56 | 50.96 | Yes | |
| GALECTIN 1 | 39.95 | 11.54 | Yes | Regulation of Cell Cycle [49] |
| IL-1α | 22.68 | 4.439 | Yes | Alarmin and Inflammatory Process [50] |
| IL-3 | 17.01 | 1.369 | Yes | Inflammatory processes, Cell Growth, and Differentiation [51] |
| IL-4 | 29.42 | 5.13 | Yes | Proliferation and Cell Survival [52] |
| IL-7 | 61.77 | 19.08 | Yes | Proliferation and Cell Survival [53] |
| MIP-1a | 54.91 | 12.89 | Yes | Immune Cell Activation and Chemotaxis [54] |
| PDGF-BB | 88.81 | 20.92 | Yes | Proliferation, Immune Cell Activation, and Differentiation [55] |
| Protein | Percentage Change | SEM (%) | Significant (p < 0.05) | Function |
|---|---|---|---|---|
| CCL20 | −6.356 | 1.787 | Yes | Immune Migration and Chemotaxis [56] |
| CINC1 | −22.5 | 6.018 | Yes | Neutrophil Chemoattractant [57] |
| CINC2 | −22.68 | 4.599 | Yes | Immune Cell Recruitment and Chemotaxis [58] |
| ICAM-1 | −14.18 | 3.261 | Yes | Cell Adhesion and Immune Cell Recruitment [59] |
| IL-13 | −12.33 | 2.959 | Yes | Anti-inflammatory Effects [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozewski, L.; Tharmalingam, S.; Michael, P.; Kumar, A.; Tai, T.C. C5a Induces Inflammatory Signaling and Apoptosis in PC12 Cells through C5aR-Dependent Signaling: A Potential Mechanism for Adrenal Damage in Sepsis. Int. J. Mol. Sci. 2024, 25, 10673. https://doi.org/10.3390/ijms251910673
Mrozewski L, Tharmalingam S, Michael P, Kumar A, Tai TC. C5a Induces Inflammatory Signaling and Apoptosis in PC12 Cells through C5aR-Dependent Signaling: A Potential Mechanism for Adrenal Damage in Sepsis. International Journal of Molecular Sciences. 2024; 25(19):10673. https://doi.org/10.3390/ijms251910673
Chicago/Turabian StyleMrozewski, Lucas, Sujeenthar Tharmalingam, Paul Michael, Aseem Kumar, and T. C. Tai. 2024. "C5a Induces Inflammatory Signaling and Apoptosis in PC12 Cells through C5aR-Dependent Signaling: A Potential Mechanism for Adrenal Damage in Sepsis" International Journal of Molecular Sciences 25, no. 19: 10673. https://doi.org/10.3390/ijms251910673
APA StyleMrozewski, L., Tharmalingam, S., Michael, P., Kumar, A., & Tai, T. C. (2024). C5a Induces Inflammatory Signaling and Apoptosis in PC12 Cells through C5aR-Dependent Signaling: A Potential Mechanism for Adrenal Damage in Sepsis. International Journal of Molecular Sciences, 25(19), 10673. https://doi.org/10.3390/ijms251910673







