Effects of PACAP Deficiency on Immune Dysfunction and Peyer’s Patch Integrity in Adult Mice
Abstract
1. Introduction
2. Results
2.1. Peyer’s Patches—Macroscopic and Microscopic Features
2.2. Peyer’s Patches—Immune Cellular Features
2.2.1. Phenotype Characteristics of Different T Cell Subpopulations
2.2.2. Immune Checkpoint Molecules
PD-1 and PD-L1 Expression by Different T Cell Subpopulations
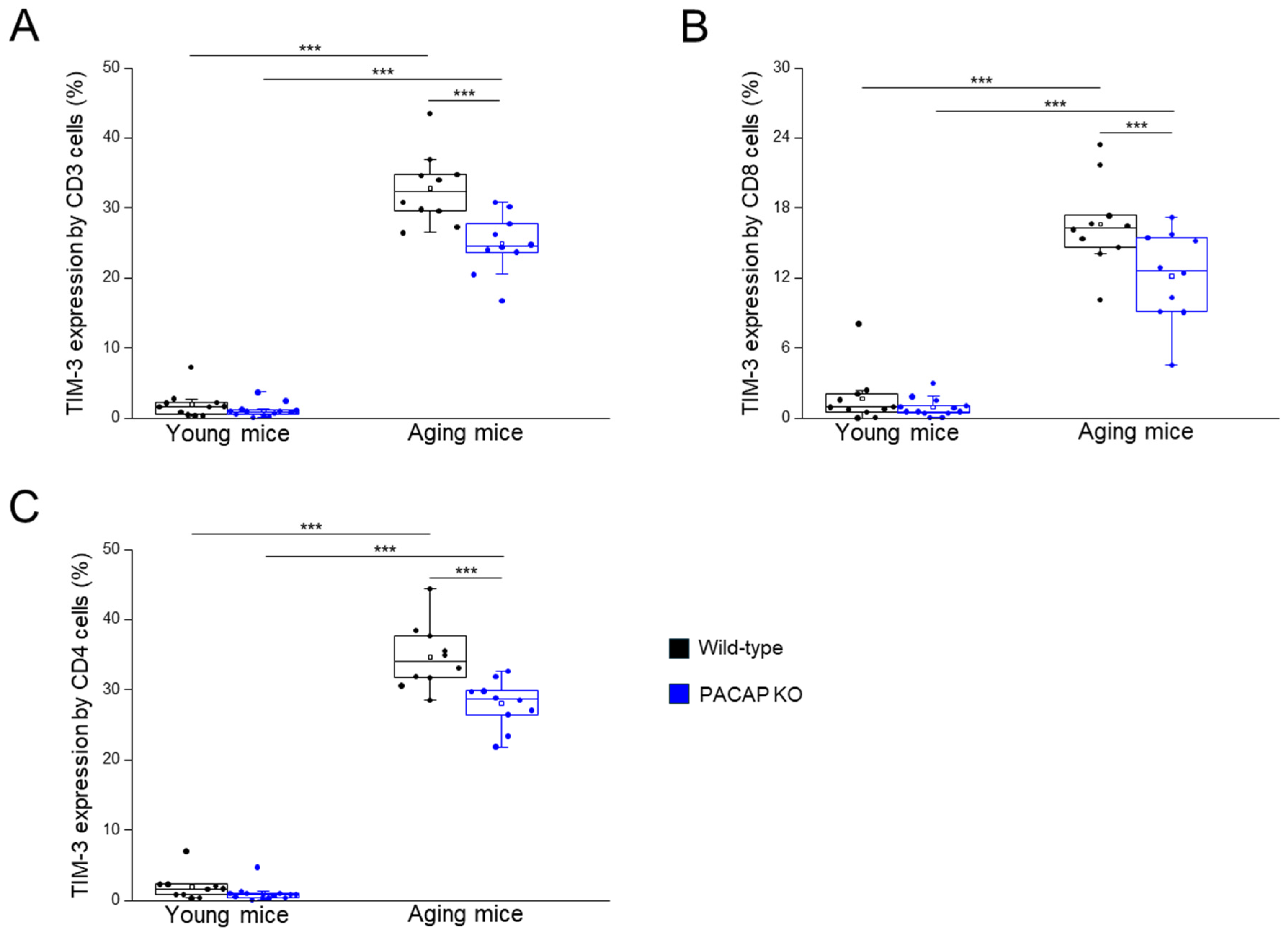
TIM-3 and Galectin-9 Expression by Different T Cell Subpopulations
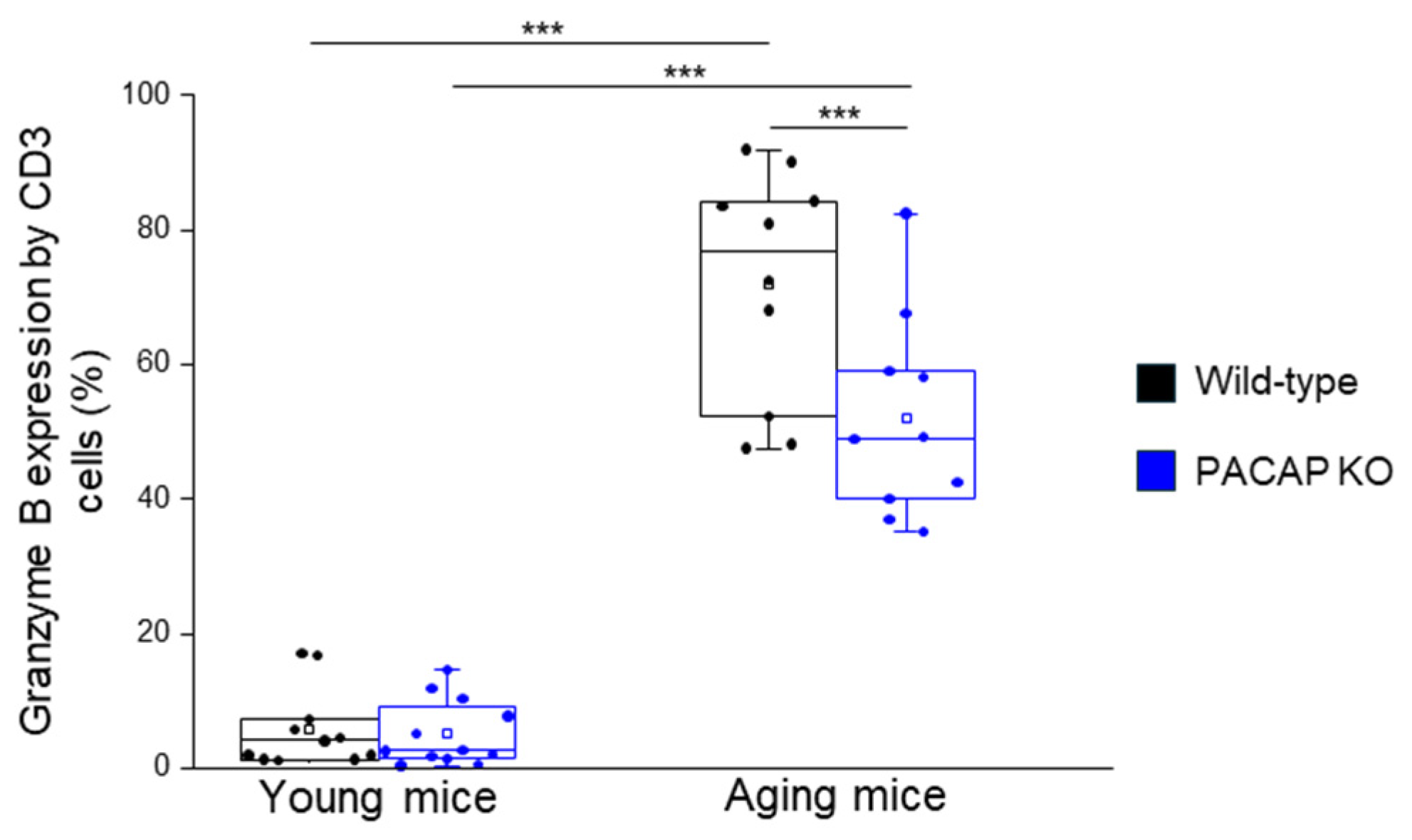
2.2.3. Expression of Intracellular Molecules by Different T Cell Subpopulations
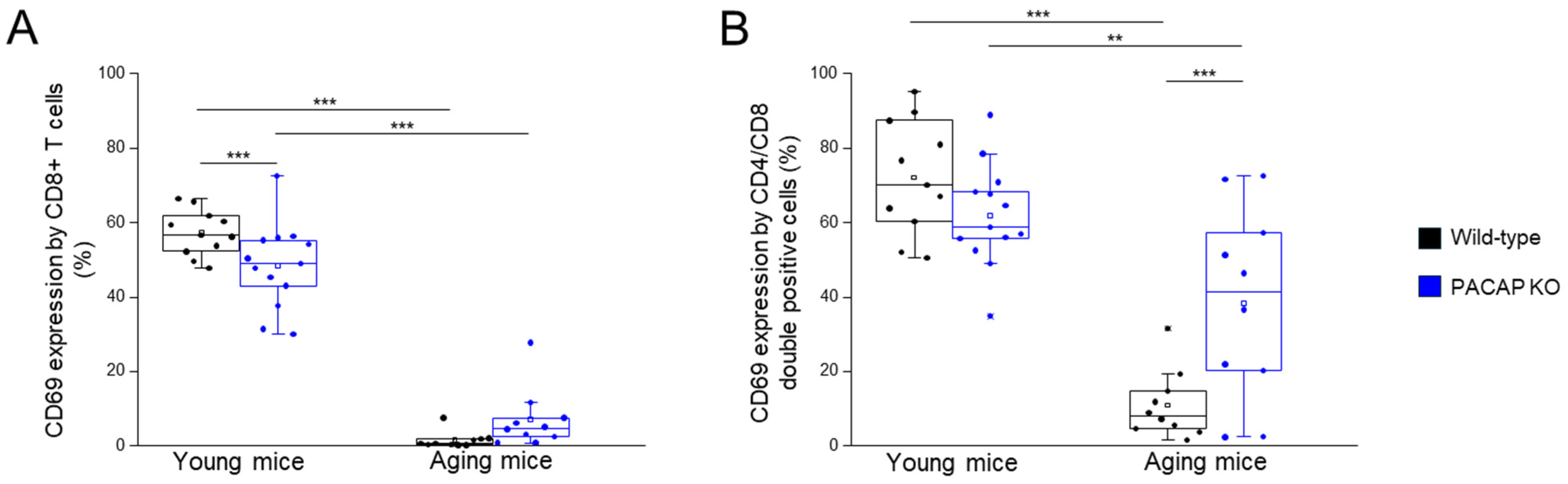
2.2.4. CD69 Expression by Different T Cell Subpopulations
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Isolation of the Peyer’s Patches from the Small Intestine
4.3. Histological Analysis
4.4. Cell Isolation from the Peyer’s Patches
4.5. Mononuclear Cell Surface Staining, Antibodies, and Flow Cytometric Analysis
4.6. Intracellular Staining of Perforin and Granzyme B
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of Mucosa-Associated Lymphoid Tissue. Mucosal Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human Gut-Associated Lymphoid Tissues (GALT); Diversity, Structure, and Function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Makala, L.H.C.; Suzuki, N.; Nagasawa, H. Peyer’s Patches: Organized Lymphoid Structures for the Induction of Mucosal Immune Responses in the Intestine. Pathobiology 2002, 70, 55–68. [Google Scholar] [CrossRef]
- Cornes, J.S. Number, Size, and Distribution of Peyer’s Patches in the Human Small Intestine: Part I The Development of Peyer’s Patches. Gut 1965, 6, 225–229. [Google Scholar] [CrossRef]
- Finke, D.; Kraehenbuhl, J.P. Formation of Peyer’s Patches. Curr. Opin. Genet. Dev. 2001, 11, 561–567. [Google Scholar] [CrossRef]
- Olaison, G.; Smedh, K.; Sjödahl, R. Natural Course of Crohn’s Disease after Ileocolic Resection: Endoscopically Visualised Ileal Ulcers Preceeding Symptoms. Gut 1992, 33, 331–335. [Google Scholar] [CrossRef]
- Gullberg, E.; Söderholm, J.D. Peyer’s Patches and M Cells as Potential Sites of the Inflammatory Onset in Crohn’s Disease. Ann. N. Y. Acad. Sci. 2006, 1072, 218–232. [Google Scholar] [CrossRef]
- Hiyama, S.; Iijima, H.; Sakakibara, Y.; Yamada, T.; Mukai, A.; Otake, Y.; Yamaguchi, T.; Araki, M.; Kawai, S.; Tsujii, Y.; et al. Endoscopic Alterations in Peyer’s Patches in Patients with Ulcerative Colitis: A Prospective, Multicenter Study. J. Gastroenterol. Hepatol. 2020, 35, 1143–1149. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front. Immunol. 2019, 10, 2345. [Google Scholar] [CrossRef]
- Block, K.E.; Zheng, Z.; Dent, A.L.; Kee, B.L.; Huang, H. Gut Microbiota Regulates K/BxN Autoimmune Arthritis through Follicular Helper T but Not Th17 Cells. J. Immunol. 2016, 196, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, B.L.; Strober, W. Distinct Populations of Dendritic Cells Are Present in the Subepithelial Dome and T Cell Regions of the Murine Peyer’s Patch. J. Exp. Med. 1996, 183, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, D.; Baloche, V.; Zhang, L.; Xu, J.; Li, B.; Zhao, X.; He, J.; Mai, H.; Chen, Q.; et al. Galectin-9 Promotes a Suppressive Microenvironment in Human Cancer by Enhancing STING Degradation. Oncogenesis 2020, 9, 65. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel Immune Checkpoint Targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Lajko, A.; Meggyes, M.; Polgar, B.; Szereday, L. The Immunological Effect of Galectin-9/TIM-3 Pathway after Low Dose Mifepristone Treatment in Mice at 14.5 Day of Pregnancy. PLoS ONE 2018, 13, e0194870. [Google Scholar] [CrossRef]
- Lajko, A.; Meggyes, M.; Fulop, B.D.; Gede, N.; Reglodi, D.; Szereday, L. Comparative Analysis of Decidual and Peripheral Immune Cells and Immune-Checkpoint Molecules during Pregnancy in Wild-Type and PACAP-Deficient Mice. Am. J. Reprod. Immunol. 2018, 80, e13035. [Google Scholar] [CrossRef]
- Du, X.; Wu, Z.; Xu, Y.; Liu, Y.; Liu, W.; Wang, T.; Li, C.; Zhang, C.; Yi, F.; Gao, L.; et al. Increased Tim-3 Expression Alleviates Liver Injury by Regulating Macrophage Activation in MCD-Induced NASH Mice. Cell Mol. Immunol. 2019, 16, 878–886. [Google Scholar] [CrossRef]
- John, S.; Mishra, R. Galectin-9: From Cell Biology to Complex Disease Dynamics. J. Biosci. 2016, 41, 507–534. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Komrokji, R.S.; Brunner, A.M. TIM-3 Pathway Dysregulation and Targeting in Cancer. Expert. Rev. Anticancer. Ther. 2021, 21, 523–534. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Liang, S.; Potter, J.; Jiang, X.; Mao, H.-Q.H.-Q.; Li, Z. Tim-3/Galectin-9 Regulate the Homeostasis of Hepatic NKT Cells in a Murine Model of Nonalcoholic Fatty Liver Disease. J. Immunol. 2013, 190, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Ishida, M.; Iwai, Y.; Tanaka, Y.; Okazaki, T.; Freeman, G.J.; Minato, N.; Honjo, T. Differential Expression of PD-L1 and PD-L2, Ligands for an Inhibitory Receptor PD-1, in the Cells of Lymphohematopoietic Tissues. Immunol. Lett. 2002, 84, 57–62. [Google Scholar] [CrossRef]
- Meggyes, M.; Miko, E.; Szigeti, B.; Farkas, N.; Szereday, L. The Importance of the PD-1/PD-L1 Pathway at the Maternal-Fetal Interface. BMC Pregnancy Childbirth 2019, 19, 74. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Li, M.; Zhang, F.; Zeng, X. The PD-1/PD-L Pathway in Rheumatic Diseases. J. Formos. Med. Assoc. 2021, 120, 48–59. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Doba, K.; Barakonyi, A.; Szereday, L. Immune Checkpoint Molecules in Reproductive Immunology. Front. Immunol. 2019, 10, 846. [Google Scholar] [CrossRef]
- Di Luccia, B.; Colonna, M. Precision Probiotic Medicine to Improve ICB Immunotherapy. Cancer Discov. 2022, 12, 1189–1190. [Google Scholar] [CrossRef]
- Maruya, M.; Kawamoto, S.; Kato, L.M.; Fagarasan, S. Impaired Selection of IgA and Intestinal Dysbiosis Associated with PD-1-Deficiency. Gut Microbes 2013, 4, 165–171. [Google Scholar] [CrossRef]
- Arimura, A. Receptors for Pituitary Adenylate Cyclase-Activating Polypeptide: Comparison with Vasoactive Intestinal Peptide Receptors. Trends Endocrinol. Metab. 1992, 3, 288–294. [Google Scholar] [CrossRef]
- Horvath, G.; Reglodi, D.; Fabian, E.; Opper, B. Effects of Pituitary Adenylate Cyclase Activating Polypeptide on Cell Death. Int. J. Mol. Sci. 2022, 23, 4953. [Google Scholar] [CrossRef]
- Läuffer, J.M.; Modlin, I.M.; Tang, L.H. Biological Relevance of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in the Gastrointestinal Tract. Regul. Pept. 1999, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karpiesiuk, A.; Palus, K. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Physiological and Pathological Processes within the Gastrointestinal Tract: A Review. Int. J. Mol. Sci. 2021, 22, 8682. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Vu, J.P.; Germano, P.M.; Pisegna, J.R. The Role of Neuropeptides in Mouse Models of Colitis. J. Mol. Neurosci. 2016, 59, 203–210. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Dunay, I.R.; Schulze, S.; Fischer, A.; Grundmann, U.; Alutis, M.; Kühl, A.A.; Tamas, A.; Toth, G.; Dunay, M.P.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide Ameliorates Experimental Acute Ileitis and Extra-Intestinal Sequelae. PLoS ONE 2014, 9, e108389. [Google Scholar] [CrossRef]
- Kono, H.; Furuya, S.; Sun, C.; Akazawa, Y.; Nakata, Y.; Fukushima, H.; Wakana, H.; Fujii, H.; Ichikawa, D. Pituitary Adenylate Cyclase-Activating Polypeptide Prevents Mortality Caused by Septic Peritonitis in Mice. Immunohorizons 2020, 4, 191–200. [Google Scholar] [CrossRef]
- Nemetz, N.; Abad, C.; Lawson, G.; Nobuta, H.; Chhith, S.; Duong, L.; Tse, G.; Braun, J.; Waschek, J.A. Induction of Colitis and Rapid Development of Colorectal Tumors in Mice Deficient in the Neuropeptide PACAP. Int. J. Cancer 2008, 122, 1803–1809. [Google Scholar] [CrossRef]
- Gomariz, R.P.; Juarranz, Y.; Abad, C.; Arranz, A.; Leceta, J.; Martinez, C. VIP-PACAP System in Immunity: New Insights for Multitarget Therapy. Ann. N. Y. Acad. Sci. 2006, 1070, 51–74. [Google Scholar] [CrossRef]
- Abad, C.; Martinez, C.; Leceta, J.; Juarranz, M.G.; Delgado, M.; Gomariz, R.P. Pituitary Adenylate-Cyclase-Activating Polypeptide Expression in the Immune System. Neuroimmunomodulation 2002, 10, 177–186. [Google Scholar] [CrossRef]
- Reglodi, D.; Atlasz, T.; Szabo, E.; Jungling, A.; Tamas, A.; Juhasz, T.; Fulop, B.D.; Bardosi, A. PACAP Deficiency as a Model of Aging. Geroscience 2018, 40, 437–452. [Google Scholar] [CrossRef]
- Abad, C.; Tan, Y.V. Immunomodulatory Roles of PACAP and VIP: Lessons from Knockout Mice. J. Mol. Neurosci. 2018, 66, 102–113. [Google Scholar] [CrossRef]
- Farkas, J.; Sandor, B.; Tamas, A.; Kiss, P.; Hashimoto, H.; Nagy, A.D.; Fulop, B.D.; Juhasz, T.; Manavalan, S.; Reglodi, D. Early Neurobehavioral Development of Mice Lacking Endogenous PACAP. J. Mol. Neurosci. 2017, 61, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Valasek, A.; Szabadfi, K.; Dénes, V.; Szalontai, B.; Tamás, A.; Kiss, P.; Szabó, A.; Setalo, G.; Reglődi, D.; Gábriel, R. Accelerated Retinal Aging in PACAP Knock-out Mice. Neuroscience 2017, 348, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.A.; Lelievre, V.; Braas, K.M.; Razinia, T.; Vizzard, M.A.; Ioffe, Y.; El Meskini, R.; Ronnett, G.V.; Waschek, J.A.; May, V. Noncompensation in Peptide/Receptor Gene Expression and Distinct Behavioral Phenotypes in VIP- and PACAP-Deficient Mice. J. Neurochem. 2006, 99, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Lajko, A.; Fulop, B.D.B.D.; Reglodi, D.; Szereday, L. Phenotypic Characterization of Testicular Immune Cells Expressing Immune Checkpoint Molecules in Wild-Type and Pituitary Adenylate Cyclase-Activating Polypeptide-Deficient Mice. Am. J. Reprod. Immunol. 2020, 83, e13212. [Google Scholar] [CrossRef]
- Nakata, M.; Kohno, D.; Shintani, N.; Nemoto, Y.; Hashimoto, H.; Baba, A.; Yada, T. PACAP Deficient Mice Display Reduced Carbohydrate Intake and PACAP Activates NPY-Containing Neurons in the Rat Hypothalamic Arcuate Nucleus. Neurosci. Lett. 2004, 370, 252–256. [Google Scholar] [CrossRef]
- Tomimoto, S.; Ojika, T.; Shintani, N.; Hashimoto, H.; Hamagami, K.I.; Ikeda, K.; Nakata, M.; Yada, T.; Sakurai, Y.; Shimada, T.; et al. Markedly Reduced White Adipose Tissue and Increased Insulin Sensitivity in Adcyap1-Deficient Mice. J. Pharmacol. Sci. 2008, 107, 41–48. [Google Scholar] [CrossRef]
- Colwell, C.S.; Michel, S.; Itri, J.; Rodriguez, W.; Tam, J.; Lelièvre, V.; Hu, Z.; Waschek, J.A. Selective Deficits in the Circadian Light Response in Mice Lacking PACAP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 1194–1201. [Google Scholar] [CrossRef]
- Arimura, A. Editorial: Impaired Adaptive Thermogenesis in Pituitary Adenylate Cyclase-Activating Polypeptide-Deficient Mice. Endocrinology 2002, 143, 3715–3716. [Google Scholar] [CrossRef][Green Version]
- Filatov, E.; Short, L.I.; Forster, M.A.M.; Harris, S.S.; Schien, E.N.; Hughes, M.C.; Cline, D.L.; Appleby, C.J.; Gray, S.L. Contribution of Thermogenic Mechanisms by Male and Female Mice Lacking Pituitary Adenylate Cyclase-Activating Polypeptide in Response to Cold Acclimation. Am. J. Physiol. Endocrinol. Metab. 2021, 320, 475–487. [Google Scholar] [CrossRef]
- Szakaly, P.; Laszlo, E.; Kovacs, K.; Racz, B.; Horvath, G.; Ferencz, A.; Lubics, A.; Kiss, P.; Tamas, A.; Brubel, R.; et al. Mice Deficient in Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Show Increased Susceptibility to in Vivo Renal Ischemia/Reperfusion Injury. Neuropeptides 2011, 45, 113–121. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, Y.; Shen, X.-D.; Gao, F.; Huang, C.Y.; Abad, C.; Busuttil, R.W.; Waschek, J.A.; Kupiec-Weglinski, J.W. Neuropeptide PACAP in Mouse Liver Ischemia and Reperfusion Injury: Immunomodulation by the CAMP-PKA Pathway. Hepatology 2013, 57, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Danyadi, B.; Tamas, A.; Helyes, Z.; Hashimoto, H.; Shintani, N.; Baba, A.; Toth, G.; et al. Mice Deficient in Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Are More Susceptible to Retinal Ischemic Injury in Vivo. Neurotox. Res. 2012, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, A.; Kiss, P.; Weber, G.; Helyes, Z.; Shintani, N.; Baba, A.; Reglodi, D. Comparison of Intestinal Warm Ischemic Injury in PACAP Knockout and Wild-Type Mice. J. Mol. Neurosci. 2010, 42, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.T.; Hagi, K.; Shintani, N.; Kuwamura, M.; Nakajima, H.; Hashimoto, H.; Baba, A.; Takeuchi, T. PACAP Provides Colonic Protection against Dextran Sodium Sulfate Induced Colitis. J. Cell Physiol. 2008, 216, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.; Jungling, A.; Kiss, G.; Hiripi, L.; Pham, D.; Tamas, A.; Hoffmann, O.; Bardosi, S.; Miseta, A.; Reglodi, D. Presence of Systemic Amyloidosis in Mice with Partial Deficiency in Pituitary Adenylate Cyclase-Activating Polypeptide (Pacap) in Aging. Appl. Sci. 2021, 11, 7373. [Google Scholar] [CrossRef]
- Baranowska-Bik, A.; Bik, W.; Wolinska-Witort, E.; Chmielowska, M.; Martynska, L.; Baranowska, B. Can PACAP-38 Modulate Immune and Endocrine Responses during Lipopolysaccharide (LPS)-Induced Acute Inflammation? Ann. N. Y. Acad. Sci. 2006, 1070, 156–160. [Google Scholar] [CrossRef]
- Waschek, J.A. VIP and PACAP: Neuropeptide Modulators of CNS Inflammation, Injury, and Repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef]
- Van, C.; Condro, M.C.; Lov, K.; Zhu, R.; Ricaflanca, P.T.; Ko, H.H.; Diep, A.L.; Hoang, A.Q.; Pisegna, J.; Rohrer, H.; et al. PACAP/PAC1 Regulation of Inflammation via Catecholaminergic Neurons in a Model of Multiple Sclerosis. J. Mol. Neurosci. 2019, 68, 439–451. [Google Scholar] [CrossRef]
- Bik, W.; Wolinska-Witort, E.; Pawlak, J.; Skwarlo-Sonta, K.; Chmielowska, M.; Martynska, L.; Baranowska-Bik, A.; Baranowska, B. PACAP 38 as a Modulator of Immune and Endocrine Responses during LPS-Induced Acute Inflammation in Rats. J. Neuroimmunol. 2006, 177, 76–84. [Google Scholar] [CrossRef]
- Reglodi, D.; Jungling, A.; Longuespée, R.; Kriegsmann, J.; Casadonte, R.; Kriegsmann, M.; Juhasz, T.; Bardosi, S.; Tamas, A.; Fulop, B.D.; et al. Accelerated Pre-Senile Systemic Amyloidosis in PACAP Knockout Mice–a Protective Role of PACAP in Age-Related Degenerative Processes. J. Pathol. 2018, 245, 478–490. [Google Scholar] [CrossRef]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Illes, A.; Heimesaat, M.M.; Bardosi, A.; Bardosi, S.; Tamas, A.; Fulop, B.D.; Opper, B.; Nemeth, J.; Ferencz, A.; et al. Protective Intestinal Effects of Pituitary Adenylate Cyclase Activating Polypeptide. In Opper Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer, G., Horvath, A., Illes, M.M., Heimesaat, A., Bardosi, S., Bardosi, A., Tamas, B.D., Fulop, B., Eds.; Springer: New York, NY, USA, 2016; pp. 271–288. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Reifenberger, G.; Vicena, V.; Illes, A.; Horvath, G.; Tamas, A.; Fulop, B.D.; Bereswill, S.; Reglodi, D. Intestinal Microbiota Changes in Mice Lacking Pituitary Adenylate Cyclase Activating Polypeptide (PACAP)-Bifidobacteria Make the Difference. Eur. J. Microbiol. Immunol. 2017, 7, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fujihashi, K.; Kato, R.; Dohi, T.; Fujihashi, K.; Hagiwara, Y.; Kataoka, K.; Kobayashi, R.; McGhee, J.R. Lack of Oral Tolerance in Aging Is Due to Sequential Loss of Peyer’s Patch Cell Interactions. Int. Immunol. 2003, 15, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Gaytan, F.; Martinez-Fuentes, A.J.; Garcia-Navarro, F.; Vaudry, H.; Aguilar, E. Pituitary Adenylate Cyclase-Activating Peptide (PACAP) Immunolocalization in Lymphoid Tissues of the Rat. Cell Tissue Res. 1994, 276, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Squillacioti, C.; Colitti, M.; Germano, G.; Pelagalli, A.; Paino, G. Pituitary Adenylate Cyclase Activating Peptide (PACAP) Immunoreactivity and MRNA Expression in the Duck Gastrointestinal Tract. Cell Tissue Res. 2002, 308, 347–359. [Google Scholar] [CrossRef]
- Schulz, S.; Mann, A.; Novakhov, B.; Piggins, H.D.; Lupp, A. VPAC2 Receptor Expression in Human Normal and Neoplastic Tissues: Evaluation of the Novel MAB SP235. Endocr. Connect. 2014, 4, 18–26. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System. Nat. Immunol. 2017, 19, 10–19. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J.; Li, G.; Uhrlaub, J.L.; Renkema, K.R.; Smithey, M.J. Age-Related Changes in CD8 T Cell Homeostasis and Immunity to Infection. Semin. Immunol. 2012, 24, 356–364. [Google Scholar] [CrossRef]
- Jiang, J.; Fisher, E.M.; Murasko, D.M. Intrinsic Defects in CD8 T Cells with Aging Contribute to Impaired Primary Antiviral Responses. Exp. Gerontol. 2013, 48, 579–586. [Google Scholar] [CrossRef][Green Version]
- Jergović, M.; Thompson, H.L.; Renkema, K.R.; Smithey, M.J.; Nikolich-Žugich, J. Defective Transcriptional Programming of Effector CD8 T Cells in Aged Mice Is Cell-Extrinsic and Can Be Corrected by Administration of IL-12 and IL-18. Front. Immunol. 2019, 10, 469166. [Google Scholar] [CrossRef]
- Decman, V.; Laidlaw, B.J.; DiMenna, L.J.; Abdulla, S.; Mozdzanowska, K.; Erikson, J.; Ertl, H.C.J.; Wherry, E.J. Cell-Intrinsic Defects in the Proliferative Response of Antiviral Memory CD8 T Cells in Aged Mice upon Secondary Infection. J. Immunol. 2010, 184, 5151–5159. [Google Scholar] [CrossRef]
- Weinberger, B.; Lazuardi, L.; Weiskirchner, I.; Keller, M.; Neuner, C.; Fischer, K.H.; Neuman, B.; Würzner, R.; Grubeck-Loebenstein, B. Healthy Aging and Latent Infection with CMV Lead to Distinct Changes in CD8+ and CD4+ T-Cell Subsets in the Elderly. Hum. Immunol. 2007, 68, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Lee, W.W.; Weyand, C.M. Aging and T-Cell Diversity. Exp. Gerontol. 2007, 42, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Czesnikiewicz-Guzik, M.; Lee, W.W.; Cui, D.; Hiruma, Y.; Lamar, D.L.; Yang, Z.Z.; Ouslander, J.G.; Weyand, C.M.; Goronzy, J.J. T Cell Subset-Specific Susceptibility to Aging. Clin. Immunol. 2008, 127, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zöphel, D.; Angenendt, A.; Kaschek, L.; Ravichandran, K.; Hof, C.; Janku, S.; Hoth, M.; Lis, A. Faster Cytotoxicity with Age: Increased Perforin and Granzyme Levels in Cytotoxic CD8+ T Cells Boost Cancer Cell Elimination. Aging Cell 2022, 21, e13668. [Google Scholar] [CrossRef]
- Smithey, M.J.; Renkema, K.R.; Rudd, B.D.; Nikolich-Žugich, J. Increased Apoptosis, Curtailed Expansion and Incomplete Differentiation of CD8+ T Cells Combine to Decrease Clearance of L. Monocytogenes in Old Mice. Eur. J. Immunol. 2011, 41, 1352–1364. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shintani, N.; Tanaka, K.; Mori, W.; Hirose, M.; Matsuda, T.; Sakaue, M.; Miyazaki, J.-i.; Niwa, H.; Tashiro, F.; et al. Altered Psychomotor Behaviors in Mice Lacking Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP). Proc. Natl. Acad. Sci. USA 2001, 98, 13355–13360. [Google Scholar] [CrossRef]
- Lefrançois, L.; Lycke, N. Isolation of Mouse Small Intestinal Intraepithelial Lymphocytes, Peyer’s Patch, and Lamina Propria Cells. Curr. Protoc. Immunol. 1996, 17, 3.19.1–3.19.16. [Google Scholar] [CrossRef]
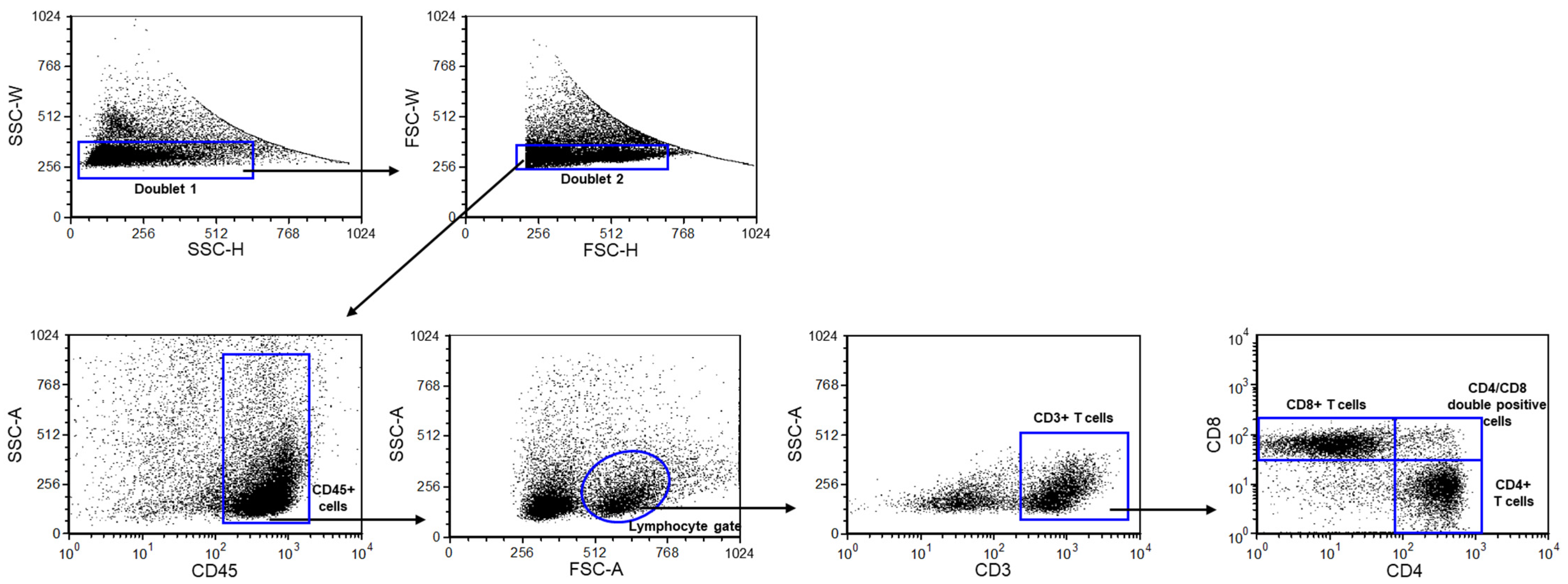

| WT Young | PACAP KO Young | WT Aging | PACAP KO Aging | p Value | |
|---|---|---|---|---|---|
| CD3+ T-cells | 50.38 ± 13.52 | 53.24 ± 17.71 | 42.18 ± 13.39 | 37.76 ± 9.68 | young KO vs. aging KO p = 0.013 |
| CD8+ T-cells | 15.33 ± 10.40 | 14.23 ± 9.93 | 5.03 ± 2.27 | 8.57 ± 5.68 | young WT vs. aging WT p = 0.006 |
| CD4+ T-cells | 28.67 ± 11.63 | 27.40 ± 10.88 | 32.64 ± 11.21 | 20.67 ± 5.30 | aging WT vs. aging KO p = 0.012 |
| CD8+ T-cells ratio in CD3+ T-cells | 28.74 ± 17.70 | 26.94 ± 14.62 | 12.20 ± 6.22 | 21.44 ± 9.23 | young WT vs. aging WT p = 0.006 |
| CD4+ T-cells ratio in CD3+ T-cells | 59.27 ± 23.33 | 53.83 ± 19.79 | 77.19 ± 9.96 | 55.70 ± 11.07 | aging WT vs. aging KO p = 0.009 young WT vs. aging WT p = 0.024 |
| CD4+ T-cells ratio in CD8+ T-cells | 3.75 ± 5.10 | 8.63 ± 8.13 | 1.12 ± 1.11 | 3.40 ± 2.33 | young KO vs. aging KO p = 0.023 young WT vs. Young KO p = 0.029 |
| PD-1 expression by CD3+ T-cells | 32.18 ± 11.64 | 31.48 ± 13.58 | 30.27 ± 9.80 | 19.88 ± 7.75 | aging WT vs. aging KO p = 0.043 young KO vs. aging KO p = 0.018 |
| PD-1 expression by CD4+ T-cells | 36.53 ± 11.49 | 32.85 ± 11.46 | 33.32 ± 8.72 | 21.08 ± 5.65 | aging WT vs. aging KO p = 0.008 young KO vs. aging KO p = 0.007 |
| TIM-3 expression by CD4+/CD8+ T-cells | 7.07 ± 6.96 | 2.36 ± 1.48 | 30.94 ± 8.33 | 22.01 ± 4.32 | aging WT vs. aging KO p = 0.001 young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| CD69 expression by CD3+ T-cells | 41.26 ± 10.23 | 38.47 ± 12.94 | 7.53 ± 1.52 | 9.46 ± 6.54 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| CD69 expression by CD4+ T-cells | 30.61 ± 5.31 | 27.92 ± 11.09 | 6.00 ± 1.49 | 4.11 ± 2.77 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| Gal-9 expression by CD3+ T-cells | 46.12 ± 17.70 | 55.44 ± 13.68 | 10.32 ± 8.12 | 14.97 ± 10.06 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| Gal-9 expression by CD8+ T-cells | 52.40 ± 15.63 | 59.17 ± 12.08 | 12.54 ± 9.07 | 13.71 ± 8.05 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| Gal-9 expression by CD4+ T-cells | 40.05 ± 13.98 | 50.20 ± 17.24 | 7.60 ± 6.84 | 10.94 ± 5.90 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| Gal-9 expression by CD4+/CD8+ T-cells | 78.59 ± 12.11 | 73.70 ± 16.55 | 29.08 ± 21.25 | 44.15 ± 22.00 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| PD-L1 expression by CD3+ T-cells | 48.26 ± 30.09 | 57.54 ± 23.75 | 35.52 ± 10.32 | 30.51 ± 8.38 | young KO vs. aging KO p = 0.004 |
| PD-L1 expression by CD4+ T-cells | 49.14 ± 30.57 | 58.81 ± 23.82 | 30.81 ± 11.38 | 23.89 ± 9.69 | young KO vs. aging KO p < 0.001 |
| PD-L1 expression by CD8+ T-cells | 46.53 ± 28.39 | 55.28 ± 23.80 | 35.23 ± 10.38 | 31.44 ± 8.19 | young KO vs. aging KO p = 0.009 |
| PD-L1 expression by CD4+/CD8+ T-cells | 63.53 ± 33.92 | 69.05 ± 23.98 | 53.40 ± 12.19 | 53.87 ± 14.07 | NS |
| perforin expression by CD3+ T-cells | 42.41 ± 24.32 | 60.93 ± 18.97 | 43.54 ± 8.21 | 49.57 ± 21.71 | young WT vs. Young KO p = 0.026 |
| perforin expression by CD8+ T-cells | 51.12 ± 27.26 | 67.91 ± 16.65 | 47.84 ± 8.32 | 55.35 ± 23.29 | young WT vs. Young KO p = 0.05 |
| Granzyme B expression by CD8+ T-cells | 8.58 ± 7.72 | 7.61 ± 5.02 | 74.39 ± 14.67 | 71.13 ± 15.99 | young WT vs. aging WT p < 0.001 young KO vs. aging KO p < 0.001 |
| Number of Peyer’s patches (PP) | 12.40 ± 2.72 | 10.46 ± 1.69 | 10.20 ± 1.69 | 10.11 ± 1.81 | young WT vs. aging WT p = 0.016 young WT vs. Young KO p = 0.015 |
| Antigen | Format | Clone | Isotype | Company | CAT |
|---|---|---|---|---|---|
| CD3 | BV510 | 145-2C11 | Armenian Hamster IgG1, κ | BD Biosciences | 563024 |
| CD4 | FITC | GK1.5 | Lewis IgG2b, κ | BD Biosciences | 557307 |
| CD8 | APC-H7 | 53-6.7 | Louvain, LOU/C, LOU/M IgG2a, κ | BD Biosciences | 560247 |
| CD45 | PerCp | 30-F11 | Louvain, LOU/C, LOU/M IgG2b, κ | BD Biosciences | 561047 |
| CD69 | PE-Cy7 | H1.2F3 | Armenian Hamster IgG1, λ3 | BD Biosciences | 552879 |
| Galectin-9 | BV421 | RG9-35 | Rat IgG2a, κ | BD Biosciences | 566028 |
| GranzymeB | FITC | REA226 | recombinant human IgG1 | Miltenyi Biotec. | 130-118-341 |
| PD-1 | BV421 | J43 | Armenian Hamster IgG2, κ | BD Biosciences | 562584 |
| PD-L1 | APC | MIH5 | Sprague-Dawley (outbred) IgG2a, λ | BD Biosciences | 564715 |
| Perforin | APC | S16009A | Rat IgG2a, κ | Biolegend | 154304 |
| TIM-3 | APC | 215008 | Rat IgG2A | R&D Systems | FAB1529A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparks, J.; Meggyes, M.; Makszin, L.; Jehn, V.; Lugosi, H.; Reglodi, D.; Szereday, L. Effects of PACAP Deficiency on Immune Dysfunction and Peyer’s Patch Integrity in Adult Mice. Int. J. Mol. Sci. 2024, 25, 10676. https://doi.org/10.3390/ijms251910676
Sparks J, Meggyes M, Makszin L, Jehn V, Lugosi H, Reglodi D, Szereday L. Effects of PACAP Deficiency on Immune Dysfunction and Peyer’s Patch Integrity in Adult Mice. International Journal of Molecular Sciences. 2024; 25(19):10676. https://doi.org/10.3390/ijms251910676
Chicago/Turabian StyleSparks, Jason, Matyas Meggyes, Lilla Makszin, Viktoria Jehn, Hedvig Lugosi, Dora Reglodi, and Laszlo Szereday. 2024. "Effects of PACAP Deficiency on Immune Dysfunction and Peyer’s Patch Integrity in Adult Mice" International Journal of Molecular Sciences 25, no. 19: 10676. https://doi.org/10.3390/ijms251910676
APA StyleSparks, J., Meggyes, M., Makszin, L., Jehn, V., Lugosi, H., Reglodi, D., & Szereday, L. (2024). Effects of PACAP Deficiency on Immune Dysfunction and Peyer’s Patch Integrity in Adult Mice. International Journal of Molecular Sciences, 25(19), 10676. https://doi.org/10.3390/ijms251910676






