Functional Characterization of the Gibberellin (GA) Receptor ScGID1 in Sugarcane
Abstract
1. Introduction
2. Results
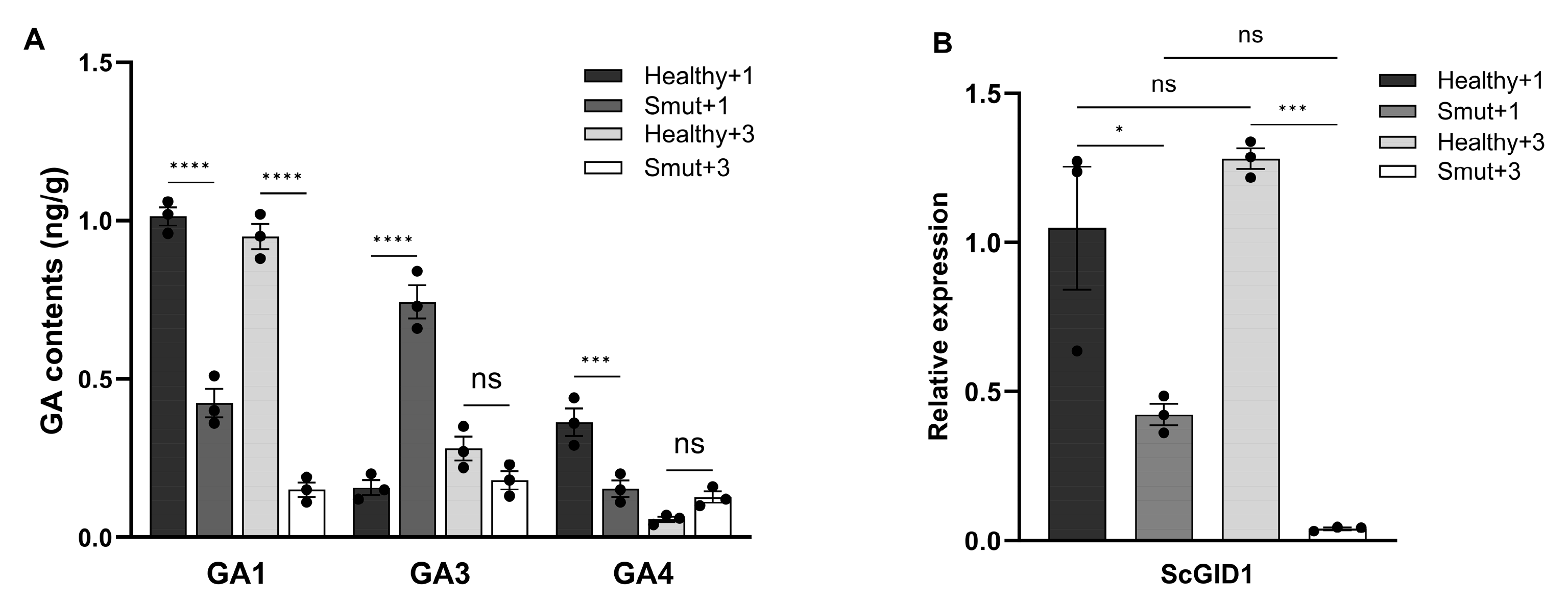
2.1. The Sugarcane Gibberellin Signaling Pathway Responded to Sporisorium scitamineum Infection
2.2. Identification of the ScGID1 Gene
2.3. Tissue Expression and Subcellular Location Analysis of ScGID1
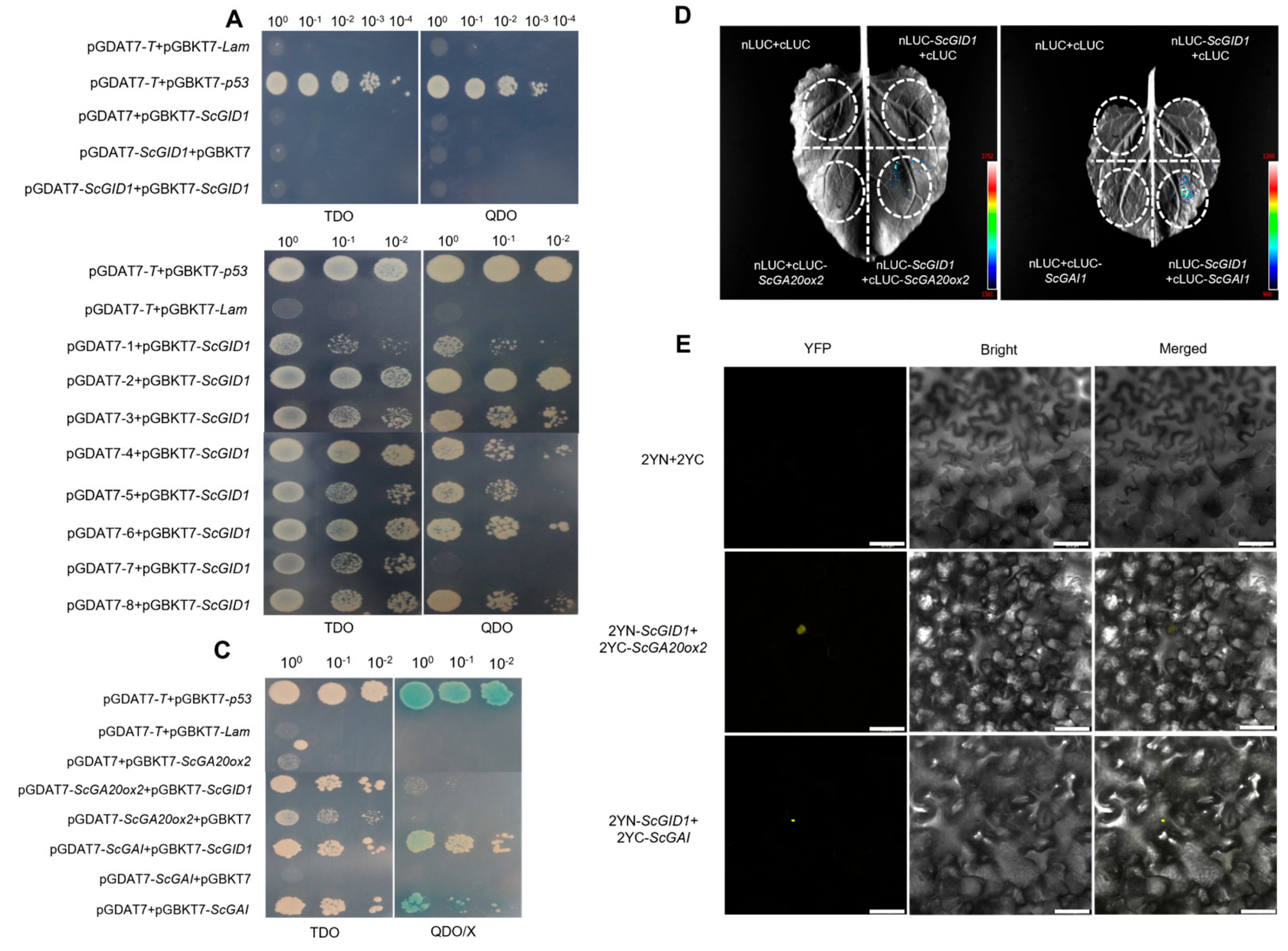
2.4. ScGID1 Interacts with Key Proteins in Gibberellin Biosynthesis and Metabolism Pathways
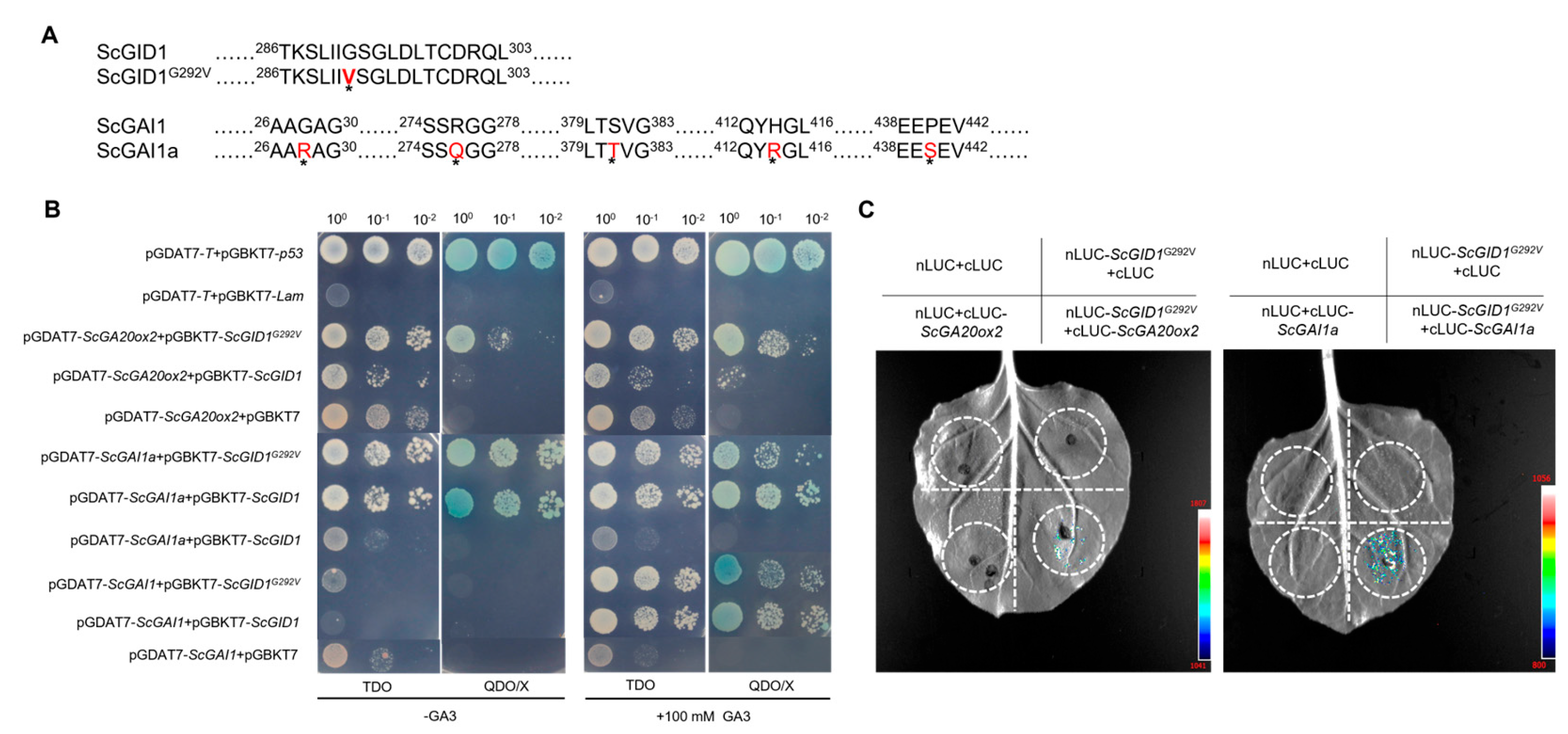
2.5. A Novel GAI1a Homologue in Sugarcane Interacts with ScGID1 and Its Mutants in a Gibberellin-Independent Manner
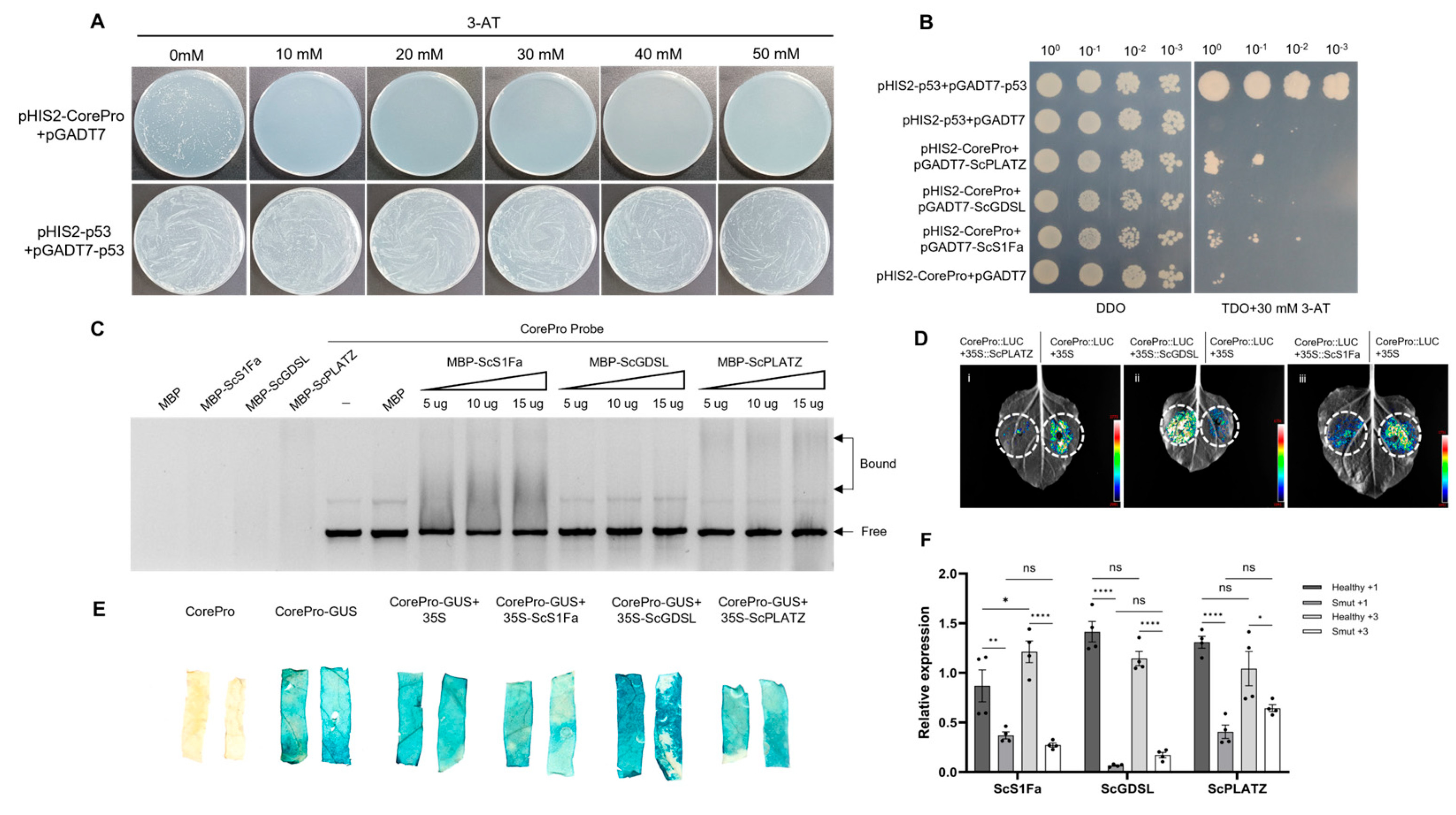
2.6. Potential Promoter of ScGID1 and Its Upstream Regulatory Transcription Factors
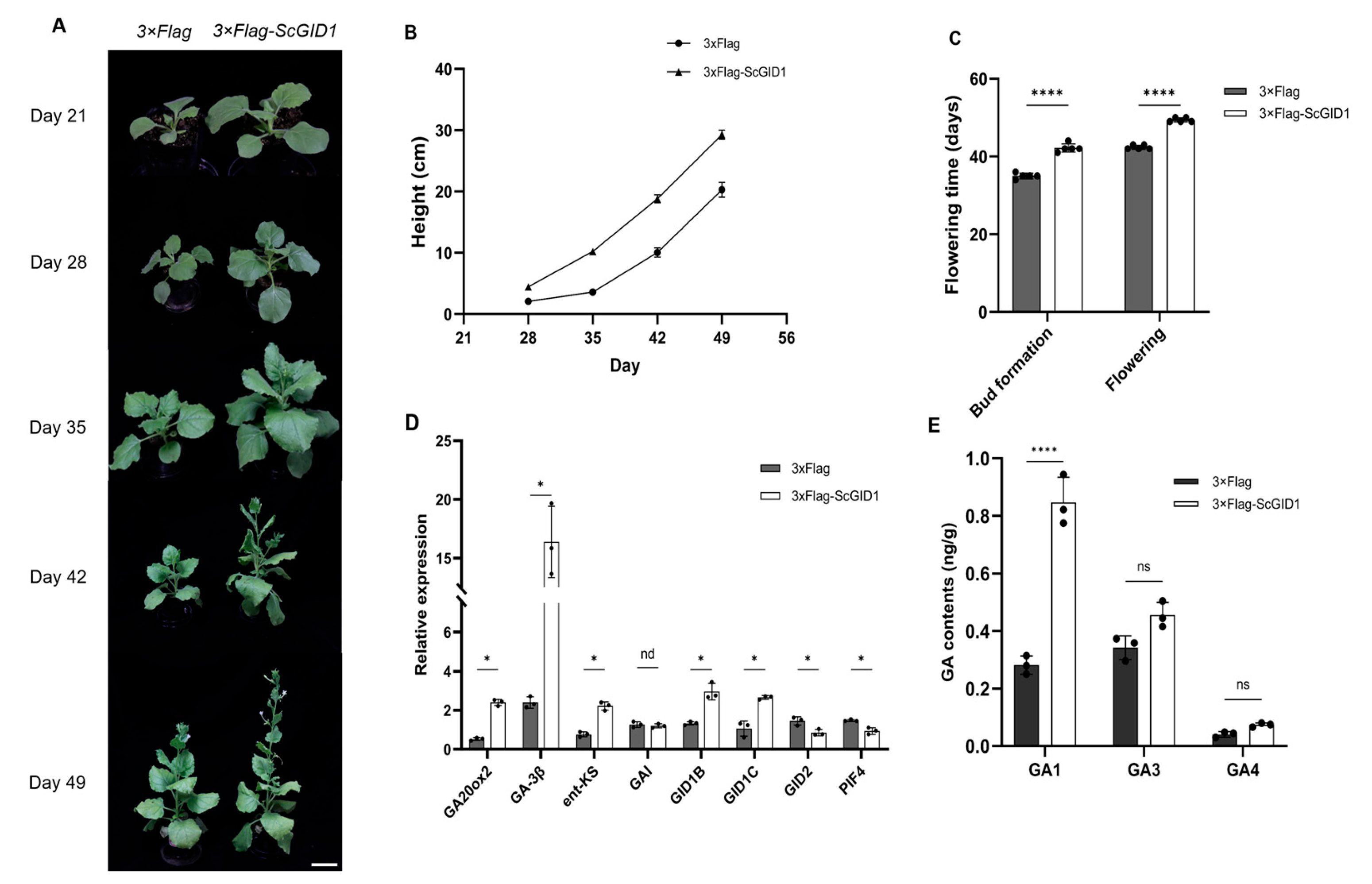
2.7. Overexpression of ScGID1 Promotes the Growth of N. benthamiana
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Gene Cloning and Sequence Analysis
4.3. Real-Time Fluorescence Quantitative PCR (RT-qPCR)
4.4. Subcellular Localization of ScGID1
4.5. Bimolecular Fluorescence Complementation Assays
4.6. Yeast Two-Hybrid and Yeast One-Hybrid Assays
4.7. Split Luciferase Assays
4.8. Promoter Cloning and Cis-Acting Regulatory Element Analysis
4.9. Histochemical GUS Staining Assay
4.10. Electrophoretic Mobility Shift Assays
4.11. Dual-Luciferase Reporter Assay
4.12. Constructs and Transformation of N. benthamiana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajput, M.A.; Rajput, N.A.; Syed, R.N.; Lodhi, A.M.; Que, Y. Sugarcane Smut: Current Knowledge and the Way Forward for Management. J. Fungi 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Deng, Y.; Cai, E.; Yan, M.; Li, L.; Chen, B.; Chang, C.; Jiang, Z. The Farnesyltransferase β-Subunit Ram1 Regulates Sporisorium scitamineum Mating, Pathogenicity and Cell Wall Integrity. Front. Microbiol. 2019, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Deng, Y.; Cai, E.; Yan, M.; Cui, G.; Wang, Z.; Zou, C.; Zhang, B.; Xi, P.; Chang, C.; et al. Identification and Functional Analysis of the Pheromone Response Factor Gene of Sporisorium scitamineum. Front. Microbiol. 2019, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.R.; Appezzato-da-Glória, B.; Piepenbring, M.; Massola, N.S., Jr.; Monteiro-Vitorello, C.B.; Vieira, M.L.C. Sugarcane smut: Shedding light on the development of the whip-shaped sorus. Ann. Bot. 2017, 119, 815–827. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Shen, X.; Guo, F.; Zhou, C.; Li, R.; Chen, B. SsPEP1, an Effector with Essential Cellular Functions in Sugarcane Smut Fungus. J. Fungi 2021, 7. [Google Scholar] [CrossRef]
- Shuai, L.; Huang, H.; Liao, L.; Duan, Z.; Zhang, X.; Wang, Z.; Lei, J.; Huang, W.; Chen, X.; Huang, D.; et al. Variety-Specific Flowering of Sugarcane Induced by the Smut Fungus Sporisorium scitamineum. Plants 2023, 12. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas Stephen, G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Peng, J.; Harberd, N.P. The role of GA-mediated signalling in the control of seed germination. Curr. Opin. Plant Biol. 2002, 5, 376–381. [Google Scholar] [CrossRef]
- Shani, E.; Hedden, P.; Sun, T.P. Highlights in gibberellin research: A tale of the dwarf and the slender. Plant Physiol. 2024, 195, 111–134. [Google Scholar] [CrossRef]
- Li, Q.F.; Zhou, Y.; Xiong, M.; Ren, X.Y.; Han, L.; Wang, J.D.; Zhang, C.Q.; Fan, X.L.; Liu, Q.Q. Gibberellin recovers seed germination in rice with impaired brassinosteroid signalling. Plant Sci. Int. J. Exp. Plant Biol. 2020, 293, 110435. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, D.; Gil, J.; Blázquez, M.A.; García-Martínez, J.L. Gibberellins repress photomorphogenesis in darkness. Plant Physiol. 2004, 134, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chen, H.; Li, T.; Xu, F.; Mao, Z.; Cao, X.; Miao, L.; Du, S.; Hua, J.; Zhao, J.; et al. Blue light-dependent interactions of CRY1 with GID1 and DELLA proteins regulate gibberellin signaling and photomorphogenesis in Arabidopsis. Plant Cell 2021, 33, 2375–2394. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Ohashi, Y.; Takahashi, R.; Nakai, K.; Takahashi, Y. DELLA degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. Plant Cell 2021, 33, 2258–2272. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Kwon, C.-T.; Paek, N.-C. Gibberellic Acid: A Key Phytohormone for Spikelet Fertility in Rice Grain Production. Int. J. Mol. Sci. 2016, 17, 794. [Google Scholar] [CrossRef]
- Plackett, A.R.; Thomas, S.G.; Wilson, Z.A.; Hedden, P. Gibberellin control of stamen development: A fertile field. Trends Plant Sci. 2011, 16, 568–578. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Beauvoit, B.P.; Takahara, M.; Hao, S.; Ezura, K.; Andrieu, M.H.; Nishida, K.; Mori, K.; Suzuki, Y.; Kuhara, S.; et al. Fruit setting rewires central metabolism via gibberellin cascades. Proc. Natl. Acad. Sci. USA 2020, 117, 23970–23981. [Google Scholar] [CrossRef]
- Serrani, J.C.; Sanjuán, R.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Gibberellin regulation of fruit set and growth in tomato. Plant Physiol. 2007, 145, 246–257. [Google Scholar] [CrossRef]
- Achard, P.; Cheng, H.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van Der Straeten, D.; Peng, J.; Harberd, N.P. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006, 311, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Fang, J.; Huang, C.; Huang, R.; Tan, X.; Chen, B.; Yao, W.; Zhang, M. A novel transcription factor, ScAIL1, modulates plant defense responses by targeting DELLA and regulating gibberellin and jasmonic acid signaling in sugarcane. J. Exp. Bot. 2022, 73, 6727–6743. [Google Scholar] [CrossRef]
- Li, P.; Guo, L.; Lang, X.; Li, M.; Wu, G.; Wu, R.; Wang, L.; Zhao, M.; Qing, L. Geminivirus C4 proteins inhibit GA signaling via prevention of NbGAI degradation, to promote viral infection and symptom development in N. benthamiana. PLoS Pathog. 2022, 18, e1010217. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Nakajima, M.; Katoh, E.; Ohmiya, H.; Asano, K.; Saji, S.; Hongyu, X.; Ashikari, M.; Kitano, H.; Yamaguchi, I.; et al. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 2007, 19, 2140–2155. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Nakajima, M.; Motoyuki, A.; Matsuoka, M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 2007, 58, 183–198. [Google Scholar] [CrossRef]
- Hauvermale, A.L.; Cárdenas, J.J.; Bednarek, S.Y.; Steber, C.M. GA signaling expands: The plant UBX domain-containing protein 1 is a binding partner for the GA receptor. Plant Physiol. 2022, 190, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Hirano, Y.; Sun, T.P.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hirai, T.; Yamamoto, E.; Kawamura, M.; Sato, T.; Kitano, H.; Matsuoka, M.; Ueguchi-Tanaka, M. A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins. Plant Cell 2010, 22, 3589–3602. [Google Scholar] [CrossRef]
- Chai, Z.; Fang, J.; Yao, W.; Zhao, Y.; Cheng, G.; Akbar, S.; Khan, M.T.; Chen, B.; Zhang, M. ScGAIL, a sugarcane N-terminal truncated DELLA-like protein, participates in gibberellin signaling in Arabidopsis. J. Exp. Bot. 2022, 73, 3462–3476. [Google Scholar] [CrossRef]
- Fan, Y.; Qiu, L.; Huang, X.; Zhou, H.; Gan, C.; Li, Y.; Yang, R.; Wu, J.; Chen, R. Expression Analysis of Key Genes in Gibberellin Biosynthesis and Related Phytohormonal Dynamics During Sugarcane Internode Elongation. Chin. Bull. Bot. 2019, 54, 486–496. (In Chinese) [Google Scholar] [CrossRef]
- Su, Y.; Xu, L.; Wang, Z.; Peng, Q.; Yang, Y.; Chen, Y.; Que, Y. Comparative proteomics reveals that central metabolism changes are associated with resistance against Sporisorium scitamineum in sugarcane. BMC Genom. 2016, 17, 800. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Que, Y.; Su, Y.; Guo, J.; Wu, Q.; Xu, L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. PLoS ONE 2014, 9, e106476. [Google Scholar] [CrossRef]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.P.; et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef]
- Acheampong, A.K.; Hu, J.; Rotman, A.; Zheng, C.; Halaly, T.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Lichter, A.; Sun, T.P.; et al. Functional characterization and developmental expression profiling of gibberellin signalling components in Vitis vinifera. J. Exp. Bot. 2015, 66, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y.; Yamaguchi, S. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, B.; Achard, P. Long-distance transport of phytohormones through the plant vascular system. Curr. Opin. Plant Biol. 2016, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Shimada, A.; Takashi, Y.; Kim, Y.C.; Park, S.H.; Ueguchi-Tanaka, M.; Suzuki, H.; Katoh, E.; Iuchi, S.; Kobayashi, M.; et al. Identification and characterization of Arabidopsis gibberellin receptors. Plant J. Cell Mol. Biol. 2006, 46, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Ueguchi-Tanaka, M.; Matsuoka, M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008, 13, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Willige, B.C.; Ghosh, S.; Nill, C.; Zourelidou, M.; Dohmann, E.M.; Maier, A.; Schwechheimer, C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 2007, 19, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Livne, S.; Weiss, D. Cytosolic activity of the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1A. Plant Cell Physiol. 2014, 55, 1727–1733. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Cai, J.; Zhao, Y.; Cui, J.; Li, Y. Cloning and Characterization of EuGID1 in Eucommia ulmoides Oliver. Phyton-Int. J. Exp. Bot. 2022, 91, 999–1013. [Google Scholar] [CrossRef]
- Phillips, A.L.; Ward, D.A.; Uknes, S.; Appleford, N.E.; Lange, T.; Huttly, A.K.; Gaskin, P.; Graebe, J.E.; Hedden, P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995, 108, 1049–1057. [Google Scholar] [CrossRef]
- Fukazawa, J.; Mori, M.; Watanabe, S.; Miyamoto, C.; Ito, T.; Takahashi, Y. DELLA-GAF1 Complex Is a Main Component in Gibberellin Feedback Regulation of GA20 Oxidase 2. Plant Physiol. 2017, 175, 1395–1406. [Google Scholar] [CrossRef]
- Pyvovarenko, T.; Lopato, S. Isolation of plant transcription factors using a yeast one-hybrid system. Methods Mol. Biol. 2011, 754, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.; Briones-Moreno, A.; Blázquez, M.A. Origin and evolution of gibberellin signaling and metabolism in plants. Semin. Cell Dev. Biol. 2021, 109, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Niu, Y.; Dong, H.; Jia, Y.; Wang, Y. Characterization of the Function of Two S1Fa-Like Family Genes From Populus trichocarpa. Front. Plant Sci. 2021, 12, 753099. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.; Ashrafi-Dehkordi, E.; Shahriari, A.G.; Mazloomi, S.M.; Ebrahimie, E. Integrative meta-analysis of transcriptomic responses to abiotic stress in cotton. Prog. Biophys. Mol. Biol. 2019, 146, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.; Zou, Z.; Kok, E.P.; Kwan, B.H.; Chow, K.; Nasu, S.; Nanzyo, M.; Kitashiba, H.; Nishio, T. Comparative transcriptome analysis of leaves and roots in response to sudden increase in salinity in Brassica napus by RNA-seq. BioMed Res. Int. 2014, 2014, 467395. [Google Scholar] [CrossRef]
- Zhou, D.X.; Bisanz-Seyer, C.; Mache, R. Molecular cloning of a small DNA binding protein with specificity for a tissue-specific negative element within the rps1 promoter. Nucleic Acids Res. 1995, 23, 1165–1169. [Google Scholar] [CrossRef]
- Gazara, R.K.; de Oliveira, E.A.G.; Rodrigues, B.C.; Nunes da Fonseca, R.; Oliveira, A.E.A.; Venancio, T.M. Transcriptional landscape of soybean (Glycine max) embryonic axes during germination in the presence of paclobutrazol, a gibberellin biosynthesis inhibitor. Sci. Rep. 2019, 9, 9601. [Google Scholar] [CrossRef]
- Arana, M.V.; Marín-de la Rosa, N.; Maloof, J.N.; Blázquez, M.A.; Alabadí, D. Circadian oscillation of gibberellin signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 9292–9297. [Google Scholar] [CrossRef]
- Volokita, M.; Rosilio-Brami, T.; Rivkin, N.; Zik, M. Combining comparative sequence and genomic data to ascertain phylogenetic relationships and explore the evolution of the large GDSL-lipase family in land plants. Mol. Biol. Evol. 2011, 28, 551–565. [Google Scholar] [CrossRef]
- Shen, G.; Sun, W.; Chen, Z.; Shi, L.; Hong, J.; Shi, J. Plant GDSL Esterases/Lipases: Evolutionary, Physiological and Molecular Functions in Plant Development. Plants 2022, 11. [Google Scholar] [CrossRef]
- Ding, L.N.; Guo, X.J.; Li, M.; Fu, Z.L.; Yan, S.Z.; Zhu, K.M.; Wang, Z.; Tan, X.L. Improving seed germination and oil contents by regulating the GDSL transcriptional level in Brassica napus. Plant Cell Rep. 2019, 38, 243–253. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Dong, Z.; Tian, Y.; Xie, K.; Wu, S.; Zhu, T.; Zhang, D.; Zhou, Y.; Niu, C.; Ma, B.; et al. ZmMs30 Encoding a Novel GDSL Lipase Is Essential for Male Fertility and Valuable for Hybrid Breeding in Maize. Mol. Plant 2019, 12, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Choi, H.W.; Hwang, I.S.; Kim, D.S.; Kim, N.H.; Choi, D.S.; Kim, Y.J.; Hwang, B.K. Function of a novel GDSL-type pepper lipase gene, CaGLIP1, in disease susceptibility and abiotic stress tolerance. Planta 2008, 227, 539–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, C.; Li, Q.; Zhou, Y.; Wu, Y. Genome-wide analysis of the plant-specific PLATZ proteins in maize and identification of their general role in interaction with RNA polymerase III complex. BMC Plant Biol. 2018, 18, 221. [Google Scholar] [CrossRef]
- Nagano, Y.; Furuhashi, H.; Inaba, T.; Sasaki, Y. A novel class of plant-specific zinc-dependent DNA-binding protein that binds to A/T-rich DNA sequences. Nucleic Acids Res. 2001, 29, 4097–4105. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.; Jun, S.E.; Park, S.; Timilsina, R.; Kwon, D.S.; Kim, Y.; Park, S.J.; Hwang, J.Y.; Nam, H.G.; et al. ORESARA15, a PLATZ transcription factor, mediates leaf growth and senescence in Arabidopsis. New Phytol. 2018, 220, 609–623. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Zhang, W.; Zhang, X.; Mo, Y.; Tranquilli, G.E.; Vanzetti, L.S.; Dubcovsky, J. Wheat plant height locus RHT25 encodes a PLATZ transcription factor that interacts with DELLA (RHT1). Proc. Natl. Acad. Sci. USA 2023, 120, e2300203120. [Google Scholar] [CrossRef]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14 (Suppl. 1), S61–S80. [Google Scholar] [CrossRef]
- Gallego-Giraldo, C.; Hu, J.; Urbez, C.; Gomez, M.D.; Sun, T.P.; Perez-Amador, M.A. Role of the gibberellin receptors GID1 during fruit-set in Arabidopsis. Plant J. Cell Mol. Biol. 2014, 79, 1020–1032. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Ban, L.; Wu, Y.; Wu, X.; Wang, Y.; Wen, H.; Chapurin, V.; Dzyubenko, N.; Li, Z.; et al. Functional characterization of a gibberellin receptor and its application in alfalfa biomass improvement. Sci. Rep. 2017, 7, 41296. [Google Scholar] [CrossRef]
- Voinnet, O.; Vain, P.; Angell, S.; Baulcombe, D.C. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 1998, 95, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qian, Y.; Wang, Y.; Li, Z.; Zhou, X. Iterons homologous to helper Geminiviruses are essential for efficient replication of betasatellites. J. Virol. 2019, 93, e01532-18. [Google Scholar] [CrossRef] [PubMed]



| Number | Gene ID | Functional Description |
|---|---|---|
| 1 | Sh_228D18_p000070 | Phospholipase A1-II 7 |
| 2 | Sh_210G02_p000050 | Malate dehydrogenase |
| 3 | Sh_265O22_p000030 | Alcohol dehydrogenase 1 |
| 4 | Sh_216J02_p000080 | E3 ubiquitin-protein ligase RGLG2 |
| 5 | Sh_254P24_contig-1_p000020 | Similar to the Ubiquitin-conjugating enzyme family protein |
| 6 | Sh_234J05_p000030 | Bisdemethoxycurcumin synthase |
| 7 | Sh_218M08_p000070 | Dormancy-associated protein 1 |
| 8 | Sh_222J11 | Aconitate hydratase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, S.; Chen, B.; Xu, X. Functional Characterization of the Gibberellin (GA) Receptor ScGID1 in Sugarcane. Int. J. Mol. Sci. 2024, 25, 10688. https://doi.org/10.3390/ijms251910688
Wang Z, Zhang S, Chen B, Xu X. Functional Characterization of the Gibberellin (GA) Receptor ScGID1 in Sugarcane. International Journal of Molecular Sciences. 2024; 25(19):10688. https://doi.org/10.3390/ijms251910688
Chicago/Turabian StyleWang, Zhiyuan, Shujun Zhang, Baoshan Chen, and Xiongbiao Xu. 2024. "Functional Characterization of the Gibberellin (GA) Receptor ScGID1 in Sugarcane" International Journal of Molecular Sciences 25, no. 19: 10688. https://doi.org/10.3390/ijms251910688
APA StyleWang, Z., Zhang, S., Chen, B., & Xu, X. (2024). Functional Characterization of the Gibberellin (GA) Receptor ScGID1 in Sugarcane. International Journal of Molecular Sciences, 25(19), 10688. https://doi.org/10.3390/ijms251910688







