Biocompatible Poly(ε-Caprolactone) Nanocapsules Enhance the Bioavailability, Antibacterial, and Immunomodulatory Activities of Curcumin
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Curcumin-Nanocapsules Preparation of Curcumin-Nanocapsules
2.2. Measurement of the Size and Potential of Curcumin-Nanocapsules
2.3. Determination of Encapsulated Curcumin
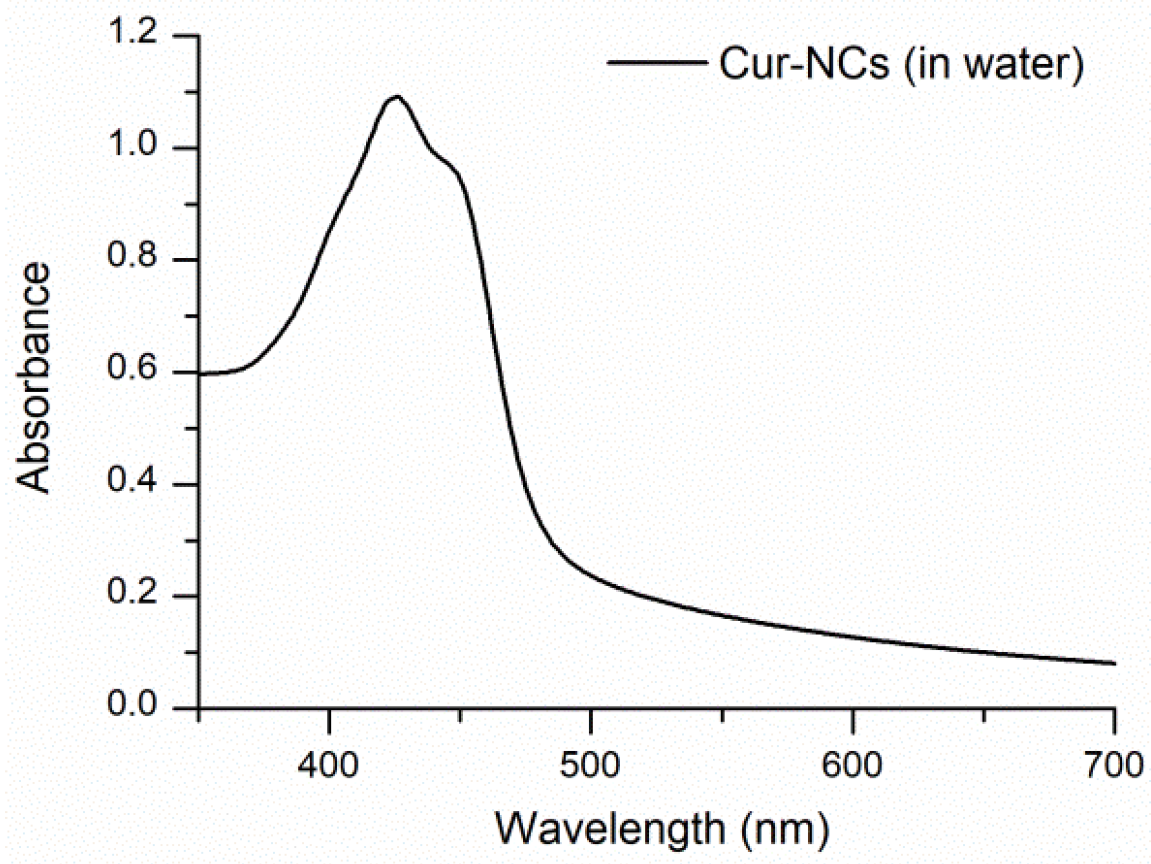
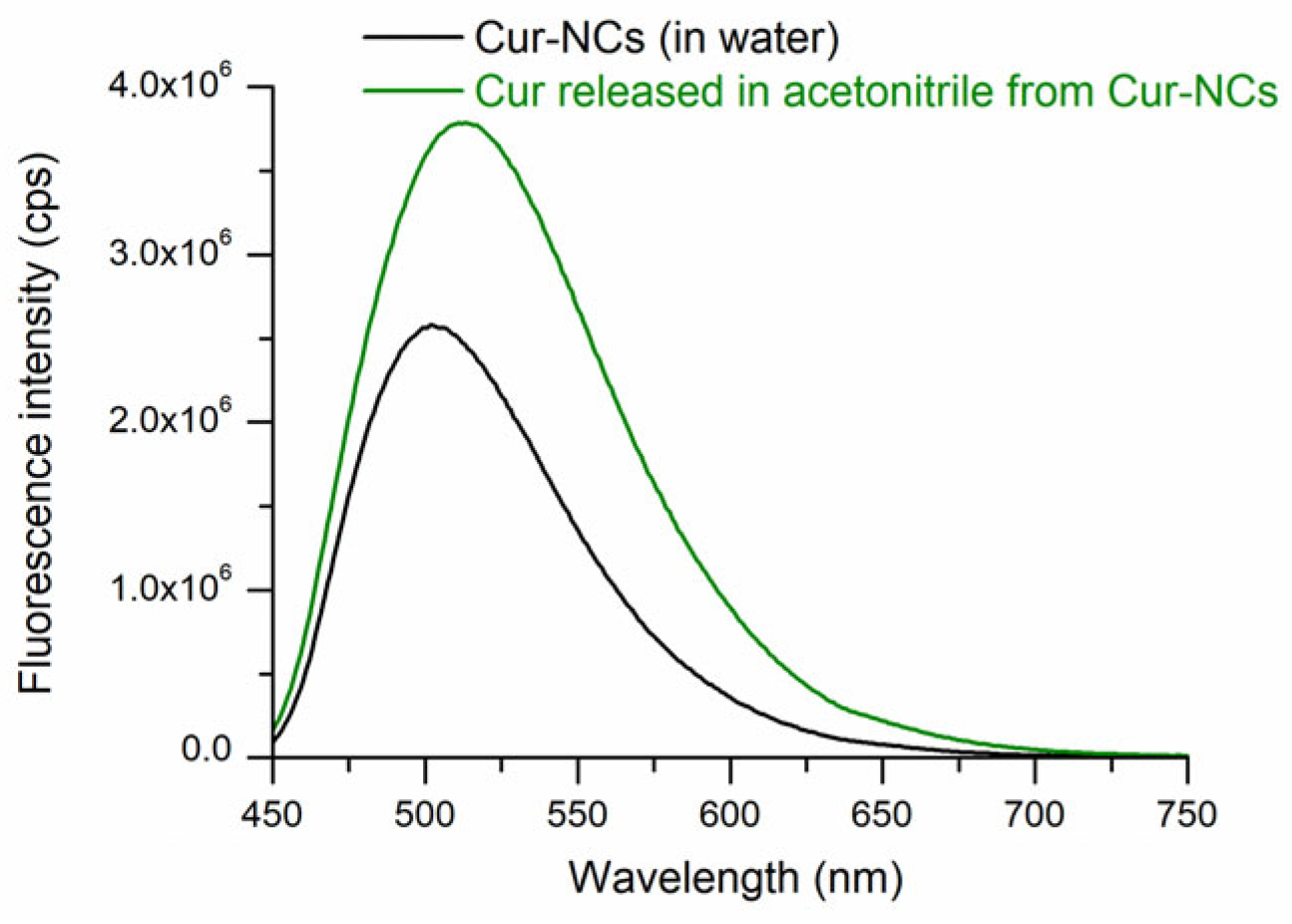
2.4. Determination of the Spectra of Curcumin-Nanocapsules
2.5. Effect of the Time on Curcumin-Nanocapsules
2.6. Determination of the Stability and Bioaccessibility of Curcumin-Nanocapsules
2.7. Antibacterial Activity of Curcumin-Nanocapsules
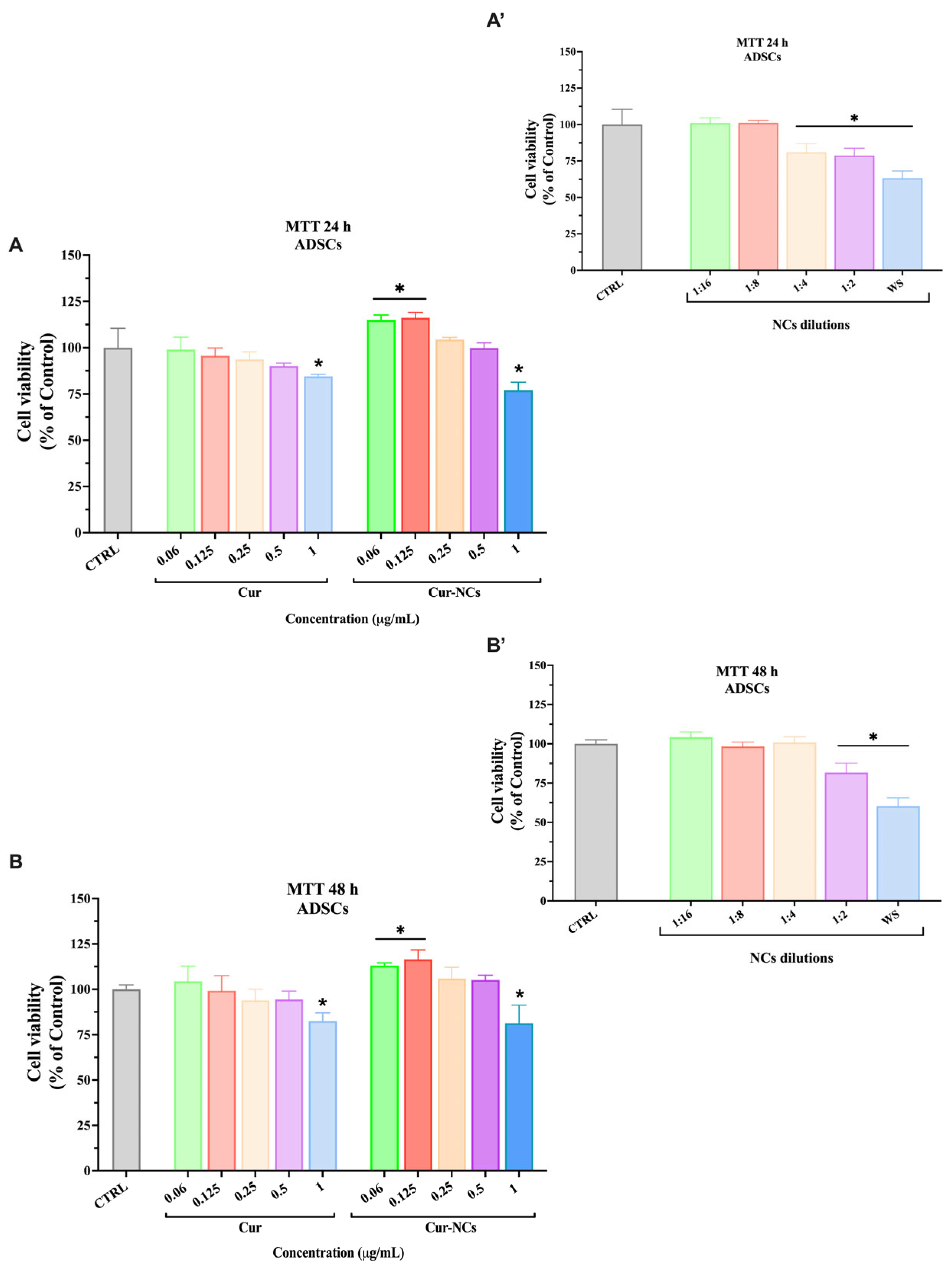
2.8. Effect of Free and Encapsulated Curcumin on Human Adipose-Derived Stem Cells Viability
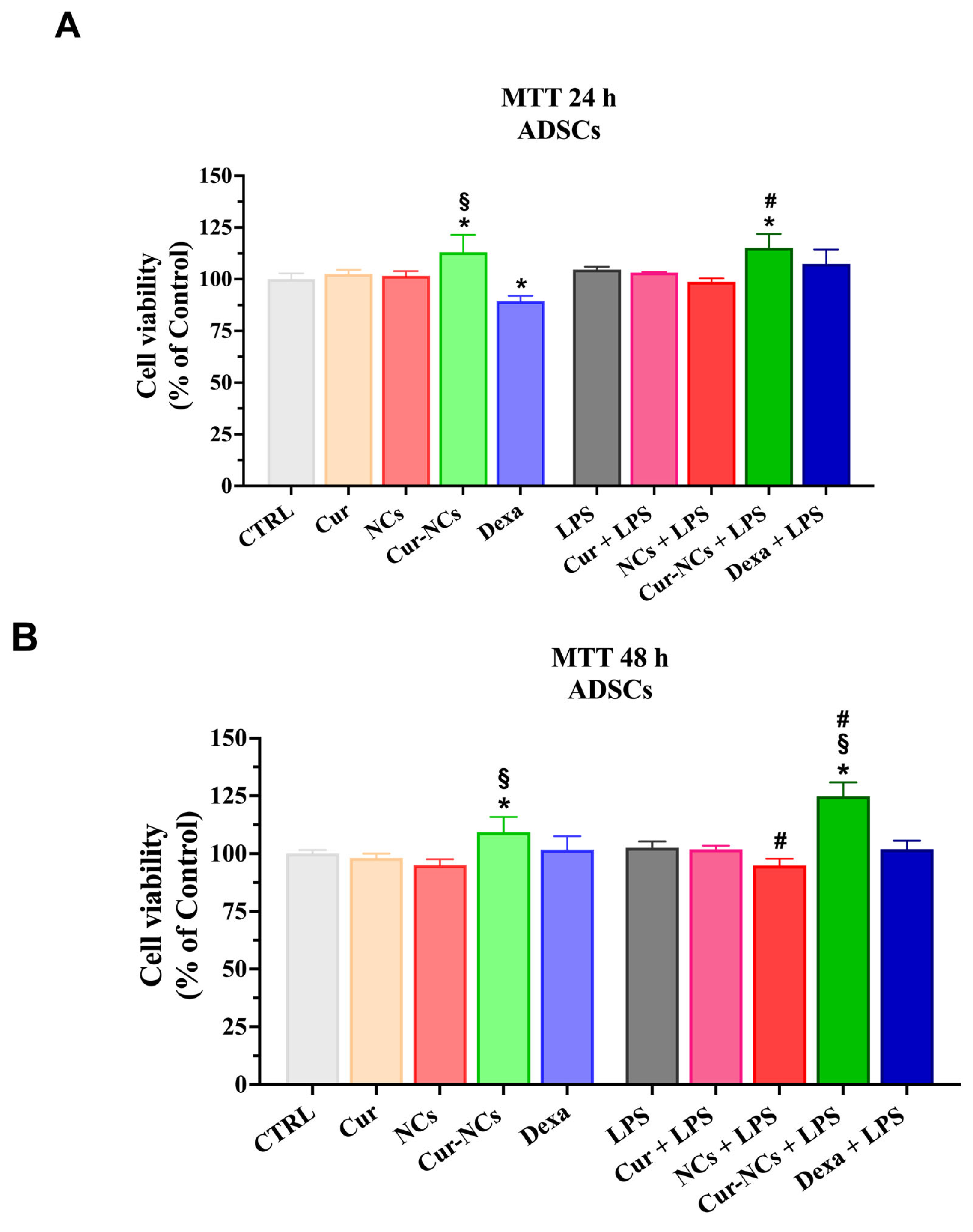
2.9. Effect of Curcumin and Curcumin-Nanocapsules on Human Adipose-Derived Stem Cells Viability in an Inflammatory Context
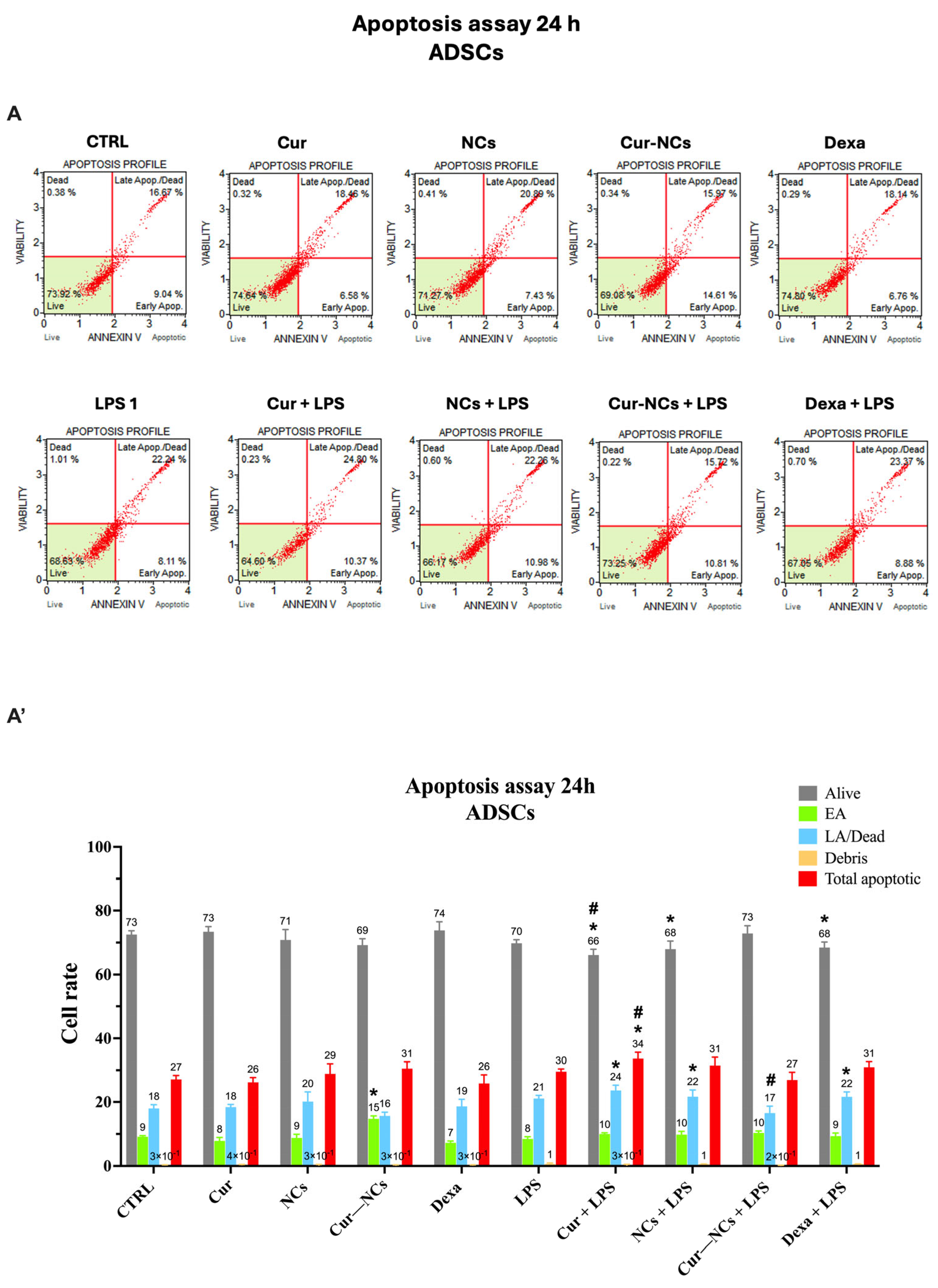
2.10. Evaluation of Apoptotic Cell Death on Human Adipose-Derived Stem Cells Exposed to Curcumin or Curcumin-Nanocapsules in the Presence or Absence of Inflammatory Stimuli
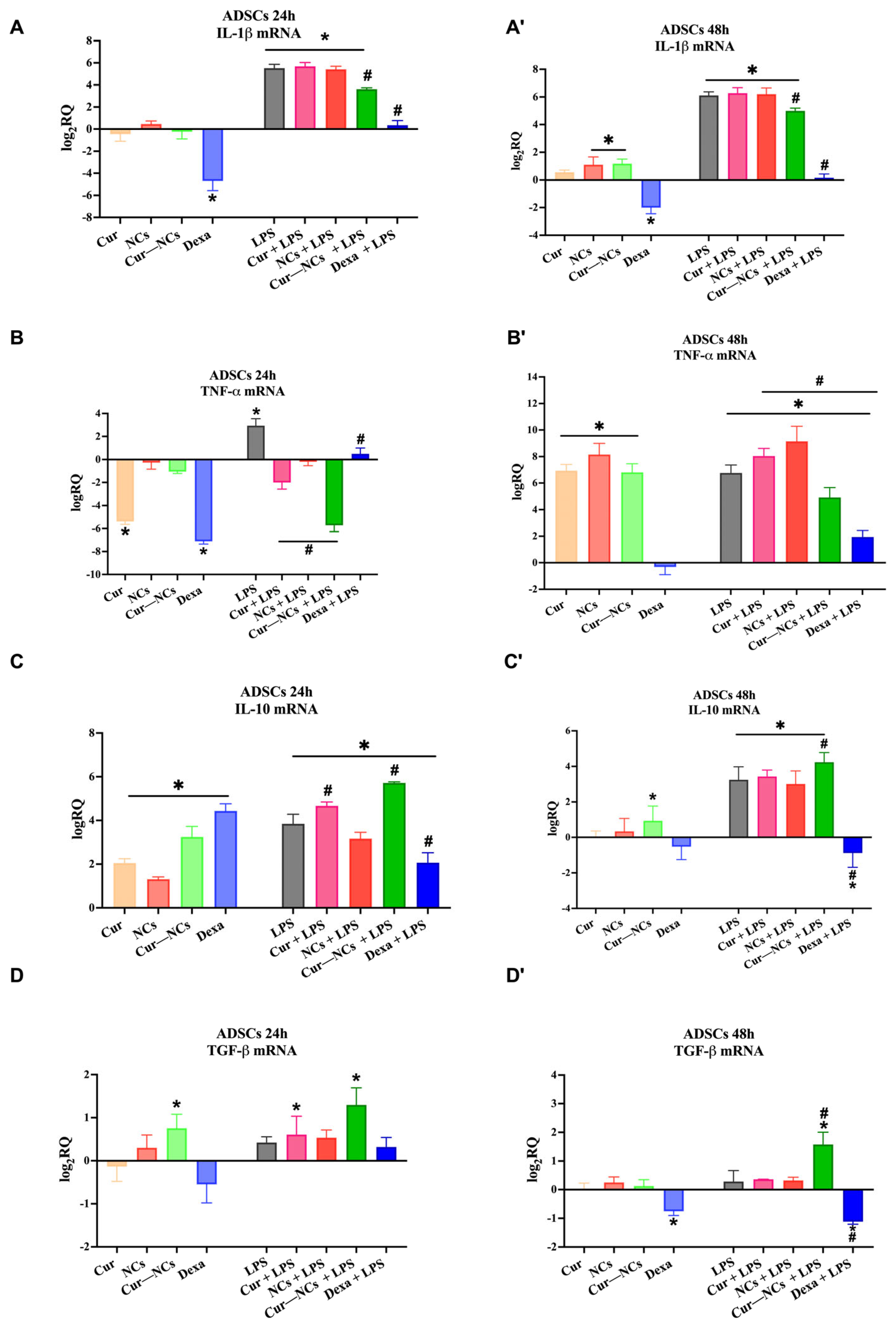
2.11. Immunomodulatory Effect of Curcumin-Nanocapsules on Human Adipose-Derived Stem Cells Grown in the Presence or Absence of Inflammatory Stimuli
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Curcumin-Nanocapsules
4.3. Characterization of Curcumin-Nanocapsules
4.3.1. Particle Size and Zeta Potential
4.3.2. Encapsulation Efficiency of Curcumin-Nanocapsules
4.3.3. Ultraviolet–Visible and Fluorescence Spectra of Curcumin-Nanocapsules
4.4. Stability of Curcumin-Nanocapsules over Time
4.5. Simulated Gastrointestinal Digestion Test of Curcumin-Nanocapsules
4.5.1. Gastric and Intestinal Phases
4.5.2. Physicochemical Characterization of Curcumin-Nanocapsules after Simulated Gastric Digestion
4.5.3. Determination of Curcumin Concentration after Digestion Phases
4.6. Microbiological Assay
4.7. Cell Culture
4.8. Cell Culture Treatments and MTT Assay
4.9. Muse Assay
4.10. Real-Time PCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Sekerkova, A. Cytokine networking of innate immunity cells: A potential target of therapy. Clin. Sci. 2014, 126, 593–612. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Ohshima, H.; Tatemichi, M.; Sawa, T. Chemical basis of inflammation-induced carcinogenesis. Arch. Biochem. Biophys. 2003, 417, 3–11. [Google Scholar] [CrossRef]
- Subeta, P.; Lana, A.J.; Schlachetzki, J.C.M. Chronic peripheral inflammation: A possible contributor to neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1711–1714. [Google Scholar]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64. [Google Scholar] [PubMed]
- Richard-Eaglin, A.; Smallheer, B.A. Immunosuppressive/Autoimmune Disorders. Nurs. Clin. N. Am. 2018, 53, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, S.; El Atat, O.; Diallo, K.; Rauwel, B.; Degboe, Y.; Cavaignac, E.; Constantin, A.; Cantagrel, A.; Trak-Smayra, V.; Alaaeddine, N.; et al. Rheumatoid Synovial Fluids Regulate the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells Through a TNF/NF-kappa B-Dependent Mechanism. Front. Immunol. 2019, 10, 1482. [Google Scholar]
- Menard, C.; Pacelli, L.; Bassi, G.; Dulong, J.; Bifari, F.; Bezier, I.; Zanoncello, J.; Ricciardi, M.; Latour, M.; Bourin, P.; et al. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: Standardization of immune quality controls. Stem Cells Dev. 2013, 22, 1789–1801. [Google Scholar] [CrossRef]
- Xie, J.; Jones, T.J.; Feng, D.N.; Cook, T.G.; Jester, A.A.; Yi, R.; Jawed, Y.T.; Babbey, C.; March, K.L.; Murphy, M.P. Human Adipose-Derived Stem Cells Suppress Elastase-Induced Murine Abdominal Aortic Inflammation and Aneurysm Expansion Through Paracrine Factors. Cell Transplant. 2017, 26, 173–189. [Google Scholar] [CrossRef]
- Lupo, G.; Agafonova, A.; Cosentino, A.; Giurdanella, G.; Mannino, G.; Lo Furno, D.; Romano, I.R.; Giuffrida, R.; D’Angeli, F.; Anfuso, C.D. Protective Effects of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells on Human Retinal Endothelial Cells in an In Vitro Model of Diabetic Retinopathy: Evidence for Autologous Cell Therapy. Int. J. Mol. Sci. 2023, 24, 913. [Google Scholar] [CrossRef]
- Agafonova, A.; Cosentino, A.; Romano, I.R.; Giurdanella, G.; D’Angeli, F.; Giuffrida, R.; Lo Furno, D.; Anfuso, C.D.; Mannino, G.; Lupo, G. Molecular Mechanisms and Therapeutic Implications of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells in an In Vitro Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2024, 25, 1774. [Google Scholar] [CrossRef]
- Yamato, M.; Sakai, Y.; Mochida, H.; Kawaguchi, K.; Takamura, M.; Usui, S.; Seki, A.; Mizukoshi, E.; Yamashita, T.; Yamashita, T.; et al. Adipose tissue-derived stem cells prevent fibrosis in murine steatohepatitis by suppressing IL-17-mediated inflammation. J. Gastroenterol. Hepatol. 2019, 34, 1432–1440. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mustapha, N.; Mokdad-Bzeouich, I.; El Gueder, D.; Ghedira, K.; Ghedira-Chekir, L. Flavones induce immunomodulatory and anti-inflammatory effects by activating cellular anti-oxidant activity: A structure-activity relationship study. Tumour Biol. 2016, 37, 6571–6579. [Google Scholar] [CrossRef]
- Gurjar, V.K.; Pal, D. Natural compounds extracted from medicinal plants and their immunomodulatory activities. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Switzerland, 2021; pp. 197–261. [Google Scholar]
- Allegra, A.; Mirabile, G.; Ettari, R.; Pioggia, G.; Gangemi, S. The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis. Int. J. Mol. Sci. 2022, 23, 14710. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Turmeric. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science & Business Media: Berlin, Germany, 2007; Volume 595. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef]
- Bonfanti, R.; Musumeci, T.; Russo, C.; Pellitteri, R. The protective effect of curcumin in Olfactory Ensheathing Cells exposed to hypoxia. Eur. J. Pharmacol. 2017, 796, 62–68. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.H.; Du, Z.Y. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Chamani, S.; Moossavi, M.; Naghizadeh, A.; Abbasifard, M.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Immunomodulatory effects of curcumin in systemic autoimmune diseases. Phytother. Res. 2022, 36, 1616–1632. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Mahjoob, M.; Stochaj, U. Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev. 2021, 69, 101364. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Y.; Song, L.; Pan, Z.; Ye, S.; Hou, Z. Design of a novel curcumin-soybean phosphatidylcholine complex-based targeted drug delivery systems. Drug Deliv. 2017, 24, 707–719. [Google Scholar] [CrossRef]
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop Based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability, and Anti-Inflammatory Effect. Mol. Pharm. 2017, 14, 1610–1622. [Google Scholar] [CrossRef]
- Granata, G.; Petralia, S.; Forte, G.; Conoci, S.; Consoli, G.M.L. Injectable supramolecular nanohydrogel from a micellar self-assembling calix[4]arene derivative and curcumin for a sustained drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110842. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.; Lo Nigro, R.; Malandrino, G.; Geraci, C. Supramolecular assembly of a succinyl-calix [4] arene derivative in multilamellar vesicles. Supramol. Chem. 2016, 28, 377–383. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- de Gomes, M.G.; Teixeira, F.E.G.; de Carvalho, F.B.; Pacheco, C.O.; da Silva Neto, M.R.; Giacomeli, R.; Ramalho, J.B.; Dos Santos, R.B.; Domingues, W.B.; Campos, V.F.; et al. Curcumin-loaded lipid-core nanocapsules attenuates the immune challenge LPS-induced in rats: Neuroinflammatory and behavioral response in sickness behavior. J. Neuroimmunol. 2020, 345, 577270. [Google Scholar] [CrossRef]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by beta-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef]
- Giacomeli, R.; Guerra Teixeira, F.E.; Carvalho, F.B.; Pacheco, C.O.; Martins Parisotto, A.J.; Funguetto Ribeiro, A.C.; Gomes de Gomes, M.; Haas, S.E. Curcumin-loaded poly(ϵ-caprolactone) lipid-core nanocapsules: Evaluation of fetal and maternal toxicity. Food Chem. Toxicol. 2020, 144, 111625. [Google Scholar] [CrossRef] [PubMed]
- Nakama, K.A.; dos Santos, R.B.; da Rosa Silva, C.E.; Izoton, J.C.; Savall, A.S.P.; Gutirrez, M.E.Z.; Roman, S.S.; Luchese, C.; Pinton, S.; Haas, S.E. Establishment of analytical method for quantification of anti-inflammatory agents co-nanoencapsulated and its application to physicochemical development and characterization of lipid-core nanocapsules. Arab. J. Chem. 2020, 13, 2456–2469. [Google Scholar] [CrossRef]
- Weiss, J.; Gaysinsky, S.; Davidson, M.; McClements, J. Nanostructured encapsulation systems: Food antimicrobials. In Global Issues in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 425–479. [Google Scholar]
- Lima, A.L.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Polymeric nanocapsules: A review on design and production methods for pharmaceutical purpose. Methods 2022, 199, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casanas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Benoit, M.-A.; Baras, B.; Gillard, J. Preparation and characterization of protein-loaded poly (ε-caprolactone) microparticles for oral vaccine delivery. Int. J. Pharm. 1999, 184, 73–84. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.R.; Guterres, S.S. Poly(ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.L.; Lo Nigro, R.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef]
- Venturini, C.G.; Jäger, E.; Oliveira, C.P.; Bernardi, A.; Battastini, A.M.; Guterres, S.S.; Pohlmann, A.R. Formulation of lipid core nanocapsules. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 200–208. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Granata, G.; Riccobene, C.; Napoli, E.; Geraci, C. Polymeric Nanocapsules Containing Fennel Essential Oil: Their Preparation, Physicochemical Characterization, Stability over Time and in Simulated Gastrointestinal Conditions. Pharmaceutics 2022, 14, 873. [Google Scholar] [CrossRef] [PubMed]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of nanomaterials: Tools and challenges. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Barakat, C. Synchronous fluorescence spectroscopic study of solvatochromic curcumin dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1034–1041. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- D’Angeli, F.; Malfa, G.A.; Garozzo, A.; Li Volti, G.; Genovese, C.; Stivala, A.; Nicolosi, D.; Attanasio, F.; Bellia, F.; Ronsisvalle, S.; et al. Antimicrobial, Antioxidant, and Cytotoxic Activities of Juglans regia L. Pellicle Extract. Antibiotics 2021, 10, 159. [Google Scholar] [CrossRef]
- Lewis, I.; James, S. Performance Standards for Antimicrobial Susceptibility Testing; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Jorgensen, J.H. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; approved guideline; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Makuch, S.; Wiecek, K.; Wozniak, M. The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis. Pharmaceuticals 2021, 14, 309. [Google Scholar] [CrossRef]
- Bucevic Popovic, V.; Karahmet Farhat, E.; Banjari, I.; Jelicic Kadic, A.; Puljak, L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals 2024, 17, 164. [Google Scholar] [CrossRef]
- Campisi, A.; Sposito, G.; Pellitteri, R.; Santonocito, D.; Bisicchia, J.; Raciti, G.; Russo, C.; Nardiello, P.; Pignatello, R.; Casamenti, F.; et al. Effect of Unloaded and Curcumin-Loaded Solid Lipid Nanoparticles on Tissue Transglutaminase Isoforms Expression Levels in an Experimental Model of Alzheimer’s Disease. Antioxidants 2022, 11, 1863. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Mkhabela, V.; Ray, S.S. Biodegradation and bioresorption of poly (ε-caprolactone) nanocomposite scaffolds. Int. J. Biol. Macromol. 2015, 79, 186–192. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017, 7, 25978–25986. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Rea, A.; Michel, S. Efficacy of a curcumin extract (Curcugen™) on gastrointestinal symptoms and intestinal microbiota in adults with self-reported digestive complaints: A randomised, double-blind, placebo-controlled study. BMC Complement. Med. Ther. 2021, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 1–28. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021, 14, 547–554. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Benameur, T.; Porro, C.; Twfieg, M.E.; Benameur, N.; Panaro, M.A.; Filannino, F.M.; Hasan, A. Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota. Brain Sci. 2023, 13, 1226. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuca, K.; Chopra, C.; Singh, R.; Kumar, H.; Sen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The Modulatory Effects of Curcumin on the Gut Microbiota: A Potential Strategy for Disease Treatment and Health Promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef] [PubMed]
- Buniowska-Olejnik, M.; Urbański, J.; Mykhalevych, A.; Bieganowski, P.; Znamirowska-Piotrowska, A.; Kačániová, M.; Banach, M. The influence of curcumin additives on the viability of probiotic bacteria, antibacterial activity against pathogenic microorganisms, and quality indicators of low-fat yogurt. Front. Nutr. 2023, 10, 1118752. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Intestinal Microbiota and Metabolic Diseases: Pharmacological Implications. Trends Pharmacol. Sci. 2016, 37, 169–171. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Sina, H.; Dah-Nouvlessounon, D.; Adjobimey, T.; Boya, B.; Dohoue, G.M.C.; N’Tcha, C.; Chidikofan, V.; Baba-Moussa, F.; Abdoulaye, I.; Adjanohoun, A.; et al. Characteristics of Escherichia coli Isolated from Intestinal Microbiota Children of 0-5 Years Old in the Commune of Abomey-Calavi. J. Pathog. 2022, 2022, 6253894. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Canica, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum beta-lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Nowrouzian, F.; Hesselmar, B.; Saalman, R.; Strannegard, I.L.; Aberg, N.; Wold, A.E.; Adlerberth, I. Escherichia coli in infants’ intestinal microflora: Colonization rate, strain turnover, and virulence gene carriage. Pediatr. Res. 2003, 54, 8–14. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.L.; Song, A.A.; Nitisinprasert, S.; Withayagiat, U. Promising discovery of beneficial Escherichia coli in the human gut. 3 Biotech 2020, 10, 296. [Google Scholar] [CrossRef]
- Blount, Z.D. The unexhausted potential of E. coli. eLife 2015, 4, e05826. [Google Scholar] [CrossRef] [PubMed]
- Moreira de Gouveia, M.I.; Bernalier-Donadille, A.; Jubelin, G. Enterobacteriaceae in the Human Gut: Dynamics and Ecological Roles in Health and Disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Martinson, J.N.V.; Walk, S.T. Escherichia coli Residency in the Gut of Healthy Human Adults. EcoSal Plus 2020, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tawfick, M.M.; Elshamy, A.A.; Mohamed, K.T.; El Menofy, N.G. Gut Commensal Escherichia coli, a High-Risk Reservoir of Transferable Plasmid-Mediated Antimicrobial Resistance Traits. Infect. Drug Resist. 2022, 15, 1077–1091. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- John, T.M.; Deshpande, A.; Brizendine, K.; Yu, P.C.; Rothberg, M.B. Epidemiology and Outcomes of Community-Acquired Escherichia coli Pneumonia. Open Forum Infect. Dis. 2022, 9, ofab597. [Google Scholar] [CrossRef]
- Kim, K.S. Human Meningitis-Associated Escherichia coli. EcoSal Plus 2016, 7, 10-1128. [Google Scholar] [CrossRef]
- Shao, Q.; Chen, D.; Chen, S.; Ru, X.; Ye, Q. Escherichia coli Infection Sepsis: An Analysis of Specifically Expressed Genes and Clinical Indicators. Diagnostics 2023, 13, 3542. [Google Scholar] [CrossRef]
- Mori, H.; Kataoka, M.; Yang, X. Past, Present, and Future of Genome Modification in Escherichia coli. Microorganisms 2022, 10, 1835. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm inhibition by biocompatible poly (ε-caprolactone) nanocapsules loaded with essential oils and their cyto/genotoxicity to human keratinocyte cell line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Obeme-Imom, J.I.; Akpor, B.O.; Rotimi, D.; Batiha, G.E.-S.; Owolabi, A. Altered redox status, DNA damage and modulation of L-tryptophan metabolism contribute to antimicrobial action of curcumin. Heliyon 2020, 6, e03495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar]
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—A structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef]
- Romano, I.R.; D’Angeli, F.; Gili, E.; Fruciano, M.; Lombardo, G.A.G.; Mannino, G.; Vicario, N.; Russo, C.; Parenti, R.; Vancheri, C.; et al. Melatonin Enhances Neural Differentiation of Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 4891. [Google Scholar] [CrossRef]
- Romano, I.R.; D’Angeli, F.; Vicario, N.; Russo, C.; Genovese, C.; Lo Furno, D.; Mannino, G.; Tamburino, S.; Parenti, R.; Giuffrida, R. Adipose-Derived Mesenchymal Stromal Cells: A Tool for Bone and Cartilage Repair. Biomedicines 2023, 11, 1781. [Google Scholar] [CrossRef]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef]
- Papadopoulos, K.S.; Piperi, C.; Korkolopoulou, P. Clinical Applications of Adipose-Derived Stem Cell (ADSC) Exosomes in Tissue Regeneration. Int. J. Mol. Sci. 2024, 25, 5916. [Google Scholar] [CrossRef]
- Mannino, G.; Cristaldi, M.; Giurdanella, G.; Perrotta, R.E.; Lo Furno, D.; Giuffrida, R.; Rusciano, D. ARPE-19 conditioned medium promotes neural differentiation of adipose-derived mesenchymal stem cells. World J. Stem Cells 2021, 13, 1783–1796. [Google Scholar] [CrossRef]
- Russo, C.; Mannino, G.; Patane, M.; Parrinello, N.L.; Pellitteri, R.; Stanzani, S.; Giuffrida, R.; Lo Furno, D.; Russo, A. Ghrelin peptide improves glial conditioned medium effects on neuronal differentiation of human adipose mesenchymal stem cells. Histochem. Cell Biol. 2021, 156, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 2019, 4, e131273. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Tunger, A.; Wobus, M.; von Bonin, M.; Towers, R.; Bornhauser, M.; Dazzi, F.; Wehner, R.; Schmitz, M. Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front. Cell Dev. Biol. 2021, 9, 637725. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Finosh, G.; Panicker, N.; Ramachandran, R.; Varghese, A. Cytotoxic effects of curcumin at various concentrations and role of curcumin on lipid peroxidation and activities of antioxidant enzymes of the rat peripheral blood lymphocytes. Blood 2011, 118, 4933. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria. Infect. Immun. 2020, 88, 10-1128. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Davinelli, S.; Saso, L.; D’Angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a Modulator of Nrf2, NF-kappa B, and Their Crosstalk: Molecular Mechanisms and Possible Clinical Applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef]
- Wu, S.-C.; Kuo, P.-J.; Rau, C.-S.; Huang, L.-H.; Lin, C.-W.; Wu, Y.-C.; Wu, C.-J.; Tsai, C.-W.; Hsieh, T.-M.; Liu, H.-T.; et al. Increased Angiogenesis by Exosomes Secreted by Adipose-Derived Stem Cells upon Lipopolysaccharide Stimulation. Int. J. Mol. Sci. 2021, 22, 8877. [Google Scholar] [CrossRef] [PubMed]
- Afarin, R.; Aslani, F.; Asadizade, S.; Jaberian Asl, B.; Mohammadi Gahrooie, M.; Shakerian, E.; Ahangarpour, A. The Effect of Lipopolysaccharide-Stimulated Adipose-Derived Mesenchymal Stem Cells on NAFLD Treatment in High-Fat Diet-Fed Rats. Iran. J. Pharm. Res. 2023, 22, e134807. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Saeedi, M.; Mohammadamini, H.; Zakaryaei, A.S. A comprehensive review of the therapeutic potential of curcumin nanoformulations. Phytother. Res. 2021, 35, 5527–5563. [Google Scholar] [CrossRef]
- de Almeida, M.; da Rocha, B.A.; Francisco, C.R.L.; Miranda, C.G.; Santos, P.D.d.F.; de Araújo, P.H.H.; Sayer, C.; Leimann, F.V.; Gonçalves, O.H.; Bersani-Amado, C.A. Evaluation of thein vivoacute antiinflammatory response of curcumin-loaded nanoparticles. Food Funct. 2018, 9, 440–449. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Hanafy, N.A.N.; Li, B.; Mehanni, A.-H.E.; Taha, E.M.; Chen, W. An updated and comprehensive review on the potential health effects of curcumin-encapsulated micro/nanoparticles. Crit. Rev. Food Sci. Nutr. 2022, 63, 9731–9751. [Google Scholar] [CrossRef]
- D’Angeli, F.; Scalia, M.; Cirnigliaro, M.; Satriano, C.; Barresi, V.; Musso, N.; Trovato-Salinaro, A.; Barbagallo, D.; Ragusa, M.; Di Pietro, C.; et al. PARP-14 Promotes Survival of Mammalian alpha but Not beta Pancreatic Cells Following Cytokine Treatment. Front. Endocrinol. 2019, 10, 271. [Google Scholar]
- Sahoo, M.; Ceballos-Olvera, I.; del Barrio, L.; Re, F. Role of the inflammasome, IL-1β, and IL-18 in bacterial infections. Sci. World J. 2011, 11, 2037–2050. [Google Scholar] [CrossRef]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Caporarello, N.; D’Angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From "Mural" Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Jančinová, V.; Perečko, T.; Nosáľ, R.; Košťálová, D.; Bauerová, K.; Drábiková, K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur. J. Pharmacol. 2009, 612, 161–166. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Singh, S.; Dubey, S.K.; Misra, K.; Khar, A. Immunomodulatory and therapeutic activity of curcumin. Int. Immunopharmacol. 2011, 11, 331–341. [Google Scholar] [CrossRef]
- Ali Reza, A.S.M.; Nasrin, M.S.; Hossen, M.A.; Rahman, M.A.; Jantan, I.; Haque, M.A.; Sobarzo-Sánchez, E. Mechanistic insight into immunomodulatory effects of food-functioned plant secondary metabolites. Crit. Rev. Food Sci. Nutr. 2021, 63, 5546–5576. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Q.; Duan, P.; Yang, L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2018, 40, 476–482. [Google Scholar] [CrossRef]
- Lee, J.-J.; Huang, W.-T.; Shao, D.-Z.; Liao, J.-F.; Lin, M.-T. Blocking NF-κB Activation May Be an Effective Strategy in the Fever Therapy. Jpn. J. Physiol. 2003, 53, 367–375. [Google Scholar]
- Baliga, M.S.; Joseph, N.; Venkataranganna, M.V.; Saxena, A.; Ponemone, V.; Fayad, R. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: Preclinical and clinical observations. Food Funct. 2012, 3, 1109–1117. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Zarrin, V.; Moghadam, E.R.; Hashemi, F.; Makvandi, P.; Samarghandian, S.; Khan, H.; Hashemi, F.; et al. Toward Regulatory Effects of Curcumin on Transforming Growth Factor-Beta Across Different Diseases: A Review. Front. Pharmacol. 2020, 11, 585413. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Gong, Y.; Zhang, J.; Mu, X.; Song, Z.; Feng, R.; Zhang, H. A novel curcumin-loaded nanoparticle restricts atherosclerosis development and promotes plaques stability in apolipoprotein E deficient mice. J. Biomater. Appl. 2018, 33, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jung, E.; Hyeon, H.; Seon, S.; Lee, D. Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102104. [Google Scholar] [CrossRef]
- Javadi, M.; Khadem Haghighian, H.; Goodarzy, S.; Abbasi, M.; Nassiri-Asl, M. Effect of curcumin nanomicelle on the clinical symptoms of patients with rheumatoid arthritis: A randomized, double-blind, controlled trial. Int. J. Rheum. Dis. 2019, 22, 1857–1862. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef]
- Lu, L.; Qi, S.; Chen, Y.; Luo, H.; Huang, S.; Yu, X.; Luo, Q.; Zhang, Z. Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials 2020, 245, 119987. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Dal Ben, S.; Voinovich, D.; Dall’Acqua, S. Untargeted UPLC-MS metabolomics reveals multiple changes of urine composition in healthy adult volunteers after consumption of curcuma longa L. extract. Food Res. Int. 2020, 127, 108730. [Google Scholar] [CrossRef]
- Zhai, S.S.; Ruan, D.; Zhu, Y.W.; Li, M.C.; Ye, H.; Wang, W.C.; Yang, L. Protective effect of curcumin on ochratoxin A–induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult. Sci. 2020, 99, 1124–1134. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M.; Schrenzel, J.; de Vos, W.M.; François, P.; Muccioli, G.G.; Girard, M.; Valet, P.; Knauf, C.; Van Roye, M.; et al. Altered Gut Microbiota and Endocannabinoid System Tone in Obese and Diabetic Leptin-Resistant Mice: Impact on Apelin Regulation in Adipose Tissue. Front. Microbiol. 2011, 2, 149. [Google Scholar]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzinska, B. Curcumin and Its Potential Impact on Microbiota. Nutrients 2021, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, V.S.; Mohanan, P.V. Degradation of Poly(epsilon-caprolactone) and bio-interactions with mouse bone marrow mesenchymal stem cells. Colloids Surf. B Bio Interfaces 2018, 163, 107–118. [Google Scholar]
- Ude, C.C.; Sulaiman, S.B.; Min-Hwei, N.; Hui-Cheng, C.; Ahmad, J.; Yahaya, N.M.; Saim, A.B.; Idrus, R.B. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS ONE 2014, 9, e98770. [Google Scholar] [CrossRef]
- D’Angeli, F.; Genovese, C.; Distefano, A.; Malik, A.; Khan, A.A.; Ronsisvalle, S.; Sipala, F.; Volti, G.L. Antibacterial, Antitumor (Lung Cancer Cell H292) and Antioxidant Properties of Sicilian Prickly Pear Cactus (Opuntia Ficus-Indica) Cladode Extracts. J. Biol. Regul. Homeost. Agents 2024, 38, 1943–1960. [Google Scholar]



| Storage Time (Days) | 0 | 7 | 15 | 21 | 30 |
|---|---|---|---|---|---|
| Z-average diameter (nm) | 223 ± 13 a | 222 ± 14 a | 224 ± 16 a | 221 ± 11 a | 226 ± 15 a |
| PDI | 0.09 ± 0.03 a | 0.07 ± 0.02 a | 0.07 ± 0.03 a | 0.06 ± 0.02 a | 0.05 ± 0.02 a |
| ζ (mV) | −17 ± 3 a | −16 ± 4 a | −16 ± 1 a | −17 ± 2 a | −15 ± 3 a |
| Cur encapsulated amount (mg/mL) | 1.00 ± 0.02 a | 1.00 ± 0.07 a | 1.00 ± 0.06 a | 1.00 ± 0.07 a | 0.97 ± 0.05 a |
| Storage Time (Days) | 0 | 7 | 15 | 21 | 30 |
|---|---|---|---|---|---|
| Z-average diameter (nm) | 223 ± 13 a | 218 ± 14 a | 222 ± 13 a | 220 ± 14 a | 223 ± 14 a |
| PDI | 0.09 ± 0.03 a | 0.07 ± 0.04 a | 0.07 ± 0.03 a | 0.05 ± 0.01 a | 0.05 ± 0.03 a |
| ζ (mV) | −17 ± 3 a | −17 ± 4 a | −19 ± 1 a | −18 ± 2 a | −17 ± 2 a |
| Cur encapsulated amount (mg/mL) | 1.00 ± 0.02 a | 1.00 ± 0.07 a | 1.00 ± 0.04 a | 0.93 ± 0.04 a | 0.75 ± 0.06 b |
| Cur-NCs after SGD Test | pH 1.5 | pH 7.0 |
|---|---|---|
| Z-average diameter (nm) | 210 ± 5 | 204 ± 4 |
| PDI | 0.09 ± 0.04 | 0.07 ± 0.04 |
| ζ (mV) | −3 ± 1 | −24 ± 1 |
| MIC 1 (µg/mL) | |||||
|---|---|---|---|---|---|
| NCs 2 | Cur 3 | Cur-NCs 4 | Gen 5 | ||
| Bacterial Strains | [0.97–500.00] | [0.97–500.00] | [0.25–128.00] | I.C. 6 | |
| Gram-positive | |||||
| Staphylococcus aureus ATCC 6538 | n.e. 7 | 62.50 | 500.00 | 0.50 | S |
| Enterococcus faecalis ATCC 29212 | n.e. | 7.81 | >500.00 | 32.00 | n.r. 8 |
| Lactobacillus delbrueckii ATCC 11842 | n.e. | 250.00 | >500.00 | 8.00 | I |
| Lactobacillus plantarum ATCC BAA 793 | n.e. | >500.00 | >500.00 | >128.00 | R |
| Lactobacillus reuteri ATCC 23272 | n.e. | >500.00 | >500.00 | 128.00 | R |
| Lactobacillus rhamnosus GG ATCC 53103 | n.e. | >500.00 | >500.00 | 128.00 | R |
| Gram-negative | |||||
| Escherichia coli ATCC 8728 | n.e. | 62.50 | 31.25 | 2.00 | S |
| Pseudomonas aeruginosa ATCC 9027 | n.e. | 250.00 | >500.00 | 0.50 | S |
| Gene Symbol | Forward | Reverse |
|---|---|---|
| IL-1β 1 | AGCTCGCCAGTGAAATGATG | GTCGGAGATTCGTAGCTGGA |
| TGF-β 2 | CAATTCCTGGCGATACCTCAG | GCACAACTCCGGTGACATCAA |
| TNF-α 3 | GCAACAAGACCACCACTTCG | GATCAAAGCTGTAGGCCCCA |
| IL-10 4 | GACTTTAAGGGTTACCTGGGTTG | TCACATGCGCCTTGATGTCTG |
| β-actin | ACGTTGCTATCCAGGCTGTGCTAT | TTAATGTCACGCACGATTTCCCGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angeli, F.; Granata, G.; Romano, I.R.; Distefano, A.; Lo Furno, D.; Spila, A.; Leo, M.; Miele, C.; Ramadan, D.; Ferroni, P.; et al. Biocompatible Poly(ε-Caprolactone) Nanocapsules Enhance the Bioavailability, Antibacterial, and Immunomodulatory Activities of Curcumin. Int. J. Mol. Sci. 2024, 25, 10692. https://doi.org/10.3390/ijms251910692
D’Angeli F, Granata G, Romano IR, Distefano A, Lo Furno D, Spila A, Leo M, Miele C, Ramadan D, Ferroni P, et al. Biocompatible Poly(ε-Caprolactone) Nanocapsules Enhance the Bioavailability, Antibacterial, and Immunomodulatory Activities of Curcumin. International Journal of Molecular Sciences. 2024; 25(19):10692. https://doi.org/10.3390/ijms251910692
Chicago/Turabian StyleD’Angeli, Floriana, Giuseppe Granata, Ivana Roberta Romano, Alfio Distefano, Debora Lo Furno, Antonella Spila, Mariantonietta Leo, Chiara Miele, Dania Ramadan, Patrizia Ferroni, and et al. 2024. "Biocompatible Poly(ε-Caprolactone) Nanocapsules Enhance the Bioavailability, Antibacterial, and Immunomodulatory Activities of Curcumin" International Journal of Molecular Sciences 25, no. 19: 10692. https://doi.org/10.3390/ijms251910692









