Synthesis, Structure, and Actual Applications of Double Metal Cyanide Catalysts
Abstract
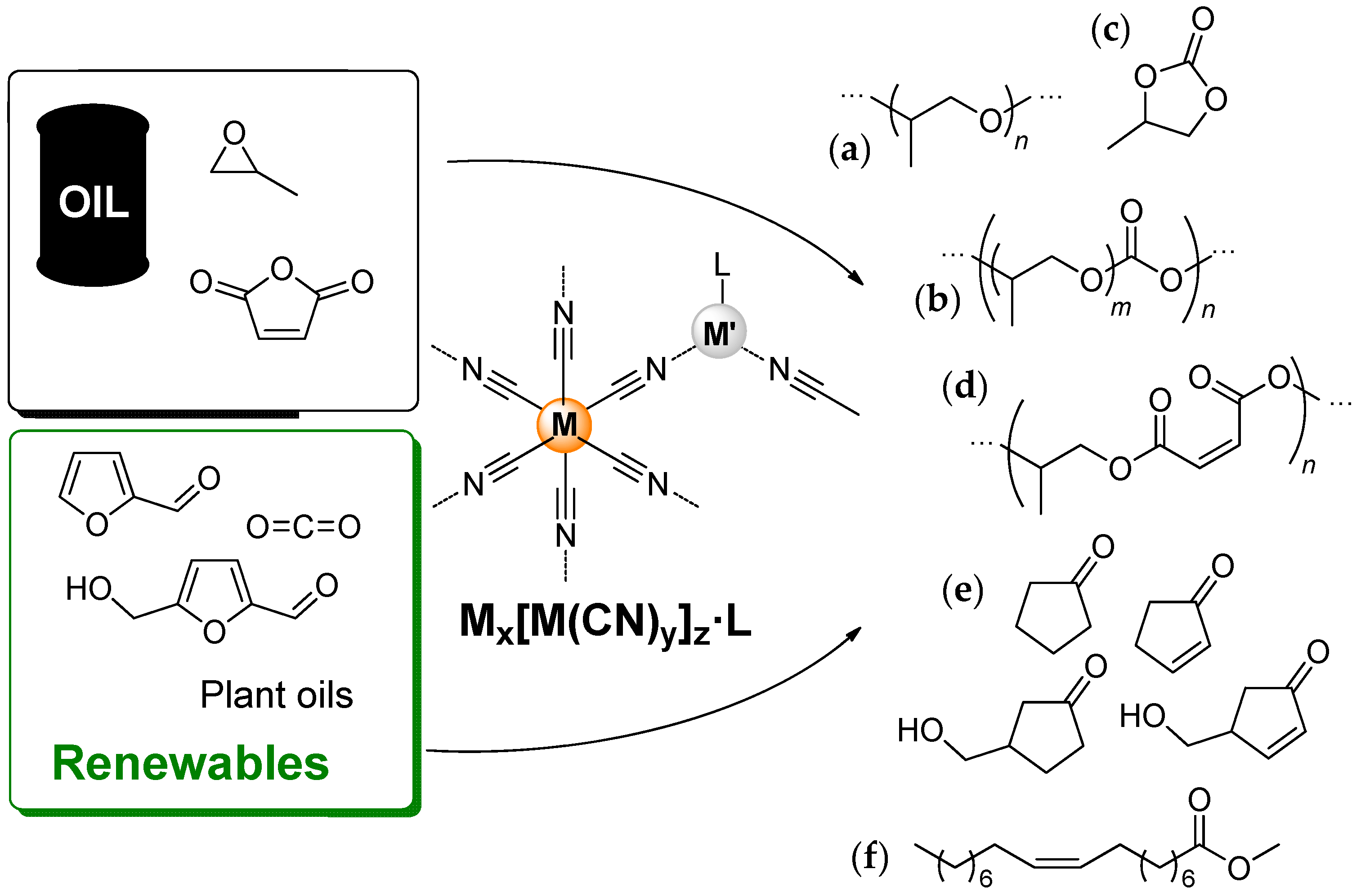
:1. Introduction
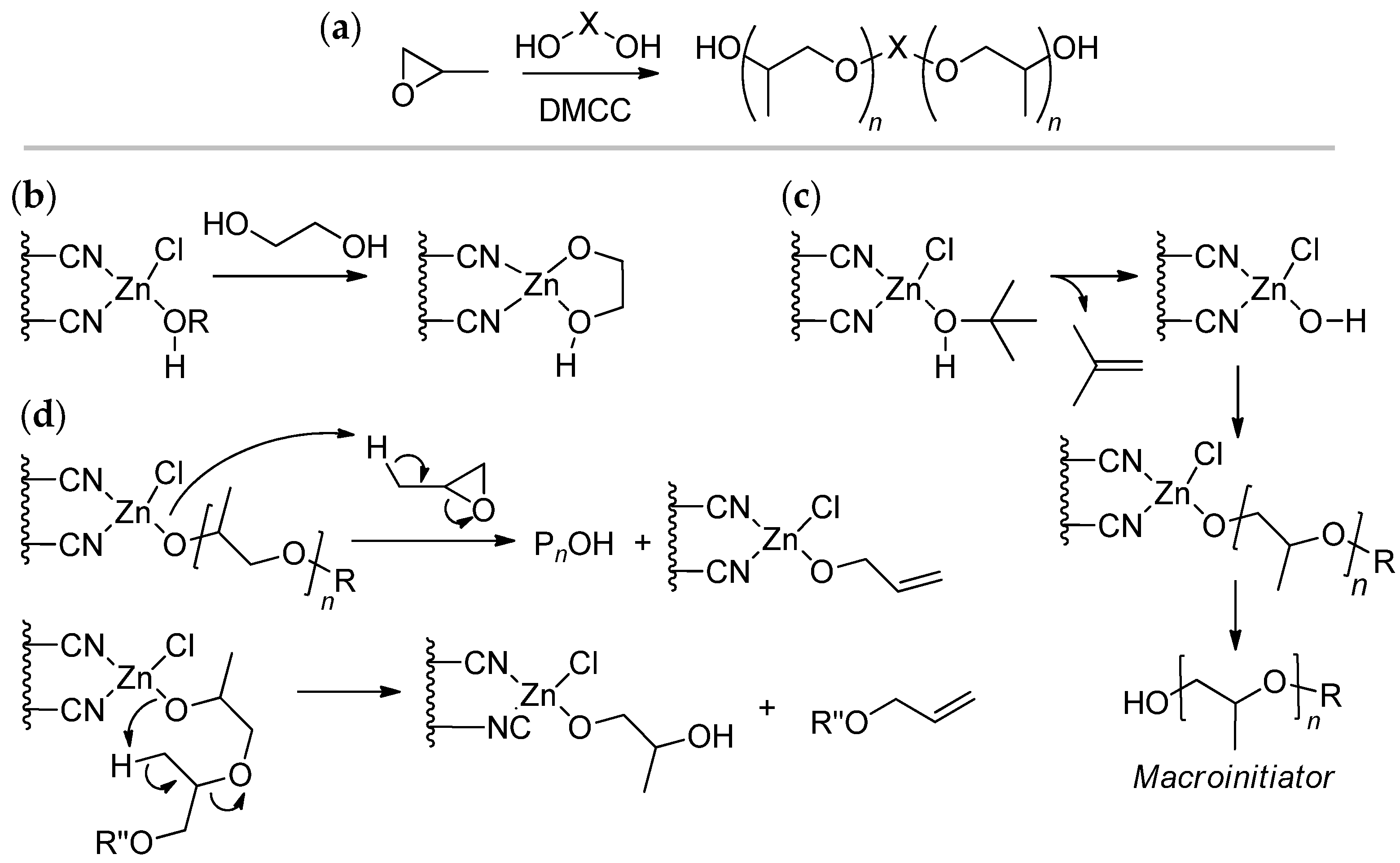
2. Preparation and Structure of DMC Catalysts
2.1. Preparation of DMC Complexes
2.1.1. Synthesis of Zn(II)-Co(III) DMCCs
2.1.2. Synthesis of Zn(II)-Fe(III) DMCCs
2.1.3. Synthesis of Other DMC Complexes
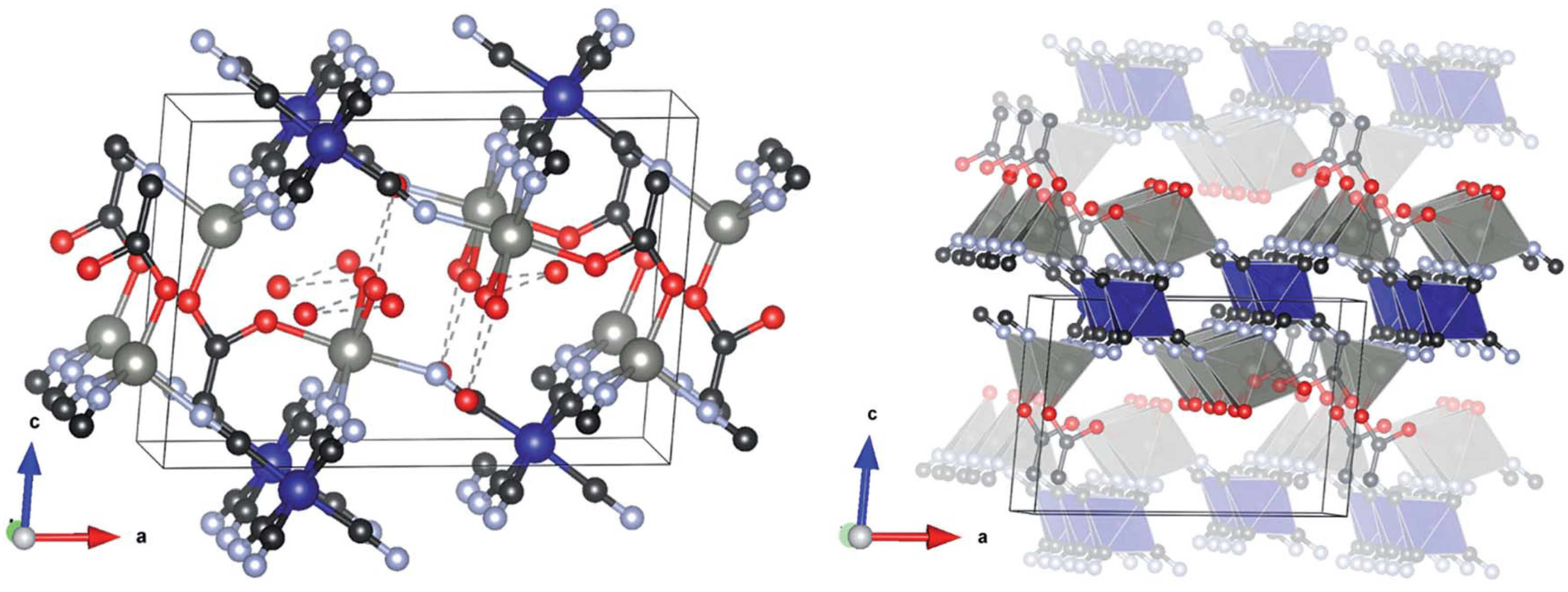
2.2. Structure of DMC Complexes
- Elemental analysis to determine metal and Cl content;
- Scanning electron microscopy (SEM), including the use of energy-dispersive X-ray spectroscopy (EDX) to determine the size and morphology of the catalyst particles;
- X-ray diffraction (XRD) methods to determine the type of the crystal lattice and degree of crystallinity;
- FT-IR spectroscopy, which gives information about the chemical bonding (M–C≡N…M’ fragments, CAs);
- Differential scanning calorimetry and thermogravimetry analysis (DCS/TGA), which reveals the quantity of CAs and the strength of their binding;
- New spectral methods such as extended X-ray fine structure/X-ray near edge structure (EXAFS/XANES) determination that allow determination of the ligand environment of the metal atoms;
- X-ray photoelectron spectroscopy (XPS), for the same purpose.
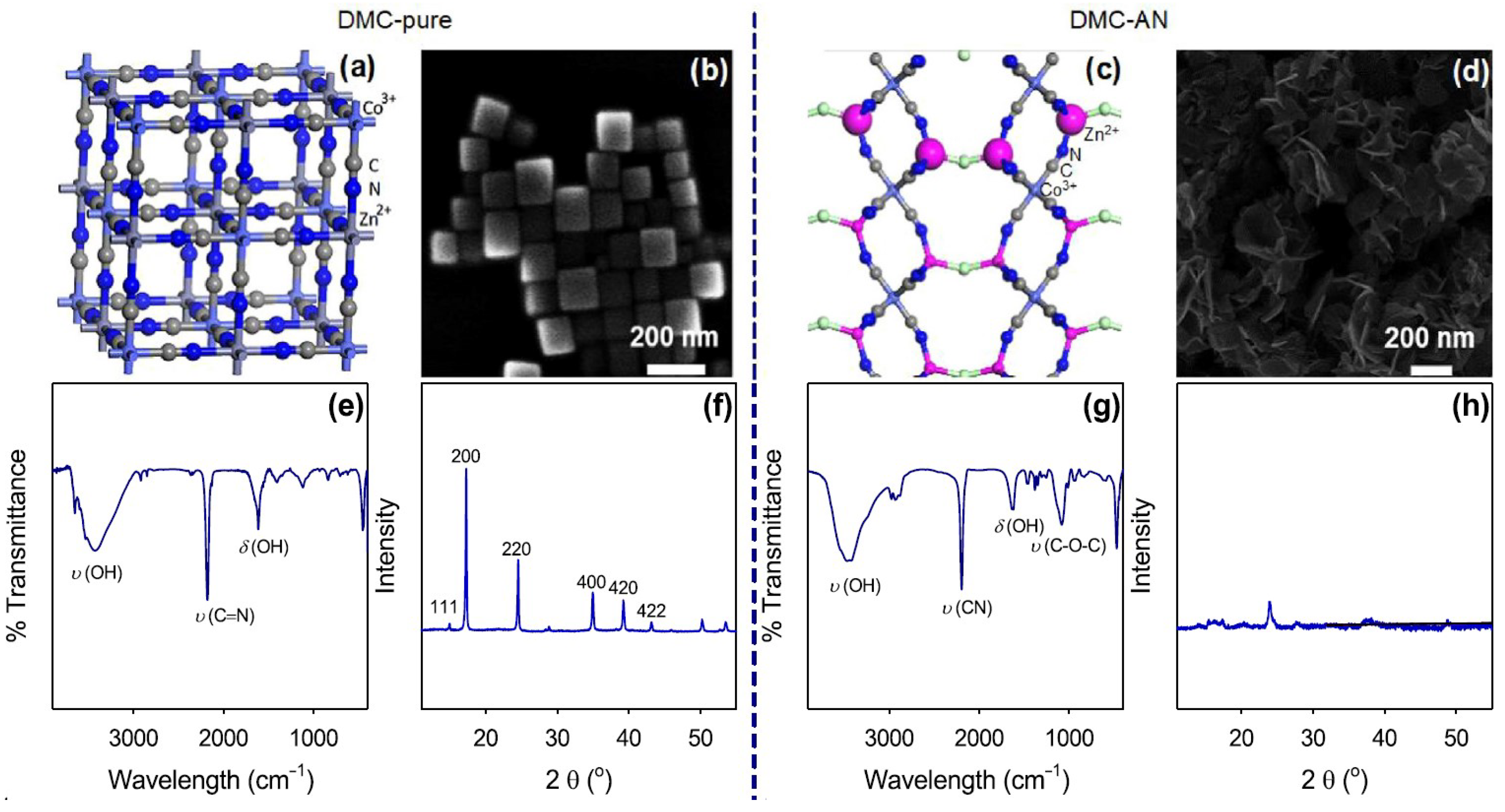
2.2.1. Structure of Zn/Co DMC Complexes
2.2.2. Structure of Other DMC Complexes
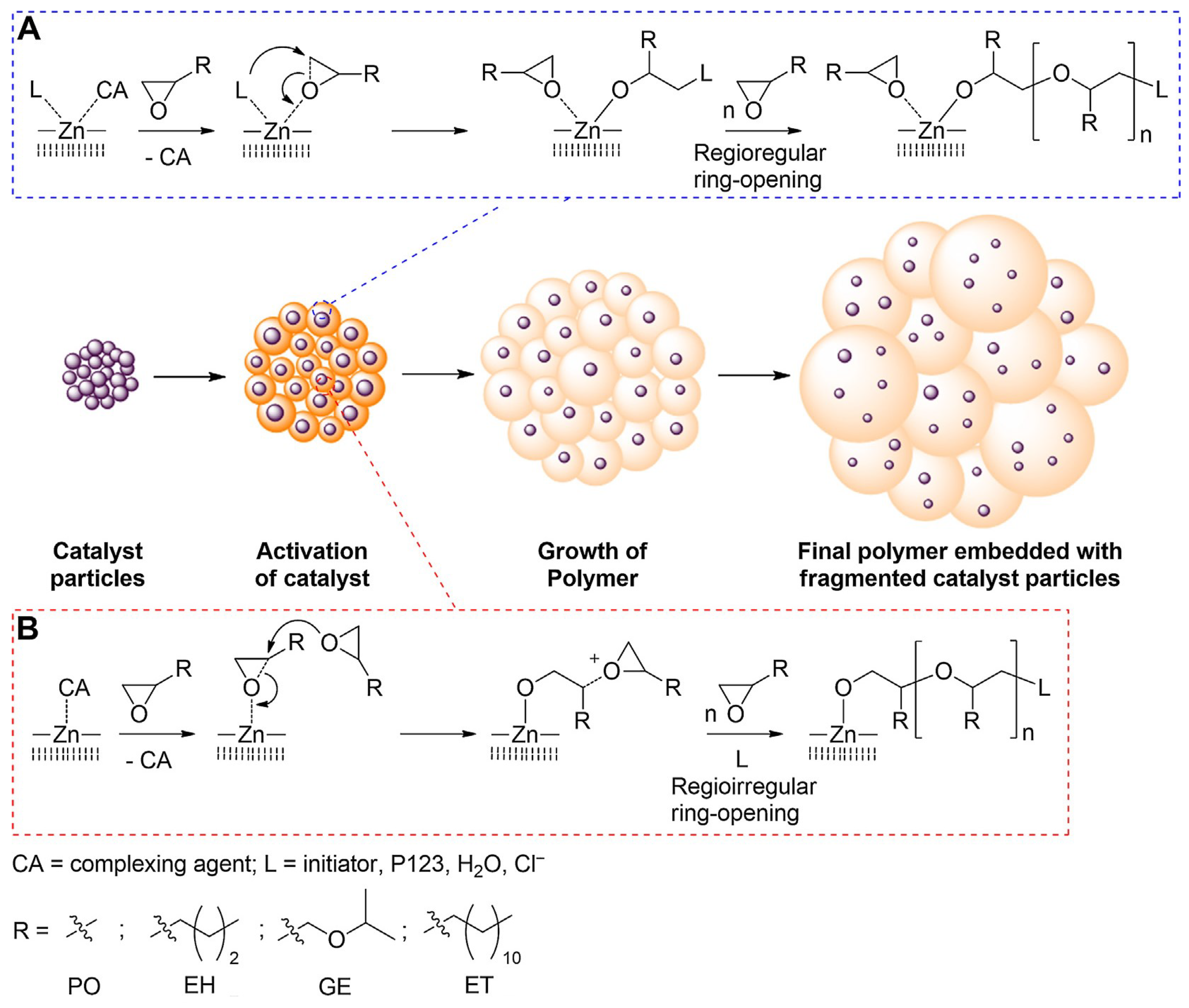
3. Mechanistic Insights of the Action of DMC Catalysts
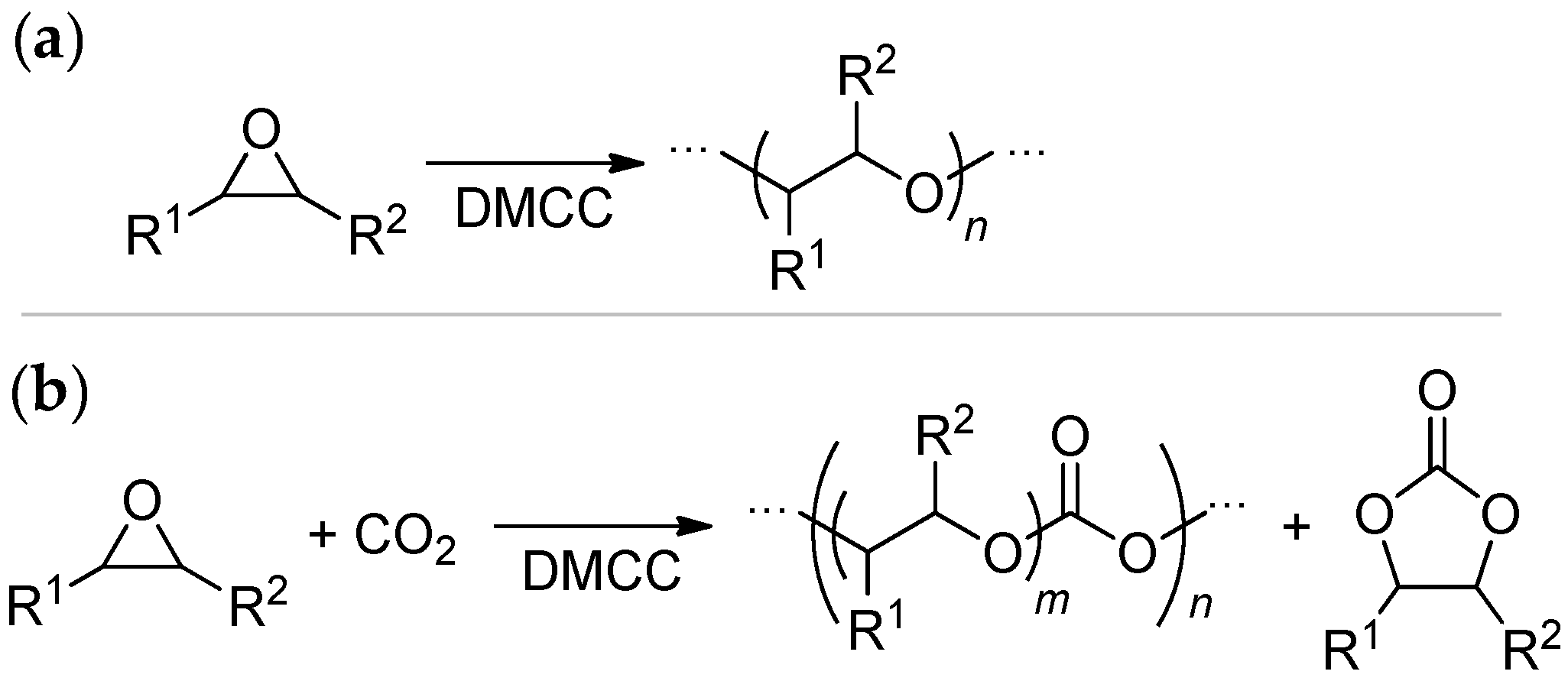
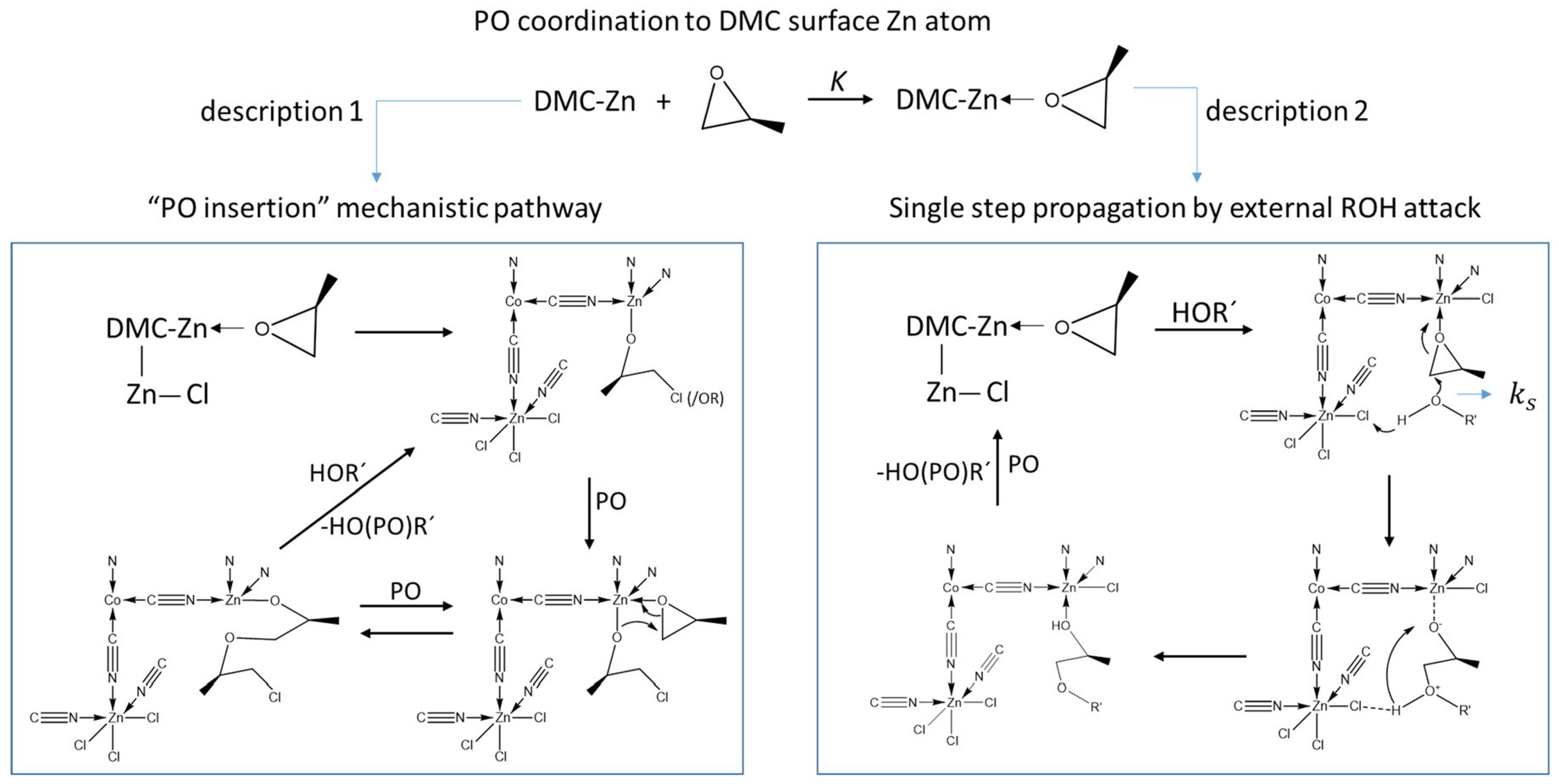
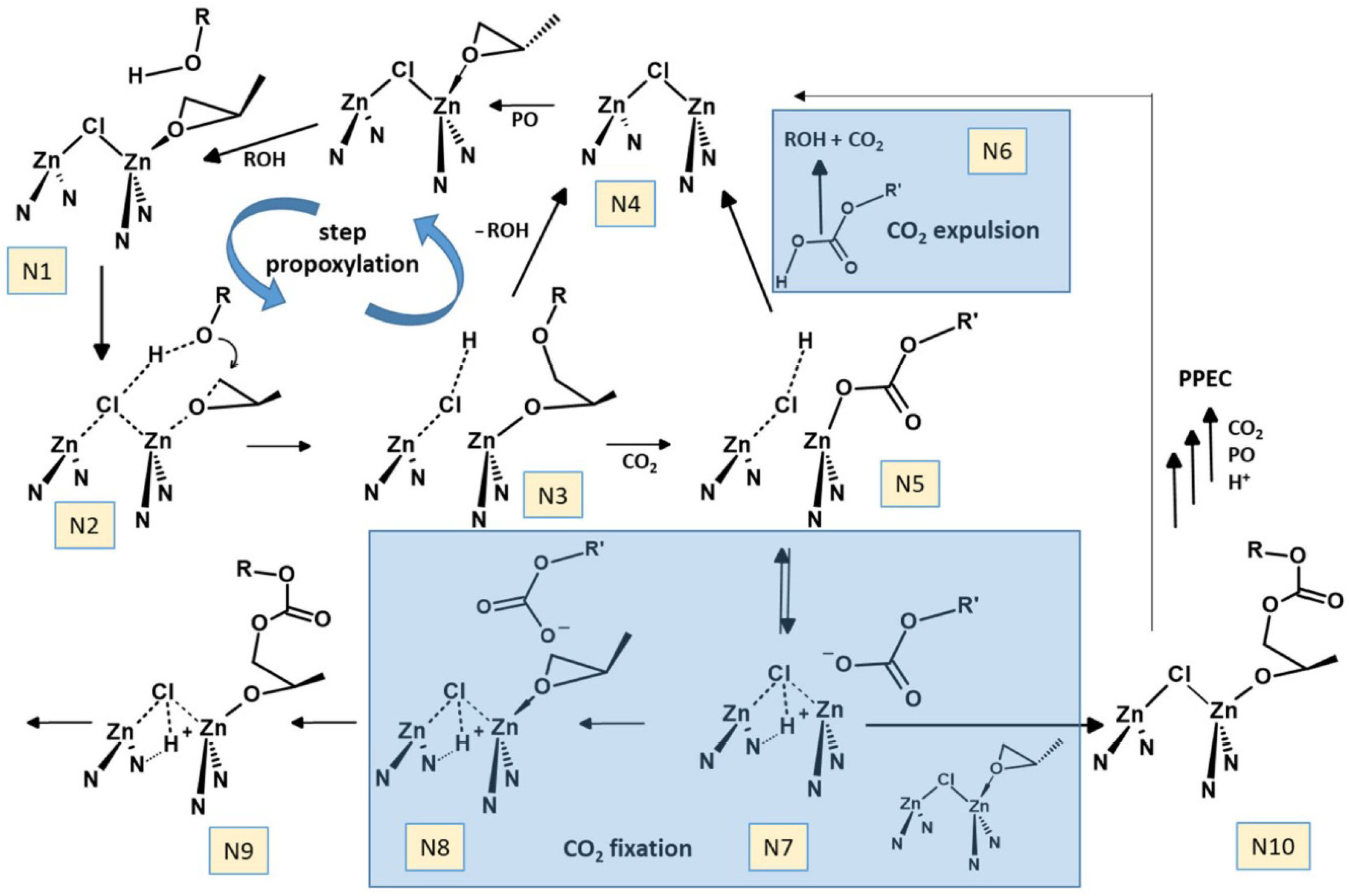
3.1. DMC Catalysts in Polymerization of Oxiranes
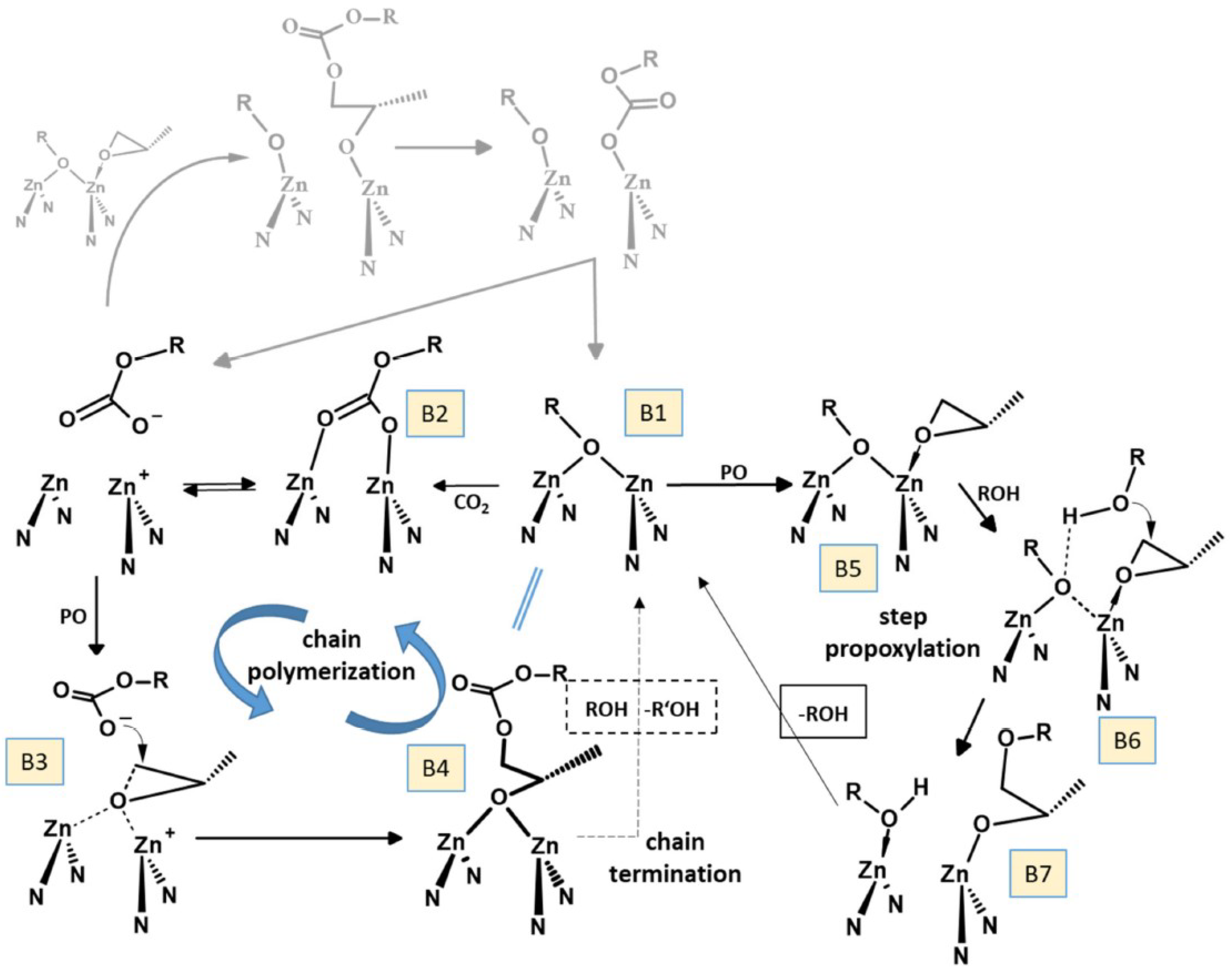
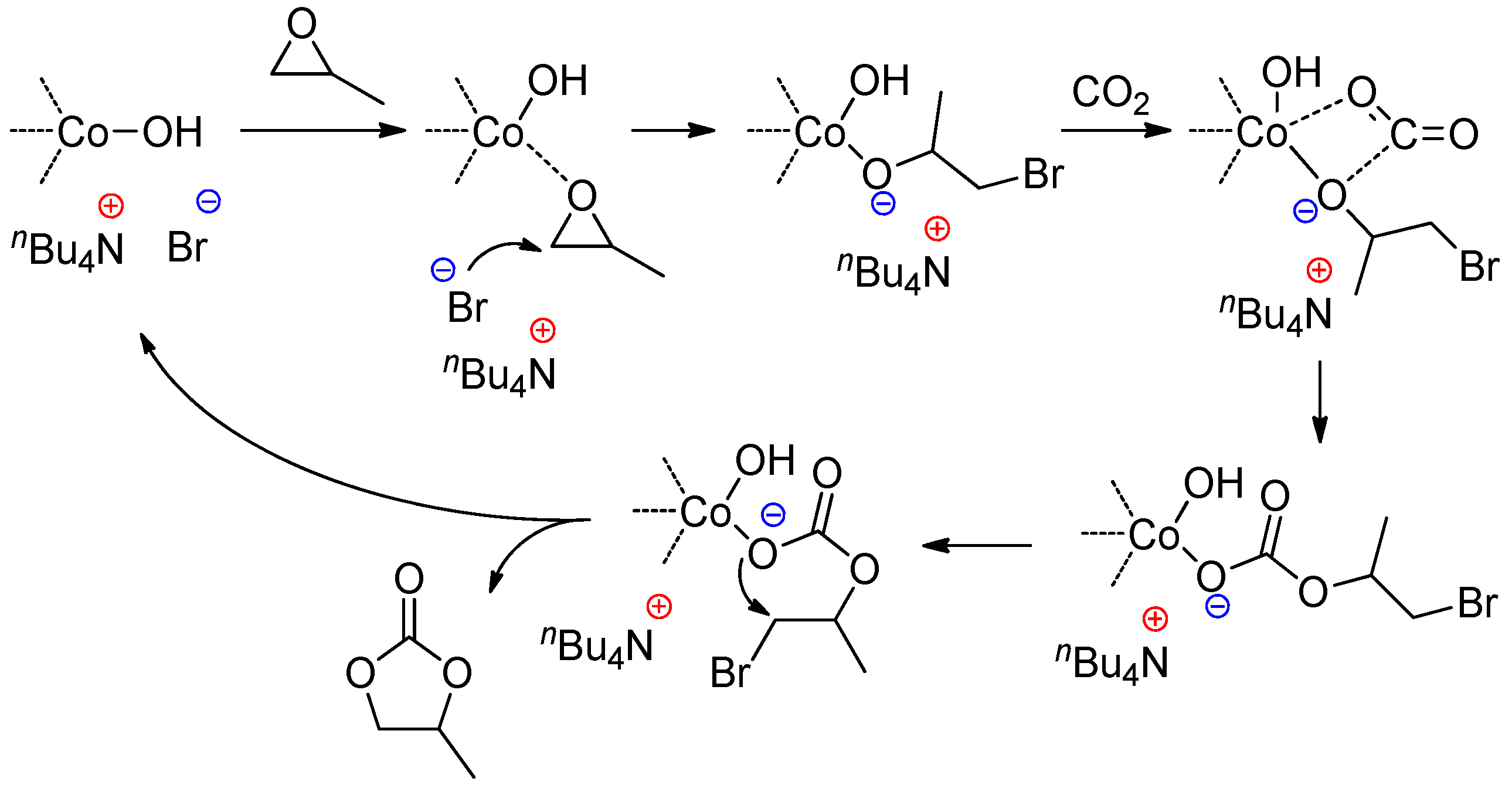
3.2. DMC Catalysts in Copolymerization of Oxiranes with CO2
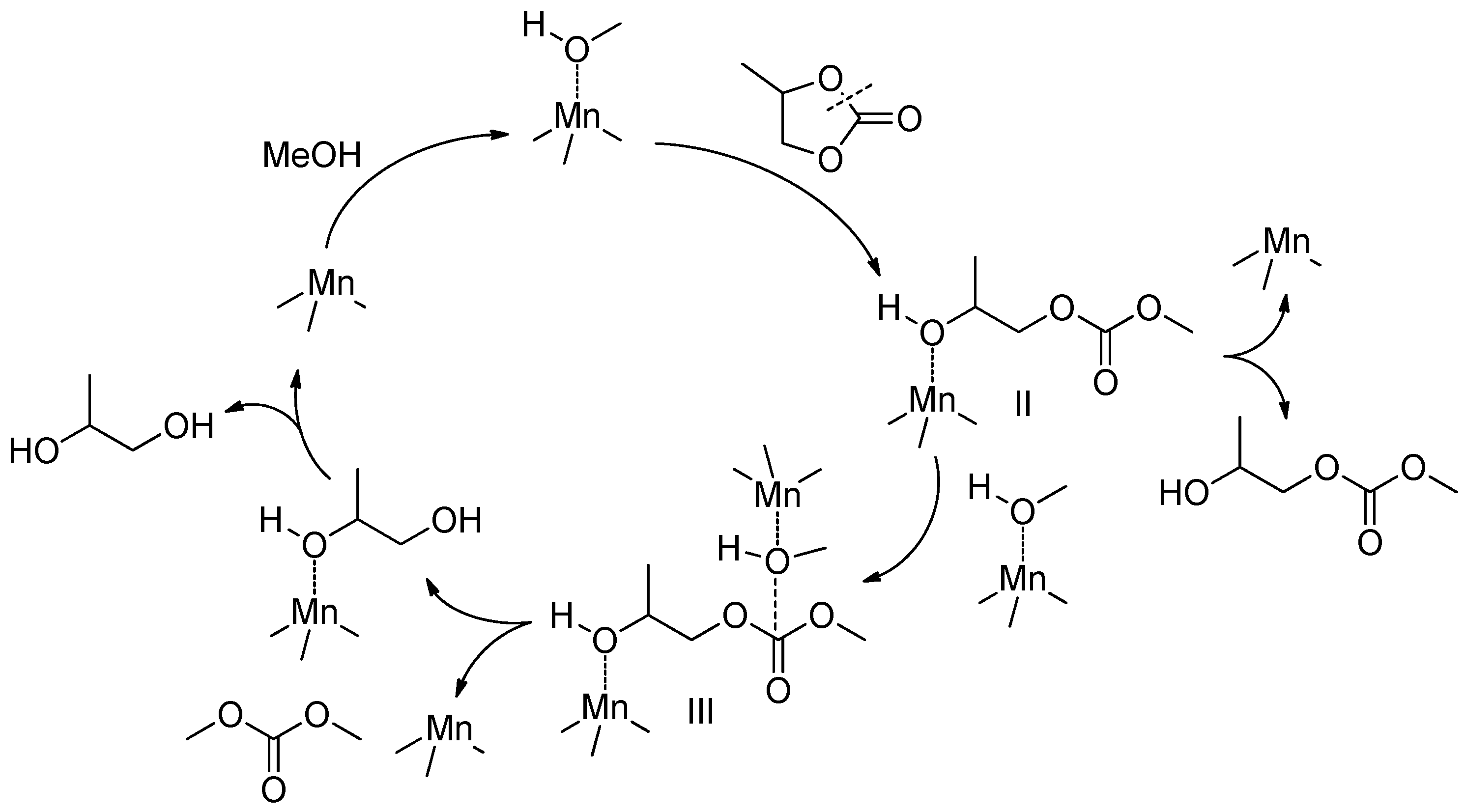
3.3. Mechanism of Transesterification of Cyclic Carbonates
4. Actual Processes and Products
4.1. Preparation of Polyols
4.1.1. Zn/Co DMCCs in the Synthesis of Polyols
4.1.2. Other DMCCs in the Synthesis of Polyols
4.1.3. Post-Functionalization of PEs
4.2. Copolymerization of Oxiranes with CO2 and Other C1 Comonomers
4.2.1. Zn/Co DMCCs in Copolymerization of Propylene Oxide and CO2
4.2.2. Other DMCCs in Copolymerization of Propylene Oxide and CO2
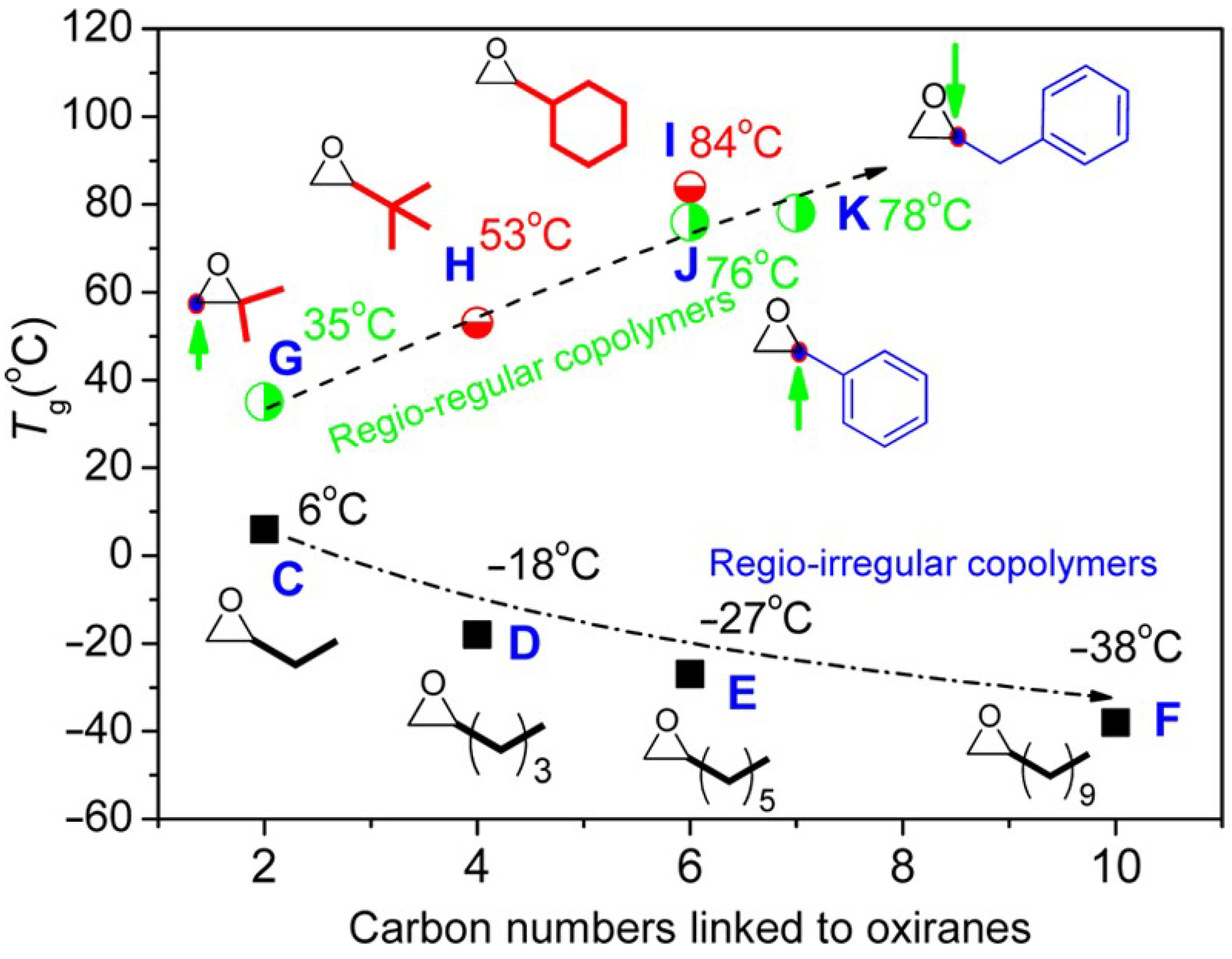
4.2.3. Copolymerization of Other Oxiranes and CO2
4.2.4. Copolymerization of Oxiranes with Other C1 Monomers
4.2.5. Copolymers of Two Different Oxiranes and CO2
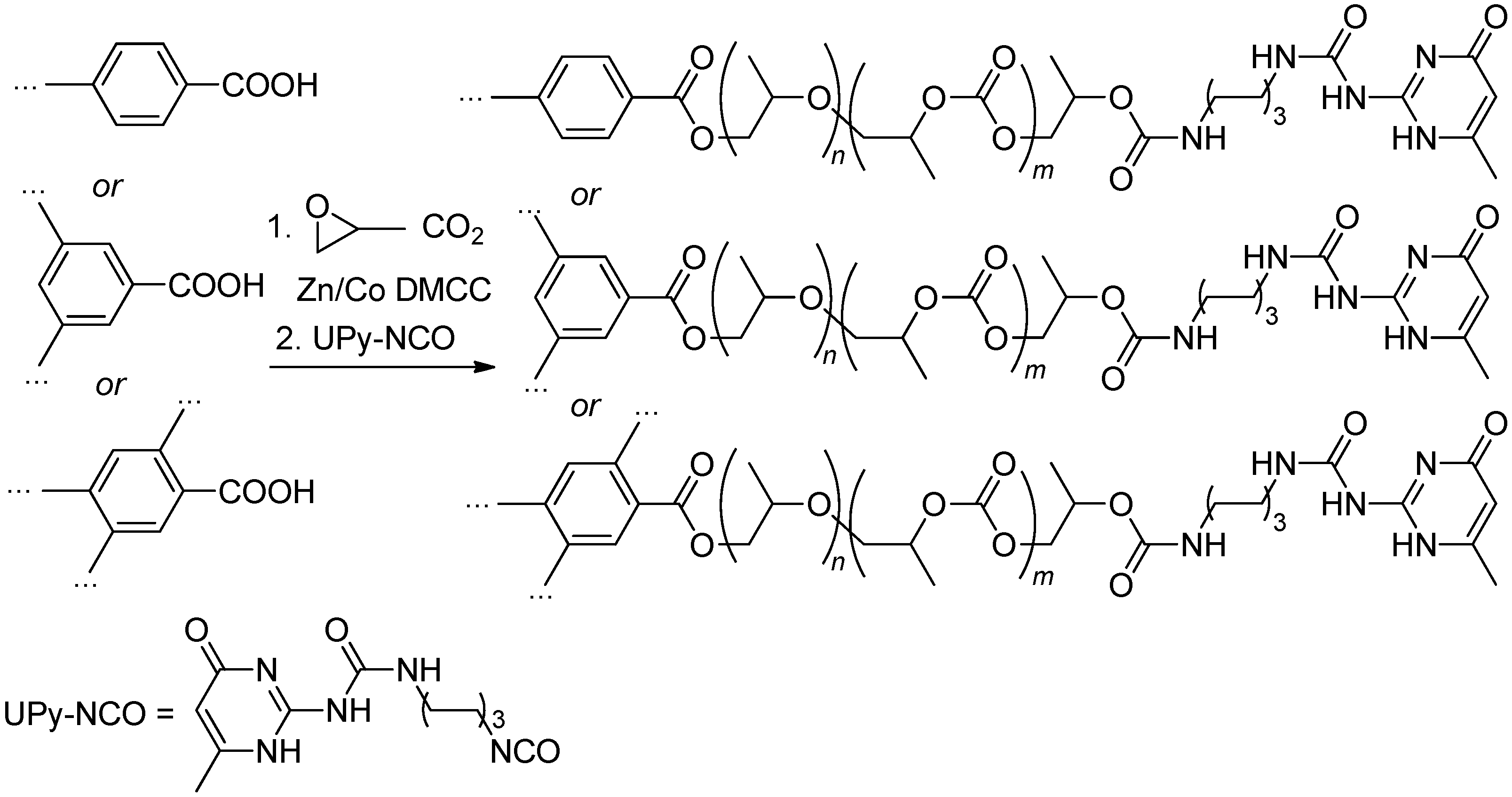
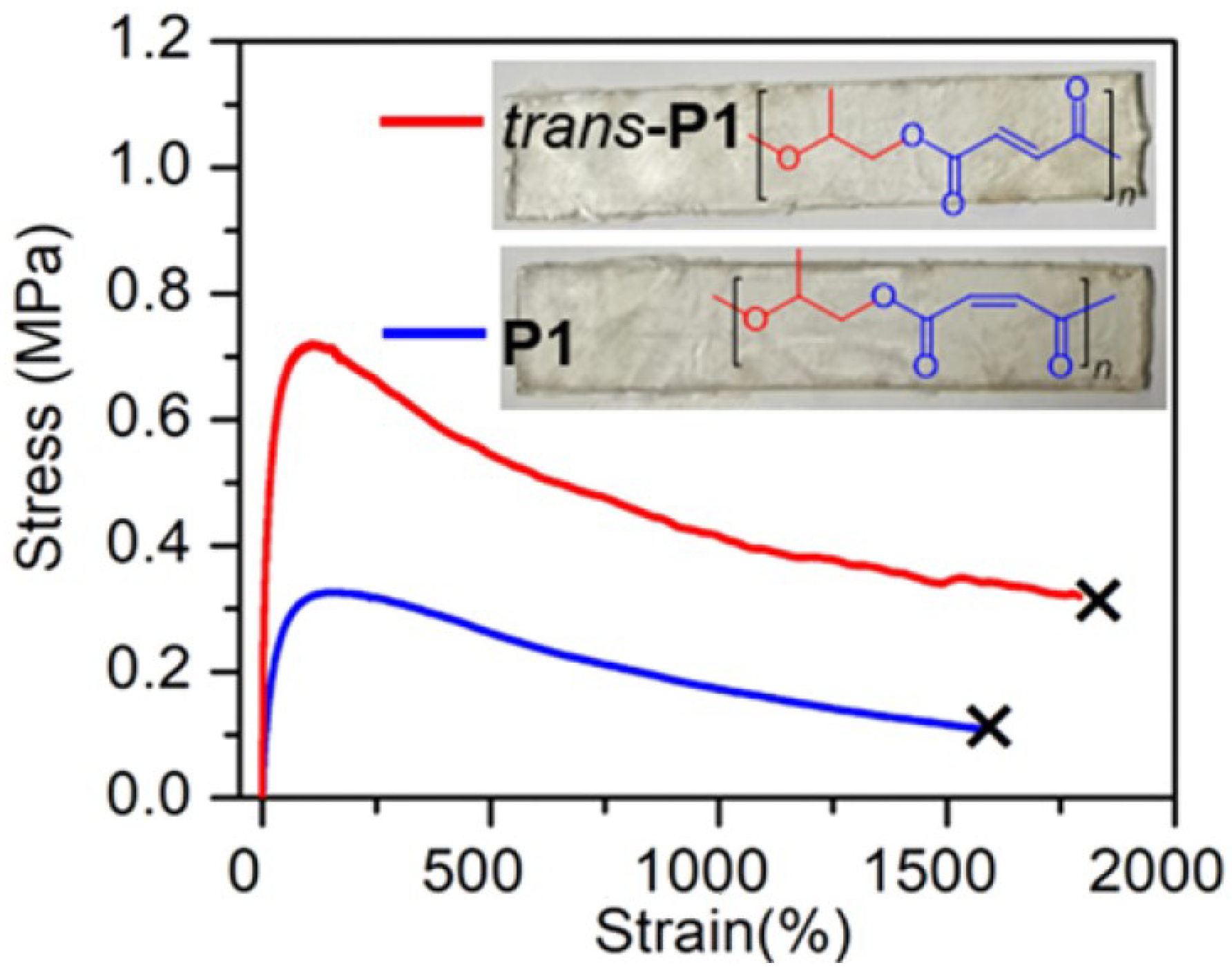
4.2.6. Functional Derivatives of PECs
4.3. Other Copolymers Based on Oxiranes
4.3.1. Copolymerization of Oxiranes with Other Monomers
4.3.2. Copolymers of Oxiranes, CO2 and Anhydrides
4.3.3. Copolymers of Oxiranes, CO2 and Cyclic Esters
4.4. Ring-Opening Polymerization of Cyclic Substrates Different from Oxiranes
4.5. Other Copolymers
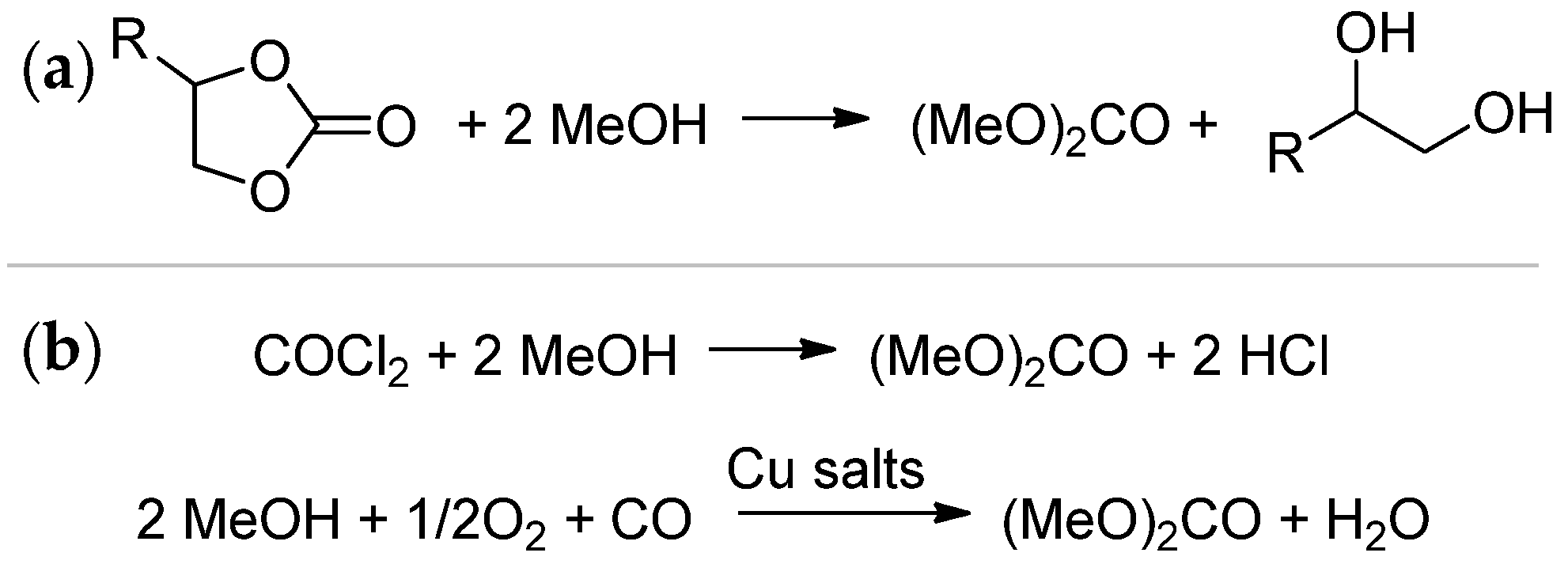
4.6. Synthesis and Transesterification of Cyclic Carbonates
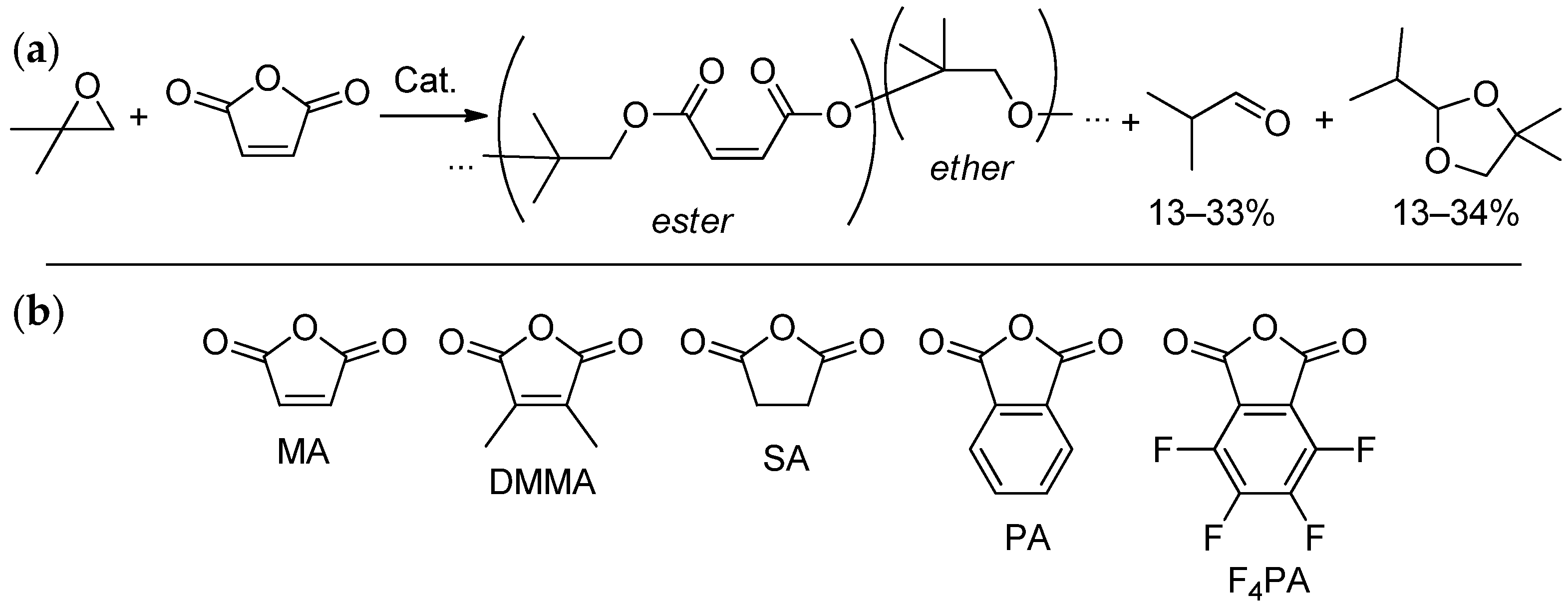
4.6.1. Synthesis of Cyclic Carbonates
4.6.2. Transesterification of Cyclic Carbonates
4.7. The Use of DMCCs in Preparative Organic Chemistry
4.7.1. Hydroamination
4.7.2. Reductive Amination
4.7.3. Transesterification
4.7.4. Acid-Nitrile Exchange
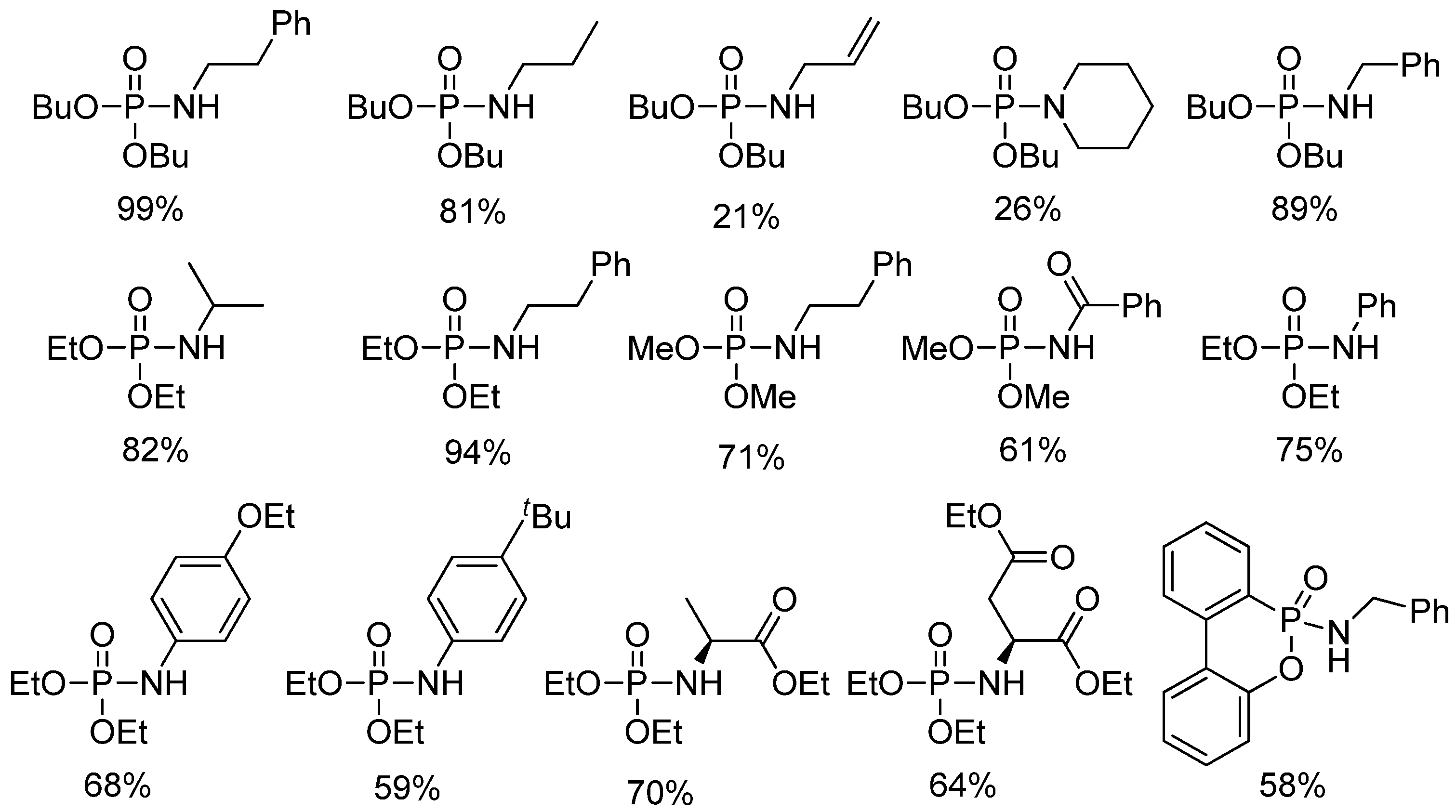
4.7.5. Synthesis of Phosphoramidates
4.7.6. Epoxidation
4.7.7. Catalytic Rearrangements of Biomass-Derived Furans
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Gao, Y.; Tang, Y. Sustainable developments in polyolefin chemistry: Progress, challenges, and outlook. Prog. Polym. Sci. 2023, 143, 101713. [Google Scholar] [CrossRef]
- Hayes, G.; Laurel, M.; MacKinnon, D.; Zhao, T.; Houck, H.A.; Becer, C.R. Polymers without Petrochemicals: Sustainable Routes to Conventional Monomers. Chem. Rev. 2023, 123, 2609–2734. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, X. Carbon dioxide copolymers: Emerging sustainable materials for versatile applications. SusMat 2021, 1, 88–104. [Google Scholar] [CrossRef]
- Liang, J.; Ye, S.; Wang, S.; Xiao, M.; Meng, Y. Design and structure of catalysts: Syntheses of carbon dioxide-based copolymers with cyclic anhydrides and/or cyclic esters. Polym. J. 2021, 53, 3–27. [Google Scholar] [CrossRef]
- Pyatakov, D.A.; Nifant’ev, I.E. (Co)polymerization Reactions with Participation of Cyclic Monomers Catalyzed by Double Metal Cyanide Catalysts. Polym. Sci. Ser. B 2023, 65, 717–732. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Grefe, L.; Mejía, E. Earth-abundant bimetallic and multimetallic catalysts for Epoxide/CO2 ring-opening copolymerization. Tetrahedron 2021, 98, 132433. [Google Scholar] [CrossRef]
- Mbabazi, R.; Wendt, O.F.; Nyanzi, S.A.; Naziriwo, B.; Tebandeke, E. Advances in carbon dioxide and propylene oxide copolymerization to form poly(propylene carbonate) over heterogeneous catalysts. Results Chem. 2022, 4, 100542. [Google Scholar] [CrossRef]
- Pyatakov, D.A.; Nifant’ev, I.E. Double Metal Cyanide (DMC) Catalysts: Synthesis, Structure, and Action Mechanism (A Review). Pet. Chem. 2023, 63, 1170–1193. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
- Li, W.-J.; Han, C.; Cheng, G.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Chemical Properties, Structural Properties, and Energy Storage Applications of Prussian Blue Analogues. Small 2019, 15, 1900470. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef] [PubMed]
- Langanke, J.; Wolf, A.; Hofmann, J.; Böhm, K.; Subhani, M.A.; Müller, T.E.; Leitner, W.; Gürtler, C. Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem. 2014, 16, 1865–1870. [Google Scholar] [CrossRef]
- Cao, H.; Liu, S.; Wang, X. Environmentally benign metal catalyst for the ring-opening copolymerization of epoxide and CO2: State-of-the-art, opportunities, and challenges. Green Chem. Eng. 2022, 3, 111–124. [Google Scholar] [CrossRef]
- Valvekens, P.; De Vos, D. Chapter 1—Double Metal Cyanides as Heterogeneous Catalysts for Organic Reactions. In New Materials for Catalytic Applications; Parvulescu, V.I., Kemnitz, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–12. [Google Scholar] [CrossRef]
- Milgrom, J. Method of Making a Polyether Using a Double Metal Cyanide Complex Compound. US Patent 3278457A, 11 October 1966. [Google Scholar]
- Herold, R.J.; Livigni, R.A. Hexacyanometalate Salt Complexes as Catalysts for Epoxide Polymerizations. In Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1973; Volume 128, pp. 208–229. [Google Scholar] [CrossRef]
- Kuyper, J.; Boxhoorn, G. Hexacyanometallate salts used as alkene-oxide polymerization catalysts and molecular sieves. J. Catal. 1987, 105, 163–174. [Google Scholar] [CrossRef]
- Kim, I.; Ahn, J.-T.; Ha, C.S.; Yang, C.S.; Park, I. Polymerization of propylene oxide by using double metal cyanide catalysts and the application to polyurethane elastomer. Polymer 2003, 44, 3417–3428. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, P.; Chen, L. Fe/Zn double metal cyanide (DMC) catalyzed ring-opening polymerization of propylene oxide: Part 3. Synthesis of DMC catalysts. Prog. Org. Coat. 2004, 50, 269–272. [Google Scholar] [CrossRef]
- Chen, S.; Hua, Z.; Fang, Z.; Qi, G. Copolymerization of carbon dioxide and propylene oxide with highly effective zinc hexacyanocobaltate(III)-based coordination catalyst. Polymer 2004, 45, 6519–6524. [Google Scholar] [CrossRef]
- Yi, M.J.; Byun, S.-H.; Ha, C.-S.; Park, D.-W.; Kim, I. Copolymerization of cyclohexene oxide with carbon dioxide over nano-sized multi-metal cyanide catalysts. Solid State Ion. 2004, 172, 139–144. [Google Scholar] [CrossRef]
- Wu, L.-C.; Yu, A.-F.; Zhang, M.; Liu, B.-H.; Chen, L.-B. DMC catalyzed epoxide polymerization: Induction period, kinetics, and mechanism. J. Appl. Polym. Sci. 2004, 92, 1302–1309. [Google Scholar] [CrossRef]
- Kim, I.; Ahn, J.-T.; Lee, S.-H.; Ha, C.-S.; Park, D.-W. Preparation of multi-metal cyanide catalysts and ring-opening polymerization of propylene oxide. Catal. Today 2004, 93–95, 511–516. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.-R.; Hua, Z.-J.; Yan, H.-Q. Double metal cyanide complex based on Zn3[Co(CN)6]2 as highly active catalyst for copolymerization of carbon dioxide and cyclohexene oxide. J. Polym. Sci. A Polym. Chem. 2004, 42, 5284–5291. [Google Scholar] [CrossRef]
- Kim, I.; Yi, M.J.; Lee, K.J.; Park, D.-W.; Kim, B.U.; Ha, C.-S. Aliphatic polycarbonate synthesis by copolymerization of carbon dioxide with epoxides over double metal cyanide catalysts prepared by using ZnX2 (X = F, Cl, Br, I). Catal. Today 2006, 111, 292–296. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, S.; Wu, X.M.; Sun, X.K.; Liu, F.; Qi, G.R. Highly active double metal cyanide complexes: Effect of central metal and ligand on reaction of epoxide/CO2. Chin. Chem. Lett. 2007, 18, 887–890. [Google Scholar] [CrossRef]
- Wojdeł, J.C.; Bromley, S.T.; Illas, F.; Jansen, J.C. Development of realistic models for Double Metal Cyanide catalyst active sites. J. Mol. Model. 2007, 13, 751–756. [Google Scholar] [CrossRef]
- Le-Khac, B.; Wang, W. Double-Metal Cyanide Catalysts Which Can Be Used to prepare Polyols and the Processes Related Thereto. US Patent 6696383B1, 24 February 2004. [Google Scholar]
- An, N.; Li, Q.; Yin, N.; Kang, M.; Wang, J. Effects of addition mode on Zn–Co double metal cyanide catalyst for synthesis of oligo(propylene-carbonate) diols. Appl. Organomet. Chem. 2018, 32, e4509. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, I.K.; Choi, H.Y.; Choi, E.J.; Yoon, J.H.; Shim, S.E.; Kim, I. Double metal cyanide catalysts bearing lactate esters as eco-friendly complexing agents for the synthesis of highly pure polyols. Green Chem. 2011, 13, 631–639. [Google Scholar] [CrossRef]
- Chen, X.; Kumbhalkar, M.; Fisk, J.; Murdoch, B. In situ Monitoring of Double Metal Cyanide (DMC) Catalyst Synthesis by Raman Spectroscopy. Spectr. Suppl. 2023, 38, 5–10. [Google Scholar] [CrossRef]
- Dharman, M.M.; Ahn, J.-Y.; Lee, M.-K.; Shim, H.-L.; Kim, K.-H.; Kim, I.; Park, D.-W. Moderate route for the utilization of CO2-microwave induced copolymerization with cyclohexene oxide using highly efficient double metal cyanide complex catalysts based on Zn3[Co(CN)6]. Green Chem. 2008, 10, 678–684. [Google Scholar] [CrossRef]
- Kim, I.; Byun, S.H.; Ha, C.-S. Ring-opening polymerizations of propylene oxide by double metal cyanide catalysts prepared with ZnX2 (X = F, Cl, Br, or I). J. Polym. Sci. A Polym. Chem. 2005, 43, 4393–4404. [Google Scholar] [CrossRef]
- Le-Khac, B. Highly Active Double Metal Cyanide Catalysts. US Patent 5789626A, 4 August 1998. [Google Scholar]
- Sebastian, J.; Srinivas, D. Effects of method of preparation on catalytic activity of Co–Zn double-metal cyanide catalysts for copolymerization of CO2 and epoxide. Appl. Catal. A Gen. 2014, 482, 300–308. [Google Scholar] [CrossRef]
- Huang, Y.J.; Qi, G.R.; Chen, L.S. Effects of morphology and composition on catalytic performance of double metal cyanide complex catalyst. Appl. Catal. A Gen. 2003, 240, 263–271. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Q.; Cheng, Y.; Lu, L.; Lin, B.; Pan, L.; Xu, N. Double metal cyanide complexes synthesized by solvent-free grinding method for copolymerization of CO2 and propylene oxide. J. Appl. Polym. Sci. 2012, 123, 977–985. [Google Scholar] [CrossRef]
- Peeters, A.; Valvekens, P.; Ameloot, R.; Sankar, G.; Kirschhock, C.E.A.; De Vos, D.E. Zn–Co Double Metal Cyanides as Heterogeneous Catalysts for Hydroamination: A Structure–Activity Relationship. ACS Catal. 2013, 3, 597–607. [Google Scholar] [CrossRef]
- Peeters, A.; Valvekens, P.; Vermoortele, F.; Ameloot, R.; Kirschhock, C.; De Vos, D. Lewis acid double metal cyanidecatalysts for hydroamination of phenylacetylene. Chem. Commun. 2011, 47, 4114–4116. [Google Scholar] [CrossRef]
- Pinilla-de Dios, M.; Andrés-Iglesias, C.; Fernández, A.; Salmi, T.; Román Galdámez, J.; García-Serna, J. Effect of Zn/Co initial preparation ratio in the activity of double metal cyanide catalysts for propylene oxide and CO2 copolymerization. Eur. Polym. J. 2017, 88, 280–291. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Lin, F.; Qi, G. Preparation of double metal cyanide compleces from water-insoluble zinc compounds and their catalytic performance for copolymerization of epoxide and CO2. React. Kinet. Catal. Lett. 2007, 91, 69–75. [Google Scholar] [CrossRef]
- Varghese, J.K.; Park, D.S.; Jeon, J.Y.; Lee, B.Y. Double metal cyanide catalyst prepared using H3Co(CN)6 for high carbonate fraction and molecular weight control in carbon dioxide/propylene oxide copolymerization. J. Polym. Sci. A Polym. Chem. 2013, 51, 4811–4818. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Chen, L. Preparation of crown ether complexing highly active double metal cyanide catalysts and copolymerization of CO2 and propylene oxide. Chin. J. Catal. 2015, 36, 1304–1311. [Google Scholar] [CrossRef]
- Mellado, M.; Gonzalez, M.D. Double Metal Cyanide (DMC) Catalysts with Crown Ethers, Process to Produce Them and Applications. US Patent 20070135298A1, 14 June 2007. [Google Scholar]
- Zhao, J.; Yuan, X.; Li, P. Preparation Method of Double Metal Cyanide Catalyst. CN Patent 117820624A, 5 April 2024. [Google Scholar]
- Le-Khac, B. Highly Active Double Metal Cyanide Catalysts. US Patent 5482908A, 9 January 1996. [Google Scholar]
- Le-Khac, B. Polyether-Containing Double Metal Cyanide Catalysts. US Patent 5545601A, 13 August 1996. [Google Scholar]
- Lee, I.K.; Ha, J.Y.; Cao, C.; Park, D.-W.; Ha, C.-S.; Kim, I. Effect of complexing agents of double metal cyanide catalyst on the copolymerizations of cyclohexene oxide and carbon dioxide. Catal. Today 2009, 148, 389–397. [Google Scholar] [CrossRef]
- Wei, R.-J.; Zhang, X.-H.; Du, B.-Y.; Fan, Z.-Q.; Qi, G.-R. Highly active and selective binary catalyst system for the coupling reaction of CO2 and hydrous epoxides. J. Mol. Catal. A Chem. 2013, 379, 38–45. [Google Scholar] [CrossRef]
- Tran, C.H.; Pham, L.T.T.; Lee, Y.; Jang, H.B.; Kim, S.; Kim, I. Mechanistic insights on Zn(II)−Co(III) double metal cyanide-catalyzed ring-opening polymerization of epoxides. J. Catal. 2019, 372, 86–102. [Google Scholar] [CrossRef]
- Chruściel, A.; Hreczuch, W.; Janik, J.; Czaja, K.; Dziubek, K.; Flisak, Z.; Swinarew, A. Characterization of a Double Metal Cyanide (DMC)-Type Catalyst in the Polyoxypropylation Process: Effects of Catalyst Concentration. Ind. Eng. Chem. Res. 2014, 53, 6636–6646. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Gu, Y.; Guo, W. Preparation and Characterization of Double Metal Cyanide Complex Catalysts. Molecules 2003, 8, 67–73. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, I.K.; Ha, J.Y.; Jo, J.K.; Park, I.; Ha, C.-S.; Suh, H.; Kim, I. Tuning of the Activity and Induction Period of the Polymerization of Propylene Oxide Catalyzed by Double Metal Cyanide Complexes Bearing β-Alkoxy Alcohols as Complexing Agents. Ind. Eng. Chem. Res. 2010, 49, 4107–4116. [Google Scholar] [CrossRef]
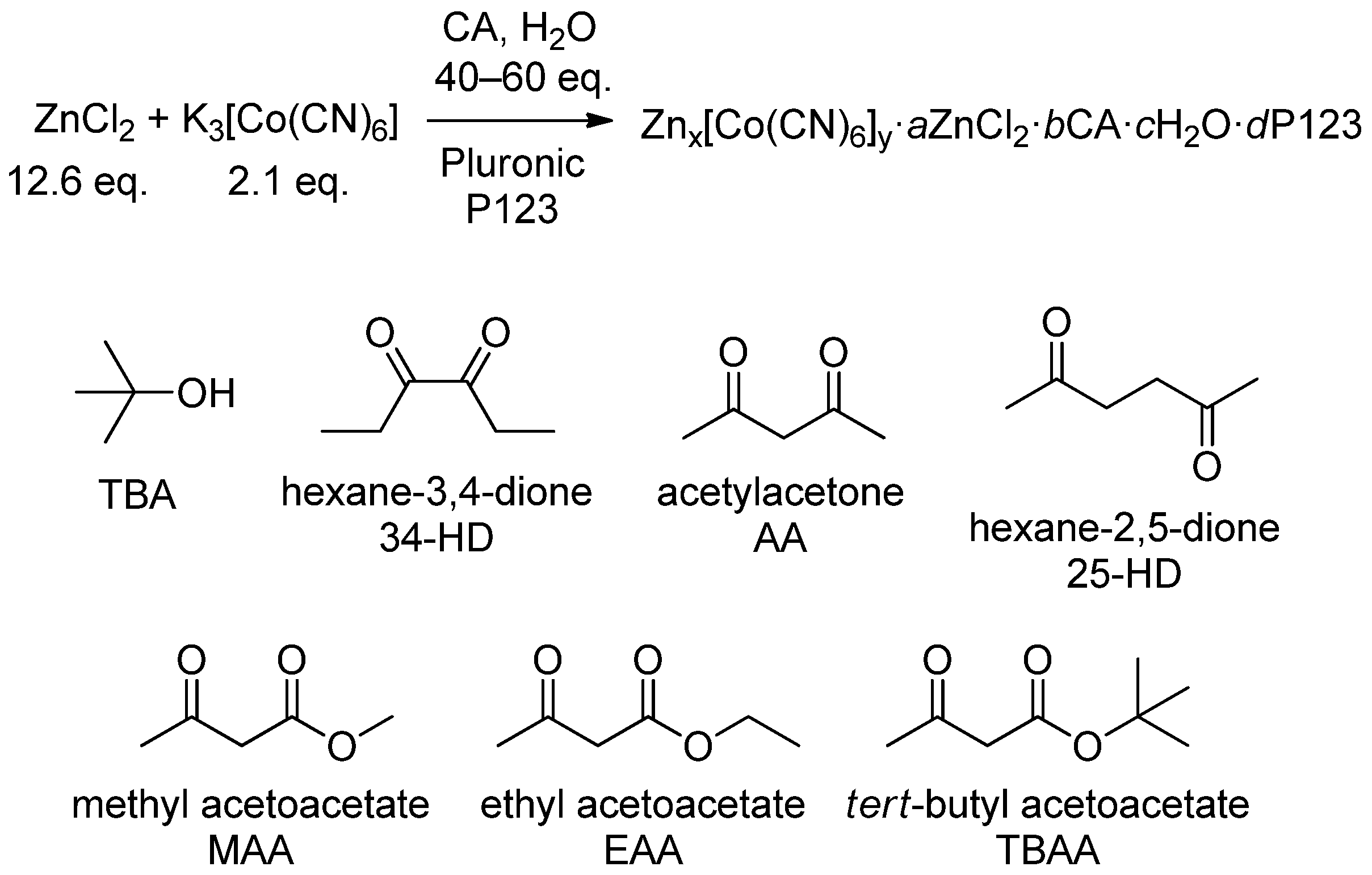
- Tran, C.H.; Kim, S.A.; Moon, Y.; Lee, Y.; Ryu, H.M.; Baik, J.H.; Hong, S.C.; Kim, I. Effect of dicarbonyl complexing agents on double metal cyanide catalysts toward copolymerization of CO2 and propylene oxide. Catal. Today 2021, 375, 335–342. [Google Scholar] [CrossRef]
- Tran, C.H.; Pham, L.T.T.; Jang, H.B.; Kim, S.A.; Kim, I. Effect of α-, β-, γ-, and δ-dicarbonyl complexing agents on the double metal cyanide-catalyzed ring-opening polymerization of propylene oxide. Catal. Today 2021, 375, 429–440. [Google Scholar] [CrossRef]
- Tran, C.H.; Jang, H.B.; Moon, B.; Lee, E.; Choi, H.; Kim, I. Organic carbonates as green and sustainable complexing agents for double metal cyanide catalysts for the synthesis of polyether and poly(ether-carbonate) polyols. Catal. Today 2024, 425, 114319. [Google Scholar] [CrossRef]
- Lee, E.-G.; Tran, C.-H.; Heo, J.-Y.; Kim, S.-Y.; Choi, H.-K.; Moon, B.-R.; Kim, I. Synthesis of Polyether, Poly(Ether Carbonate) and Poly(Ether Ester) Polyols Using Double Metal Cyanide Catalysts Bearing Organophosphorus Complexing Agents. Polymers 2024, 16, 818. [Google Scholar] [CrossRef]
- Marquez, C.; Simonov, A.; Wharmby, M.T.; Van Goethem, C.; Vankelecom, I.; Bueken, B.; Krajnc, A.; Mali, G.; De Vos, D.; De Baerdemaeker, T. Layered Zn2[Co(CN)6](CH3COO) double metal cyanide: A two-dimensional DMC phase with excellent catalytic performance. Chem. Sci. 2019, 10, 4868–4875. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Hyun, Y.B.; Lee, H.J.; Baek, J.W.; Lee, H.C.; Lee, J.H.; Lee, J.; Lee, B.Y. Preparation of double-metal cyanide catalysts with H3Co(CN)6 for propylene oxide homo- and CO2-copolymerization. J. CO2 Utiliz. 2021, 53, 101755. [Google Scholar] [CrossRef]
- Seo, Y.H.; Lee, M.R.; Lee, D.-Y.; Park, J.H.; Seo, H.J.; Park, S.U.; Kim, H.; Kim, S.-J.; Lee, B.Y. Preparation of Well-Defined Double-Metal Cyanide Catalysts for Propylene Oxide Polymerization and CO2 Copolymerization. Inorg. Chem. 2024, 63, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.-H.; Lee, M.-W.; Park, S.-W.; Jeong, J.-E.; Lee, S.-J.; Song, W.; Huh, P.H.; Kim, I. Heterogeneous Double Metal Cyanide Catalyzed Synthesis of Poly(ε-caprolactone) Polyols for the Preparation of Thermoplastic Elastomers. Catalysts 2021, 11, 1033. [Google Scholar] [CrossRef]
- Tran, C.H.; Lee, S.J.; Moon, B.; Lee, E.; Choi, H.; Kim, I. Organonitriles as complexing agents for the double metal cyanide-catalyzed synthesis of polyether, polyester, and polycarbonate polyols. Catal. Today 2023, 418, 114125. [Google Scholar] [CrossRef]
- Tran, C.-H.; Lee, M.-W.; Lee, S.-J.; Choi, J.-H.; Lee, E.-G.; Choi, H.-K.; Kim, I. Highly Active Heterogeneous Double Metal Cyanide Catalysts for Ring-Opening Polymerization of Cyclic Monomers. Polymers 2022, 14, 2507. [Google Scholar] [CrossRef]
- Tran, C.H.; Choi, H.-K.; Lee, E.-G.; Moon, B.-R.; Song, W.; Kim, I. Prussian blue analogs as catalysts for the fixation of CO2 to glycidol to produce glycerol carbonate and multibranched polycarbonate polyols. J. CO2 Utiliz. 2023, 74, 102530. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, B.; Saini, S.; Behera, B.; Saran, S.; Ganguly, S.K.; Kumar, U. Oligomeric Heterogeneous Double Metal Cyanide Catalyst for One-pot Ring-Opening Polymerization. ChemistrySelect 2023, 8, e202204760. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Nörnberg, B. Process for preparing double metal cyanide catalysts and their use in polymerization reactions. WO Patent 2016202838A1, 22 December 2022. [Google Scholar]
- Tharun, J.; Dharman, M.M.; Hwang, Y.; Roshan, R.; Parkae-Won Park, M.S. Tuning double metal cyanide catalysts with complexing agents for the selective production of cyclic carbonates over polycarbonates. Appl. Catal. A Gen. 2012, 419–420, 178–184. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, L.; Cheng, Y.; Xu, N.; Pan, L.; Lin, Q.; Wang, Y. Clean and rapid synthesis of double metal cyanide complexes using mechanochemistry. Green Chem. 2011, 13, 2701–2703. [Google Scholar] [CrossRef]
- Liu, N.; Gu, C.; Wang, Q.; Zhu, L.; Yan, H.; Lin, Q. Fabrication and characterization of the ternary composite catalyst system of ZnGA/RET/DMC for the terpolymerization of CO2, propylene oxide and trimellitic anhydride. RSC Adv. 2021, 11, 8782–8792. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, K.; Mao, Q.; Gu, Z.; Wang, F. MCM-41-supported double metal cyanide nanocomposite catalyst for ring-opening polymerisation of propylene oxide. J. Nanopart. Res. 2022, 24, 71. [Google Scholar] [CrossRef]
- Marquez, C.; Rivera-Torrente, M.; Paalanen, P.P.; Weckhuysen, B.M.; Cirujano, F.G.; De Vos, D.; De Baerdemaeker, T. Increasing the availability of active sites in Zn-Co double metal cyanides by dispersion onto a SiO2 support. J. Catal. 2017, 354, 92–99. [Google Scholar] [CrossRef]
- Dienes, Y.; Leitner, W.; Müller, M.G.J.; Offermans, W.K.; Reier, T.; Reinholdt, A.; Weirich, T.E.; Müller, T.E. Hybrid sol–gel double metal cyanidecatalysts for the copolymerisation of styrene oxide and CO2. Green Chem. 2012, 14, 1168–1177. [Google Scholar] [CrossRef]
- Subhani, M.A.; Gürtler, C.; Leitner, W.; Müller, T.E. Nanoparticulate TiO2-Supported Double Metal Cyanide Catalyst for the Copolymerization of CO2 with Propylene Oxide. Eur. J. Inorg. Chem. 2016, 2016, 1944–1949. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, L.; Cao, Y.; Liu, C.; He, P.; Li, H. Beta Zeolite-Supported Double-Metal Cyanide Catalysts with Enhanced Lewis Acidity for the Synthesis of High-Performance Poly(1,2-butylene oxide) Lubricating Oils. Ind. Eng. Chem. Res. 2024, 63, 1215–1227. [Google Scholar] [CrossRef]
- Laguta, A.; van Koningsbruggen, P. Zinc ferro- and ferri-cyanides catalyst structures from point of colloid chemistry view. Appl. Catal. A Gen. 2024, 684, 119902. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhou, S.; Zou, J.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Double-metal cyanide-supported Pd catalysts for highly efficient hydrogenative ring-rearrangement of biomass-derived furanic aldehydes to cyclopentanone compounds. J. Catal. 2019, 378, 201–208. [Google Scholar] [CrossRef]
- Verma, A.; Saini, S.; Sharma, B.; Verma, V.; Behera, B.; Singh, R.; Ganguly, S.K.; Ray, A.; Vorontsov, A.; Kumar, U. EDTA incorporated Fe-Zn double metal cyanide catalyst for the controlled synthesis of polyoxypropylene glycol. J. Polym. Res. 2023, 30, 62. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, Q. Coupling reaction of CO2 and propylene oxide catalyzed by DMC with co-complexing agents incorporated via ball milling. J. Mol. Catal. A Chem. 2014, 390, 63–68. [Google Scholar] [CrossRef]
- Zhifang, G.; Qiang, L.; Xianghui, W.; Changjiang, Y.; Jie, Z.; Yandong, S.; Ting, P. Rapid synthesis of nanoscale double metal cyanide catalysts by ball milling for the cycloaddition of CO2 and propylene oxide. Mater. Lett. 2014, 124, 184–187. [Google Scholar] [CrossRef]
- Shi, J.; Shi, Z.; Yan, H.; Wang, X.; Zhang, X.; Lin, Q.; Zhu, L. Synthesis of Zn–Fe double metal cyanide complexes with imidazolium-based ionic liquid cocatalysts via ball milling for copolymerization of CO2 and propylene oxide. RSC Adv. 2018, 8, 6565–6571. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gu, C.; Chen, M.; Zhang, J.; Yang, W.; Zhan, A.; Zhang, K.; Lin, Q.; Zhu, L. Terpolymerizations of CO2, Propylene Oxide and DL-Lactide Catalyzed by Zn-Fe DMC Catalysts with Quaternary Ammonium Salts. ChemistrySelect 2020, 5, 2388–2394. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. The role of vacancies in the hydrogen storage properties of Prussian blue analogues. Catal. Today 2007, 120, 311–316. [Google Scholar] [CrossRef]
- Yu, S.J.; Liu, Y.; Byeon, S.J.; Park, D.W.; Kim, I. Ring-opening polymerization of propylene oxide by double metal cyanide catalysts prepared by reacting CoCl2 with various metal cyanide salts. Catal. Today 2014, 232, 75–81. [Google Scholar] [CrossRef]
- Penche, G.; González-Velasco, J.R.; González-Marcos, M.P. Porous Hexacyanometallate(III) Complexes as Catalysts in the Ring-Opening Copolymerization of CO2 and Propylene Oxide. Catalysts 2021, 11, 1450. [Google Scholar] [CrossRef]
- Penche, G.; González-Marcos, M.P.; González-Velasco, J.R. Transition Metal Hexacyanoferrate(II) Complexes as Catalysts in the Ring-Opening Copolymerization of CO2 and Propylene Oxide. Top. Catal. 2022, 65, 1541–1555. [Google Scholar] [CrossRef]
- Liang, Y.; Yi, C.; Tricard, S.; Fang, J.; Zhao, J.; Shen, W. Prussian blue analogues as heterogeneous catalysts for epoxidation of styrene. RSC Adv. 2015, 5, 17993–17999. [Google Scholar] [CrossRef]
- Garcia, J.L.; Jang, E.J.; Alper, H. New heterogeneous catalysis for the synthesis of poly(ether polyol)s. J. Appl. Polym. Sci. 2002, 86, 1553–1557. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Z.; Ma, M. Copolymerization of carbon dioxide and epoxides with a novel effective Zn–Ni double-metal cyanide complex. J. Appl. Polym. Sci. 2008, 107, 3871–3877. [Google Scholar] [CrossRef]
- Alferov, K.; Wang, S.; Li, T.; Xiao, M.; Guan, S.; Meng, Y. Co-Ni Cyanide Bi-Metal Catalysts: Copolymerization of Carbon Dioxide with Propylene Oxide and Chain Transfer Agents. Catalysts 2019, 9, 632. [Google Scholar] [CrossRef]
- Robertson, N.J.; Qin, Z.; Dallinger, G.C.; Lobkovsky, E.B.; Lee, S.; Coates, G.W. Two-dimensional double metal cyanide complexes: Highly active catalysts for the homopolymerization of propylene oxide and copolymerization of propylene oxide and carbon dioxide. Dalton Trans. 2006, 45, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Zhifang, G.; Lisha, P.; Xue, X. Zn–Cr double metal cyanide catalysts synthesized by ball milling for the copolymerization of CO2/propylene oxide, phthalic anhydride/propylene oxide, and CO2/propylene oxide/phthalic anhydride. Catal. Commun. 2015, 64, 114–118. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, Y.; Zhang, C.-J.; Zhang, Y.-Y.; Cao, X.-H.; Zhang, X.-H. Synthesis of high-molecular-weight poly(ε-caprolactone) via heterogeneous zinc-cobalt(III) double metal cyanide complex. Giant 2020, 3, 100030. [Google Scholar] [CrossRef]
- Mullica, D.F.; Milligan, W.O.; Beall, G.W.; Reeves, W.L. Crystal structure of Zn3[Co(CN)6]2∙12H2O. Acta Crystallogr. 1978, B34, 3558–3561. [Google Scholar] [CrossRef]
- Simonov, A.; De Baerdemaeker, T.; Boström, H.L.B.; Ríos Gómez, M.L.; Gray, H.J.; Chernyshov, D.; Bosak, A.; Bürgi, H.-B.; Goodwin, A.L. Hidden diversity of vacancy networks in Prussian blue analogues. Nature 2020, 578, 256–260. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, T.; Yang, Z.; Yu, R.; Zeng, X.; Xu, Y.; Zhang, M.; Hu, H.; Ou, J.Z.; Zheng, L. Crystal phase-driven copolymerization of CO2 and cyclohexene oxide in Prussian blue analogue nanosheets. Appl. Mater. Today 2022, 26, 101352. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Hua, Z.-J.; Chen, S.; Liu, F.; Sun, X.-K.; Qi, G.-R. Role of zinc chloride and complexing agents in highly active double metal cyanide catalysts for ring-opening polymerization of propylene oxide. Appl. Catal. A Gen. 2007, 325, 91–98. [Google Scholar] [CrossRef]
- Almora-Barrios, N.; Pogodin, S.; Bellarosa, L.; García-Melchor, M.; Revilla-López, G.; García-Ratés, M.; Vázquez-García, A.B.; Hernández-Ariznavarreta, P.; López, N. Structure, Activity, and Deactivation Mechanisms in Double Metal Cyanide Catalysts for the Production of Polyols. ChemCatChem 2015, 7, 928–935. [Google Scholar] [CrossRef]
- Lawniczak-Jablonska, K.; Dynowska, E.; Lisowski, W.; Sobczak, J.W.; Chruściel, A.; Hreczuch, W.; Libera, J.; Reszka, A. Structural properties and chemical bonds in double metal cyanide catalysts. X-Ray Spectrom. 2015, 44, 330–338. [Google Scholar] [CrossRef]
- Lawniczak-Jablonska, K.; Chrusciel, A. Estimation of the catalytic centre in double metal cyanide catalysts by XAS. J. Phys. Conf. Ser. 2016, 712, 012062. [Google Scholar] [CrossRef]
- Chruściel, A.; Hreczuch, W.; Czaja, K.; Ławniczak-Jabłońska, K.; Janik, J. The complementary structural studies of the double metal cyanide type catalysts for the ring opening polymerization of the oxiranes. Polimery 2016, 61, 421–432. [Google Scholar] [CrossRef]
- Chruściel, A.; Hreczuch, W.; Czaja, K.; Sacher-Majewska, B. On thermal behaviour of DMC catalysts for ring opening polymerization of epoxides. Thermochim. Acta 2016, 630, 78–89. [Google Scholar] [CrossRef]
- Stahl, S.-F.; Luinstra, G.A. Analysis of propoxylation with zinc–cobalt double metal cyanide catalysts with different active surfaces and particle sizes. React. Chem. Eng. 2024, 9, 91–107. [Google Scholar] [CrossRef]
- Karadas, F.; El-Faki, H.; Deniz, E.; Yavuz, C.T.; Aparicio, S.; Atilhan, M. CO2 adsorption studies on Prussian blue analogues. Micropor. Mesopor. Mater. 2012, 162, 91–97. [Google Scholar] [CrossRef]
- Penche, G.; González-Marcos, M.P.; González-Velasco, J.R.; Vos, C.W.; Kozak, C.M. Two-dimensional (2D) layered double metal cyanides as alternative catalysts for CO2/propylene oxide copolymerization. Catal. Sci. Technol. 2023, 13, 5214–5226. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Zhang, X.-H.; Hua, Z.-J.; Chen, S.-L.; Qi, G.-R. Ring-Opening Polymerization of Propylene Oxide Catalyzed by a Calcium-Chloride-Modified Zinc-Cobalt Double Metal-Cyanide Complex. Macromol. Chem. Phys. 2010, 211, 1229–1237. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Rykov, A.I.; Fan, Q.; Xu, W.; Cong, W.; Jin, C.; Tang, H.; Zhu, K.; Ganeshraja, A.S.; et al. Zinc-modulated Fe–Co Prussian blue analogues with well-controlled morphologies for the efficient sorption of cesium. J. Mater. Chem. A 2017, 5, 3284–3292. [Google Scholar] [CrossRef]
- Fliedel, C.; Vila-Viçosa, D.; Calhorda, M.J.; Dagorne, S.; Avilés, T. Dinuclear Zinc–N-Heterocyclic Carbene Complexes for Either the Controlled Ring-Opening Polymerization of Lactide or the Controlled Degradation of Polylactide Under Mild Conditions. ChemCatChem 2014, 6, 1357–1367. [Google Scholar] [CrossRef]
- Jędrzkiewicz, D.; Adamus, G.; Kwiecień, M.; John, Ł.; Ejfler, J. Lactide as the Playmaker of the ROP Game: Theoretical and Experimental Investigation of Ring-Opening Polymerization of Lactide Initiated by Aminonaphtholate Zinc Complexes. Inorg. Chem. 2017, 56, 1349–1365. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Bagrov, V.V.; Minyaev, M.E.; Churakov, A.V.; Karchevsky, S.G.; Birin, K.P.; Ivchenko, P.V. Mono-BHT heteroleptic magnesium complexes: Synthesis, molecular structure and catalytic behavior in the ring-opening polymerization of cyclic esters. Dalton Trans. 2017, 46, 12132–12146. [Google Scholar] [CrossRef] [PubMed]
- Lapenta, R.; De Simone, N.A.; Buonerba, A.; Talotta, C.; Gaeta, C.; Neri, P.; Grassi, A.; Milione, S. Dinuclear zirconium complex bearing a 1,5-bridged-calix[8]arene ligand as an effective catalyst for the synthesis of macrolactones. Catal. Sci. Technol. 2018, 8, 2716–2727. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Gavrilov, D.; Karchevsky, S.; Ivchenko, P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers 2019, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, B.; Saini, S.; Oluokun, T.; Saini, V.; Khurana, D.; Khan, T.S.; Ganguly, S.K.; Viswanadham, N.; Kumar, U. Mechanistic insights into Schiff base incorporated double metal cyanide catalyst for the synthesis of colorless polyether polyols. Mol. Catal. 2023, 551, 113628. [Google Scholar] [CrossRef]
- Bachmann, R.; Klinger, M.; Jupke, A. Molcular Weight Distribution in Di Metal Cyanide Catalyzed Polymerization 1: Fundamental Distribution for Length Dependent Propagation Constant and Segments. Macromol. Theory Simul. 2021, 30, 2100012. [Google Scholar] [CrossRef]
- Bachmann, R.; Klinger, M.; Jupke, A. Molcular Weight Distribution in Di Metal Cyanide Catalyzed Polymerization 2: Numerical simulation of chain activation/deactivation and diffusion effects. Macromol. Theory Simul. 2021, 30, 2100013. [Google Scholar] [CrossRef]
- Stahl, S.-F.; Wietzer, M.; Luinstra, G.A. DMC-Mediated Propoxylation in Semibatch with an External Loop: Insights into the Catalytic Action. Ind. Eng. Chem. Res. 2023, 62, 11536–11548. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef]
- Sun, X.-K.; Zhang, X.-H.; Wei, R.-J.; Du, B.-Y.; Wang, Q.; Fan, Z.-Q.; Qi, G.-R. Mechanistic insight into initiation and chain transfer reaction of CO2/cyclohexene oxide copolymerization catalyzed by zinc–cobalt double metal cyanide complex catalysts. J. Polym. Sci. A Polym. Chem. 2012, 50, 2924–2934. [Google Scholar] [CrossRef]
- Stahl, S.-F.; Luinstra, G.A. DMC-Mediated Copolymerization of CO2 and PO—Mechanistic Aspects Derived from Feed and Polymer Composition. Catalysts 2020, 10, 1066. [Google Scholar] [CrossRef]
- Song, Z.; Subramaniam, B.; Chaudhari, R.V. Kinetic modeling and mechanistic investigations of transesterification of propylene carbonate with methanol over an Fe–Mn double metal cyanide catalyst. React. Chem. Eng. 2020, 5, 101–111. [Google Scholar] [CrossRef]
- Ehlers, S.; Hofmann, J.; Dietrich, M.; Gupta, P.; Steinlein, C.; Zwick, H. Method for preparing polyether polyols. US Patent 6486361B1, 26 November 2002. [Google Scholar]
- Ostrowski, T.; Ruppel, R.; Baum, E.; Harre, K. Method for Producing Polyether Alcohols. US Patent 7968754B2, 28 June 2011. [Google Scholar]
- Buckley, B.J.; Juris, C.; Davies, A.M. Polyurethane Foam Elastomers for High Temperature Applications. US Patent 20140094534A1, 3 April 2014. [Google Scholar]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology: Shawbury, UK, 2005. [Google Scholar]
- Langanke, J.; Hofmann, J.; Gürtler, C.; Wolf, A. Facile synthesis of formaldehyde-based polyether(-carbonate) polyols. J. Polym. Sci. A Polym. Chem. 2015, 53, 2071–2074. [Google Scholar] [CrossRef]
- Kunst, A.; Eling, B.; Löffler, A.; Lutter, H.-D.; Han, W.; Müller, J. Polyether Polyols, Process for Preparing Polyether Polyols and Their Use for Producing Polyurethanes. US Patent 9115246B2, 25 August 2015. [Google Scholar]
- Liu, S.; Qin, Y.; Qiao, L.; Miao, Y.; Wang, X.; Wang, F. Cheap and fast: Oxalic acid initiated CO2-based polyols synthesized by a novel preactivation approach. Polym. Chem. 2016, 7, 146–152. [Google Scholar] [CrossRef]
- Liu, S.; Qin, Y.; Chen, X.; Wang, X.; Wang, F. One-pot controllable synthesis of oligo(carbonate-ether) triol using a Zn-Co-DMC catalyst: The special role of trimesic acid as an initiation-transfer agent. Polym. Chem. 2014, 5, 6171–6179. [Google Scholar] [CrossRef]
- Liu, S.; Miao, Y.; Qiao, L.; Qin, Y.; Wang, X.; Chen, X.; Wang, F. Controllable synthesis of a narrow polydispersity CO2-based oligo(carbonate-ether) tetraol. Polym. Chem. 2015, 6, 7580–7585. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, B.; Gupta, P.; Ray, A.; Behera, B.; Kumar, U.; Viswanadham, N. Microstructural Insights into Ethylene Glycol-Mediated Polyether Polyols Using Double Metal Cyanide Catalyst. Macromol. Chem. Phys. 2024, 225, 2300319. [Google Scholar] [CrossRef]
- Safina, L.R.; Kharlampidi, K.E.; Safin, D.K. Molecular-weight characteristics and deemulsifying activity of oligourethanes based on alkylene oxide block copolymers and propylene oxide homopolymers prepared in the presence of a double metal cyanide catalyst. Russ. J. Appl. Chem. 2012, 85, 1610–1615. [Google Scholar] [CrossRef]
- Huang, Y.J.; Qi, G.R.; Wang, Y.-H. Controlled ring-opening polymerization of propylene oxide catalyzed by double metal-cyanide complex. J. Polym. Sci. A Polym. Chem. 2002, 40, 1142–1150. [Google Scholar] [CrossRef]
- Jang, E.H.; Kim, S.A.; Kim, H.; Tran, C.H.; Song, H.Y.; Hyun, K.; Seo, W.J.; Kim, I. Access to Ultra-High Molecular Weight Poly(propylene glycol)-Based Polyols Using Double Metal Cyanide Catalyst. Macromol. Res. 2020, 28, 82–85. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, X.; Sun, D.X. Synthesis of Novel Polyether Polyol by Using Double Metal Cyanide. J. Macromol. Sci. A 2012, 49, 586–590. [Google Scholar] [CrossRef]
- Wei, R.-J.; Zhang, Y.-Y.; Zhang, X.-H.; Du, B.-Y.; Fan, Z.-Q. Regio-selective synthesis of polyepichlorohydrin diol using Zn–Co(iii) double metal cyanide complex. RSC Adv. 2014, 4, 21765–21771. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, X. Novel application of double metal cyanide in the synthesis of hyperbranched polyether polyols. Des. Monomers Polym. 2013, 16, 72–78. [Google Scholar] [CrossRef]
- Tran, C.H.; Lee, M.W.; Kim, S.A.; Jang, H.B.; Kim, I. Kinetic and Mechanistic Study of Heterogeneous Double Metal Cyanide-Catalyzed Ring-Opening Multibranching Polymerization of Glycidol. Macromolecules 2020, 53, 2051–2060. [Google Scholar] [CrossRef]
- Matthes, R.; Bapp, C.; Wagner, M.; Zarbakhsh, S.; Frey, H. Unexpected Random Copolymerization of Propylene Oxide with Glycidyl Methyl Ether via Double Metal Cyanide Catalysis: Introducing Polarity in Polypropylene Oxide. Macromolecules 2021, 54, 11228–11237. [Google Scholar] [CrossRef]
- Kaushiva, B.D. Single reactor synthesis of KOH-capped polyols based on DMC-synthesized intermediates. US Patent 7005552B2, 28 February 2006. [Google Scholar]
- Raghuraman, A.; Babb, D.; Miller, M.; Paradkar, M.; Smith, B.; Nguyen, A. Sequential DMC/FAB-Catalyzed Alkoxylation toward High Primary Hydroxyl, High Molecular Weight Polyether Polyols. Macromolecules 2016, 49, 6790–6798. [Google Scholar] [CrossRef]
- Lee, D.H.; Ha, J.H.; Kim, I.; Baik, J.H.; Hong, S.C. Carbon Dioxide Based Poly(ether carbonate) Polyol in Bi-polyol Mixtures for Rigid Polyurethane Foams. J. Polym. Environ. 2020, 28, 1160–1168. [Google Scholar] [CrossRef]
- Jang, J.H.; Ha, J.H.; Kim, I.; Baik, J.H.; Hong, S.C. Facile Room-Temperature Preparation of Flexible Polyurethane Foams from Carbon Dioxide Based Poly(ether carbonate) Polyols with a Reduced Generation of Acetaldehyde. ACS Omega 2019, 4, 7944–7952. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, H.; Qiao, L.; Wang, X.; Wang, F. Near neutral waterborne cationic polyurethane from CO2-polyol, a compatible binder to aqueous conducting polyaniline for eco-friendly anti-corrosion purposes. Green Chem. 2020, 22, 7823–7831. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, Y.; Zhao, X.; Wang, F.; Wang, X. Selective synthesis of oligo(carbonate-ether) diols from copolymerization of CO2 and propylene oxide under zinc-cobalt double metal cyanide complex. J. Polym. Res. 2012, 19, 9878. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, X.; Chen, Q.; Kang, M.; Li, Q.; Wang, J. Modification of the Zn–Co double metal cyanide complex for the synthesis of carbon dioxide–derived poly(ether–carbonate) polyols. J. CO2 Utiliz. 2024, 82, 102768. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.-J.; Hwang, J.; Jung, S.M.; Park, J.D.; Baik, J.H. Enhanced Double Metal Cyanide Catalysts for Modulating the Rheological Properties of Poly(propylene carbonate) Polyols by Modifying Carbonate Contents. ACS Omega 2023, 8, 39279–39287. [Google Scholar] [CrossRef] [PubMed]
- Böhm, K.; Maerten, S.G.; Liauw, M.A.; Müller, T.E. Exploring the Sequence of Comonomer Insertion into Growing Poly(ether carbonate) Chains with Monte Carlo Methods. Macromolecules 2020, 53, 6861–6865. [Google Scholar] [CrossRef]
- Ma, K.; Bai, Q.; Zhang, L.; Liu, B. Synthesis of flame-retarding oligo(carbonate-ether) diols via double metal cyanide complex-catalyzed copolymerization of PO and CO2 using bisphenol A as a chain transfer agent. RSC Adv. 2016, 6, 48405–48410. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Su, Y.; Lee, E.G.; Duan, Z.; Kim, I.; Liu, B. Construction and arm evolution of trifunctional phenolic initiator-mediated polycarbonate polyols produced by using a double metal cyanide catalyst. Polym. Chem. 2023, 14, 1263–1274. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, L.; Qin, Y.; Wang, X.; Wang, F. Dicarboxylic acid promoted immortal copolymerization for controllable synthesis of low-molecular weight oligo(carbonate-ether) diols with tunable carbonate unit content. J. Polym. Sci. A Polym. Chem. 2012, 50, 5177–5184. [Google Scholar] [CrossRef]
- Li, X.-J.; Wen, Y.-F.; Wang, Y.; Peng, H.-Y.; Zhou, X.-P.; Xie, X.-L. CO2-based Biodegradable Supramolecular Polymers with Well-tunable Adhesive Properties. Chin. J. Polym. Sci. 2022, 40, 47–55. [Google Scholar] [CrossRef]
- Roznowska, A.; Dyduch, K.; Lee, B.Y.; Michalak, A. Theoretical study on preference of open polymer vs. cyclic products in CO2/epoxide copolymerization with cobalt(III)-salen bifunctional catalysts. J. Mol. Model. 2020, 26, 113. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, Q.; Wang, Q.; Gu, C.; Tang, A.; Liu, T.; Dai, C.; Li, H. CO2 Copolymerization Catalyzed by the Double Metal Cyanide Catalysts Zn–Fe(III)/Zn–Co(III) with Nanometer-Sized γ-Al2O3 as Co-Catalyst. Nanosci. Nanotechnol. Lett. 2018, 10, 1515–1522. [Google Scholar] [CrossRef]
- Meng, Q.; Cheng, R.; Li, J.; Wang, T.; Liu, B. Copolymerization of CO2 and propylene oxide using ZnGA/DMC composite catalyst for high molecular weight poly(propylene carbonate). J. CO2 Utiliz. 2016, 16, 86–96. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, Q.; Zhu, L.; Wang, X.; Niu, Y.; Yu, C.; Fang, T. Nanolamellar Zn–Ni/Co–Ni Catalysts Introduced by Ball Milling for the Copolymerization of CO2 with Propylene Oxide. Nanosci. Nanotechnol. Lett. 2014, 6, 353–356. [Google Scholar] [CrossRef]
- Kember, M.; Kabir, R.; Williams, C.K. Method for Preparing Polyols. US Patent 10774179B2, 15 September 2020. [Google Scholar]
- Mbabazi, R.; Nyanzi, S.A.; Naziriwo, B.; Ojwach, S.O.; Folkers, L.C.; Wendt, O.F.; Tebandeke, E. Highly efficient CO2 and propylene oxide co-polymerization using Zn glutarate/Zn-Cr double metal cyanide composite catalyst. Sust. Chem. Climate Act. 2024, 4, 100037. [Google Scholar] [CrossRef]
- Wei, R.-J.; Zhang, X.-H.; Du, B.-Y.; Sun, X.-K.; Fan, Z.-Q.; Qi, G.-R. Highly Regioselective and Alternating Copolymerization of Racemic Styrene Oxide and Carbon Dioxide via Heterogeneous Double Metal Cyanide Complex Catalyst. Macromolecules 2013, 46, 3693–3697. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Wei, R.-J.; Zhang, Y.-Y.; Du, B.-Y.; Fan, Z.-Q. Carbon Dioxide/Epoxide Copolymerization via a Nanosized Zinc–Cobalt(III) Double Metal Cyanide Complex: Substituent Effects of Epoxides on Polycarbonate Selectivity, Regioselectivity and Glass Transition Temperatures. Macromolecules 2015, 48, 536–544. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Liu, F.; Sun, X.-K.; Chen, S.; Du, B.-Y.; Qi, G.-R.; Wan, K.M. Atom-Exchange Coordination Polymerization of Carbon Disulfide and Propylene Oxide by a Highly Effective Double-Metal Cyanide Complex. Macromolecules 2008, 41, 1587–1590. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Du, B.-Y.; Wang, Q.; Fan, Z.-Q. Alternating copolymerization of carbonyl sulfide and Cyclohexene Oxide catalyzed by zinc–cobalt double metal cyanide complex. Polymer 2014, 55, 3688–3695. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Darensbourg, D.J. An Examination of the Steric and Electronic Effects in the Copolymerization of Carbonyl Sulfide and Styrene Oxide. Macromolecules 2015, 48, 6057–6062. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Darensbourg, D.J. Poly(monothiocarbonate)s from the Alternating and Regioselective Copolymerization of Carbonyl Sulfide with Epoxides. Acc. Chem. Res. 2016, 49, 2209–2219. [Google Scholar] [CrossRef]
- Khan, M.U.; Khan, S.U.; Cao, X.; Usman, M.; Yue, X.; Ghaffar, A.; Hassan, M.; Zhang, C.; Zhang, X. Copolymerization of Carbonyl Sulfide and Propylene Oxide via a Heterogeneous Prussian Blue Analogue Catalyst with High Productivity and Selectivity. Chem. Asian J. 2023, 18, e202201050. [Google Scholar] [CrossRef]
- Mo, W.; Zhuo, C.; Liu, S.; Wang, X.; Wang, F. From plastic to elastomers: Introducing reversible copper–thioether coordination in CO2-based polycarbonate. Polym. Chem. 2023, 14, 152–160. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, J.; Zhang, X.; Liu, B.; Liu, S.; Wang, X. Construction of Ultralow-Molecular-Weight CO2–Polyols with Self-Catalytic Performance in Polyurethane Preparation. Macromolecules 2024, 57, 2706–2714. [Google Scholar] [CrossRef]
- Lee, G.R.; Lee, E.J.; Shin, H.S.; Kim, J.; Kim, I.; Hong, S.C. Preparation of Non-Isocyanate Polyurethanes from Mixed Cyclic-Carbonated Compounds: Soybean Oil and CO2-Based Poly(ether carbonate). Polymers 2024, 16, 1171. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Qi, G.; Chen, S. Ring-opening copolymerization of maleic anhydride with propylene oxide by double-metal cyanide. J. Appl. Polym. Sci. 2004, 93, 1788–1792. [Google Scholar] [CrossRef]
- Hu, L.-F.; Zhang, C.-J.; Chen, D.-J.; Cao, X.-H.; Yang, J.-L.; Zhang, X.-H. Synthesis of High-Molecular-Weight Maleic Anhydride-Based Polyesters with Enhanced Properties. ACS Appl. Polym. Mater. 2020, 2, 5817–5823. [Google Scholar] [CrossRef]
- DiCiccio, A.M.; Coates, G.W. Ring-Opening Copolymerization of Maleic Anhydride with Epoxides: A Chain-Growth Approach to Unsaturated Polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhang, H.; Hu, L.; Liu, B.; Zhang, C.; Zhang, X.; Tang, B.Z. Altering Chain Flexibility of Aliphatic Polyesters for Yellow-Green Clusteroluminescence in 38 % Quantum Yield. Angew. Chem. Int. Ed. 2022, 61, e202114117. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Chu, B.; Zhang, Z.; Xie, Y.; Wang, L.; Sun, J.Z.; Zhang, H.; Zhang, X.-H.; Tang, B.Z. Manipulation of clusteroluminescence in carbonyl-based aliphatic polymers. Aggregate 2022, 3, e278. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, X.; Cao, X.; Chen, D.; Sun, Y.; Zhang, C.; Zhang, X. Alternating Copolymerization of Isobutylene Oxide and Cyclic Anhydrides: A New Route to Semicrystalline Polyesters. Macromolecules 2021, 54, 6182–6190. [Google Scholar] [CrossRef]
- Mohr, R.; Wagner, M.; Zarbakhsh, S.; Frey, H. The Unique Versatility of the Double Metal Cyanide (DMC) Catalyst: Introducing Siloxane Segments to Polypropylene Oxide by Ring-Opening Copolymerization. Macromol. Rapid Commun. 2021, 42, 2000542. [Google Scholar] [CrossRef]
- Sun, X.-K.; Zhang, X.-H.; Chen, S.; Du, B.-Y.; Wang, Q.; Fan, Z.-Q.; Qi, G.-R. One-pot terpolymerization of CO2, cyclohexene oxide and maleic anhydride using a highly active heterogeneous double metal cyanide complex catalyst. Polymer 2010, 51, 5719–5725. [Google Scholar] [CrossRef]
- Sebastian, J.; Darbha, S. Structure-induced catalytic activity of Co–Zn double-metal cyanide complexes for terpolymerization of propylene oxide, cyclohexene oxide and CO2. RSC Adv. 2015, 5, 18196–18203. [Google Scholar] [CrossRef]
- Sebastian, J.; Srinivas, D. Factors influencing catalytic activity of Co–Zn double-metal cyanide complexes for alternating polymerization of epoxides and CO2. Appl. Catal. A Gen. 2015, 506, 163–172. [Google Scholar] [CrossRef]
- Subhani, M.A.; Köhler, B.; Gürtler, C.; Leitner, W.; Müller, T.E. Transparent Films from CO2-Based Polyunsaturated Poly(ether carbonate)s: A Novel Synthesis Strategy and Fast Curing. Angew. Chem. Int. Ed. 2016, 55, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, W.-Z.; Wang, L.; Li, L.-L.; Zhang, Y.-L.; Zhao, S.-D. Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate. e-Polymers 2021, 21, 854. [Google Scholar] [CrossRef]
- Li, Y.; Hong, J.; Wei, R.; Zhang, Y.; Tong, Z.; Zhang, X.; Du, B.; Xu, J.; Fan, Z. Highly efficient one-pot/one-step synthesis of multiblock copolymers from three-component polymerization of carbon dioxide, epoxide and lactone. Chem. Sci. 2015, 6, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Ivchenko, P. Coordination Ring-Opening Polymerization of Cyclic Esters: A Critical Overview of DFT Modeling and Visualization of the Reaction Mechanisms. Molecules 2019, 24, 4117. [Google Scholar] [CrossRef]
- Choi, J.-H.; Woo, J.-J.; Kim, I. Sustainable Polycaprolactone Polyol-Based Thermoplastic Poly(ester ester) Elastomers Showing Superior Mechanical Properties and Biodegradability. Polymers 2023, 15, 3209. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.-Y.; Zhang, X.-H.; Du, B.-Y.; Fan, Z.-Q. Fixation of carbon dioxide concurrently or in tandem with free radical polymerization for highly transparent polyacrylates with specific UV absorption. Polym. Chem. 2016, 7, 3731–3739. [Google Scholar] [CrossRef]
- Dai, W.-L.; Luo, S.-L.; Yin, S.-F.; Au, C.-T. The direct transformation of carbon dioxide to organic carbonates over heterogeneous catalysts. Appl. Catal. A Gen. 2009, 366, 2–12. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, D.; Malik, N.; Kumar, S.; Malik, R.S. Metal catalyst for CO2 capture and conversion into cyclic carbonate: Progress and challenges. Mater. Today 2023, 65, 133–165. [Google Scholar] [CrossRef]
- Dharman, M.M.; Yu, J.-I.; Ahn, J.-Y.; Park, D.-W. Selective production of cyclic carbonate over polycarbonate using a double metal cyanide–quaternary ammonium salt catalyst system. Green Chem. 2009, 11, 1754–1757. [Google Scholar] [CrossRef]
- He, Z.; Yu, S.; Cai, Z.; Cao, X.; Zhang, L.; Huang, K. Modification of ZnCoPBA by different organic ligands and its application in the cycloaddition of CO2 and epoxides. J. Chem. Sci. 2022, 134, 35. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, X.; Du, B.; Fan, Z.; Qi, G. Synthesis of bis(cyclic carbonate) and propylene carbonate via a one-pot coupling reaction of CO2, bisepoxide and propylene oxide. RSC Adv. 2013, 3, 17307–17313. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Wei, R.-J.; Sun, X.-K.; Zhang, J.-F.; Du, B.-Y.; Fan, Z.-Q.; Qi, G.-R. Selective copolymerization of carbon dioxide with propylene oxide catalyzed by a nanolamellar double metal cyanide complex catalyst at low polymerization temperatures. Polymer 2011, 52, 5494–5502. [Google Scholar] [CrossRef]
- Khan, M.U.; Khan, S.U.; Kiriratnikom, J.; Zareen, S.; Zhang, X. CoCo-PBA/tetrabutylammonium bromide as highly efficient catalyst for CO2 and epoxides coupling reaction under mild conditions. Chin. Chem. Lett. 2022, 33, 1081–1086. [Google Scholar] [CrossRef]
- Khan, M.U.; Khan, S.U.; Kiriratnikom, J.; Zareen, S.; Zhang, X.-H. Mesoporous Prussian Blue as the highly effective and selective catalyst for CO2 conversion into cyclic carbonates under mild conditions. Polyhedron 2022, 214, 115640. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; Taffin de Givenchy, E.P.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Christy, S.; Noschese, A.; Lomelí-Rodriguez, M.; Greeves, N.; Lopez-Sanchez, J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, V.C.; Štangar, U.L.; Mušič, B.; Mishra, I.M.; Meng, Y. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts. Catal. Rev. 2021, 63, 363–421. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Fe–Zn double-metal cyanide complexes as novel, solid transesterification catalysts. J. Catal. 2006, 241, 34–44. [Google Scholar] [CrossRef]
- Song, Z.; Subramaniam, B.; Chaudhari, R.V. Transesterification of Propylene Carbonate with Methanol Using Fe–Mn Double Metal Cyanide Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 5698–5710. [Google Scholar] [CrossRef]
- Tran, C.H.; Kim, S.; Choi, H.-K.; Moon, B.-R.; Song, W.; Heo, J.Y.; Kim, I. Multi-role heterogeneous Zn–Co double metal cyanide catalysts valid for ring-opening polymerizations and hydrofunctionalizations. J. Ind. Eng. Chem. 2024, 137, 524–535. [Google Scholar] [CrossRef]
- Jadhav, A.R.; Bandal, H.A.; Kim, H. Synthesis of substituted amines: Catalytic reductive amination of carbonyl compounds using Lewis acid Zn–Co-double metal cyanide/polymethylhydrosiloxane. Chem. Eng. J. 2016, 295, 376–383. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel Strategies for the Production of Fuels, Lubricants, and Chemicals from Biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef]
- Belousov, A.S.; Esipovich, A.L.; Kanakov, E.A.; Otopkova, K.V. Recent advances in sustainable production and catalytic transformations of fatty acid methyl esters. Sustain. Energy Fuels 2021, 5, 4512–4545. [Google Scholar] [CrossRef]
- Len, C.; Duhan, V.; Ouyang, W.; Nguyen, R.; Lochab, B. Mechanochemistry and oleochemistry: A green combination for the production of high-value small chemicals. Front. Chem. 2023, 11, 1306182. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Ashrafur Rahman, S.M.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Qadeer, M.U.; Ayoub, M.; Komiyama, M.; Daulatzai, M.U.K.; Mukhtar, A.; Saqib, S.; Ullah, S.; Qyyum, M.A.; Asif, S.; Bokhari, A. Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process. J. Clean. Prod. 2021, 309, 127388. [Google Scholar] [CrossRef]
- Kumar, P.; Matoh, L.; Srivastava, V.C.; Štangar, U.L. Synthesis of zinc/ferrocyanide nano-composite catalysts having a high activity for transesterification reaction. Renew. Energy 2020, 148, 946–952. [Google Scholar] [CrossRef]
- Marquez, C.; Corbet, M.; Smolders, S.; Marion, P.; De Vos, D. Double metal cyanides as heterogeneous Lewis acid catalysts for nitrile synthesis via acid-nitrile exchange reactions. Chem. Commun. 2019, 55, 12984–12987. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Bugaev, A.L.; Pnevskaya, A.Y.; Janssens, K.; Marquez, C.; De Vos, D. Copper–cobalt double metal cyanides as green catalysts for phosphoramidate synthesis. Commun. Chem. 2023, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fu, Y.; Tricard, S.; Sun, A.; Mei, B.; Zheng, P.; Du, X.; Fang, J.; Zhao, J. Effects of several ionic liquids on the structures and catalytic properties of double metal cyanides. J. Mol. Liquids 2022, 345, 118252. [Google Scholar] [CrossRef]
- Wang, H.; Jin, T.; Tricard, S.; Peng, X.; Liang, K.; Zheng, P.; Fang, J.; Zhao, J. Enhancement of the Catalytic Activity of Double Metal Cyanides for the Oxidation of Styrene by the Presence of Included Alcohols. Langmuir 2022, 38, 8696–8707. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, Q.; Yu, L.; Gao, R.; Tong, Z.; Lu, C.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Double-metal cyanide as an acid and hydrogenation catalyst for the highly selective ring-rearrangement of biomass-derived furfuryl alcohol to cyclopentenone compounds. Green Chem. 2020, 22, 2549–2557. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef]
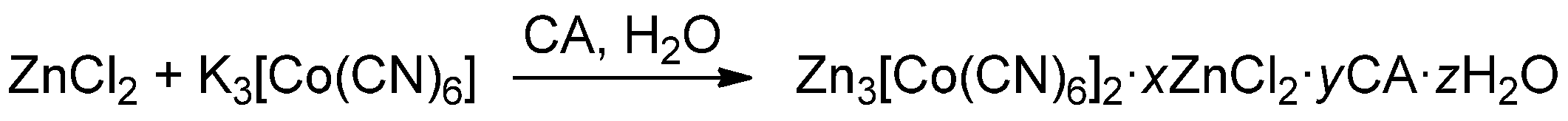



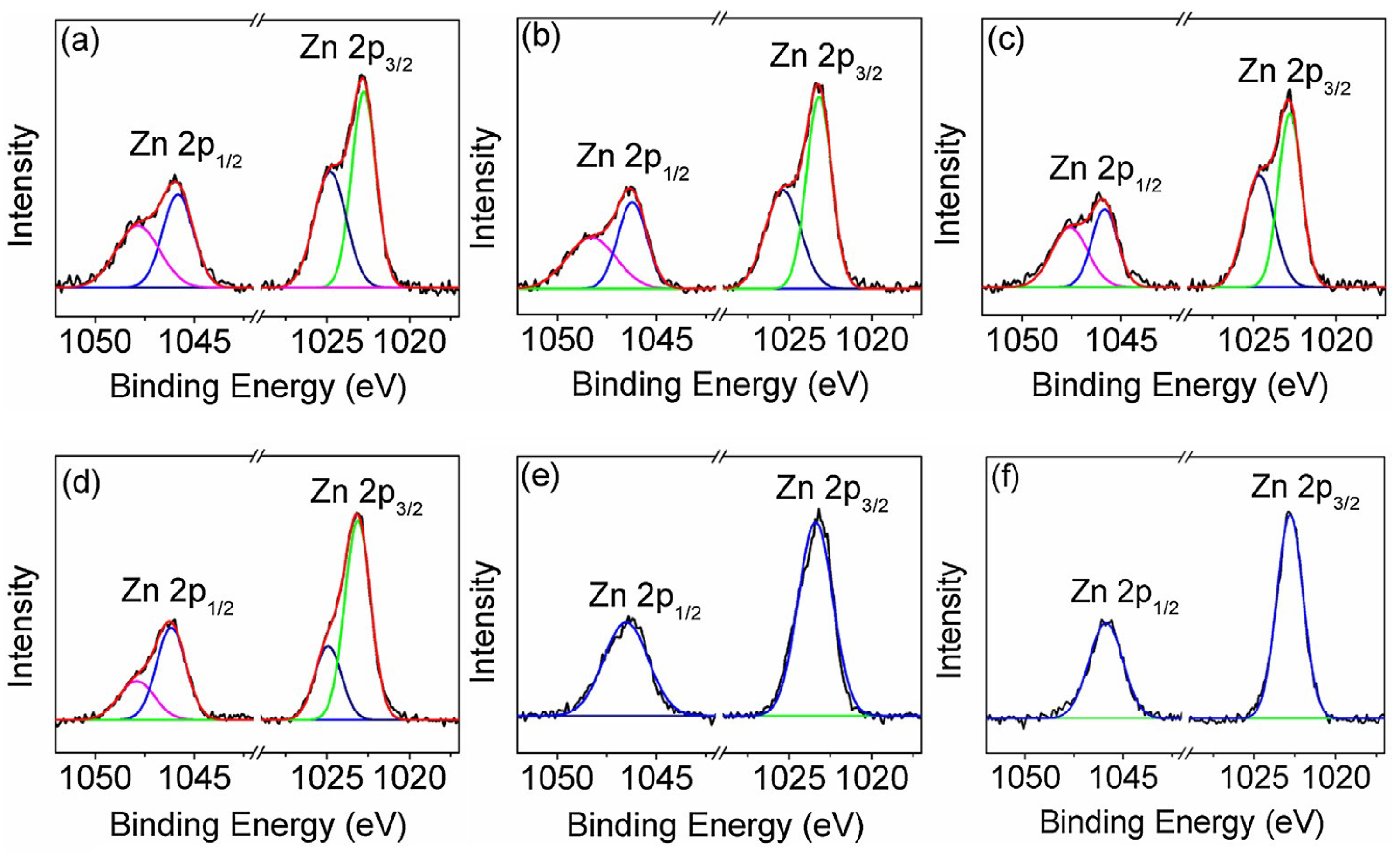




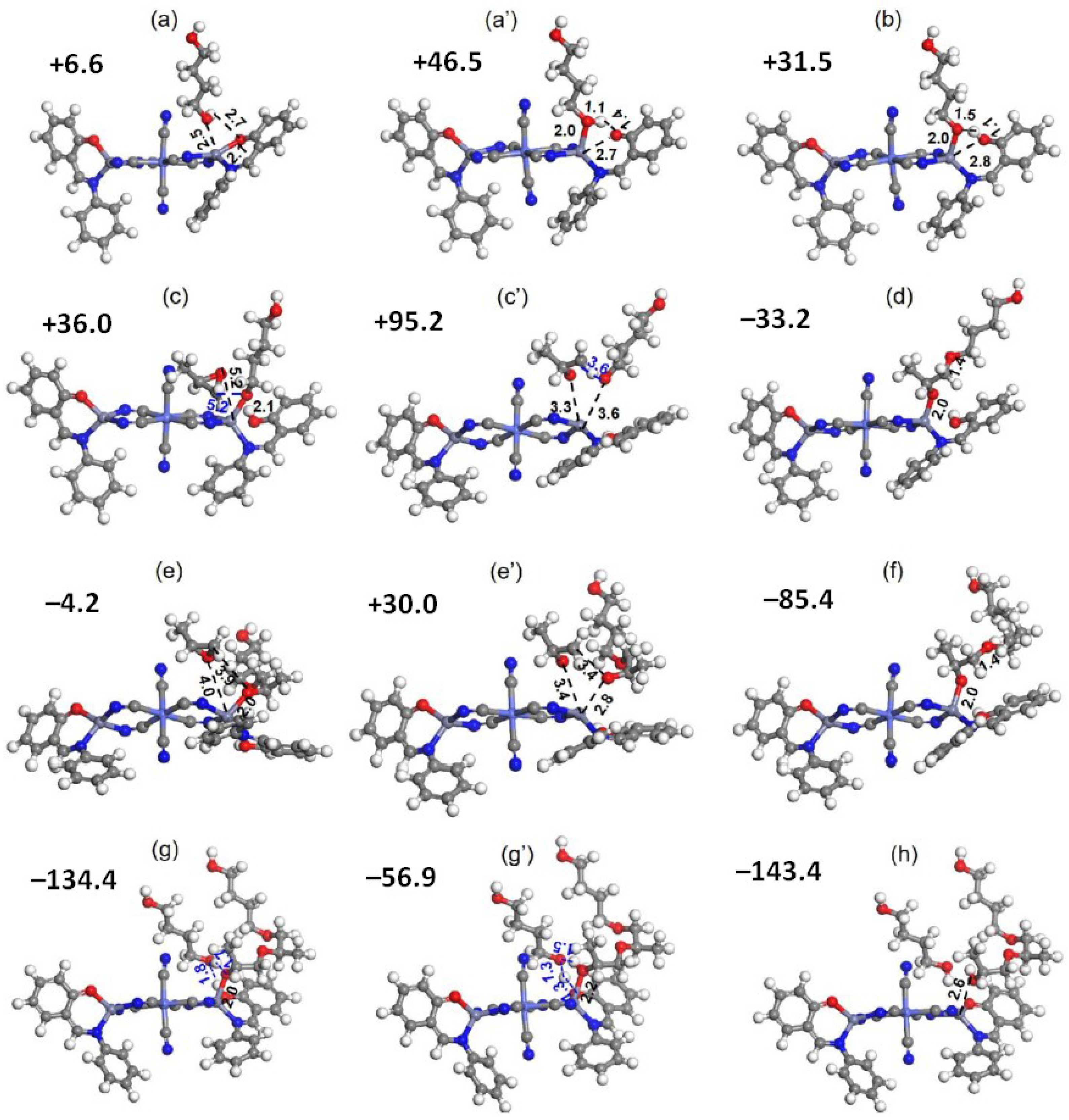










| Entry | Catalyst | Init. Time, min | Cat. Longevity, min | Polymer Yield, g | TOF, ([PO]∙ [Zn]−1∙h−1) | Unsatur. Level 2 | Mn, kDa (ÐM) |
|---|---|---|---|---|---|---|---|
| 1 | Benchmark DMC | 1 | 29 | 27.5 | 132,000 | 0.004 | 5.69 (4.88) |
| 2 | [DMC-Cl][MeOCMe2OH] | heating | 30 | 27.9 | 120,000 | 0.024 | 6.05 (1.18) |
| 3 | [DMC-Cl][tBuOH] | 4 | 29 | 28.4 | 126,000 | 0.022 | 6.17 (1.16) |
| 4 | DMC-OCMe2OMe | 3 | 25 | 23.0 | 119,000 | 0.004 | 2.62 (3.70) |
| 5 | DMC-(OCMe2OMe/Cl)(0.25/0.75) | 2 | 32 | 31.5 | 127,000 | 0.018 | 5.33 (1.40) |
| 6 | DMC-(OCMe2OMe/Cl)(0.5/0.5) | 3 | 30 | 29.9 | 129,000 | 0.014 | 6.11 (3.57) |
| 7 | DMC-(OCMe2OMe/Cl)(0.75/0.25) | 2 | 27 | 26.2 | 125,000 | 0.006 | 5.44 (2.00) |
| 8 | DMC-OtBu | heating | 27 | 26.6 | 127,000 | 0.004 | 6.07 (7.17) |
| 9 | DMC-(OtBu/Cl)(0.5/0.5) | 7 | 31 | 28.7 | 120,000 | 0.005 | 6.40 (6.44) |
| 10 | DMC-OAc (60 °C, evacuation) | 2 | 29 | 30.0 | 134,000 | 0.016 | 6.91 (2.53) |
| 11 | Zn2[Co(CN)6](OAc)·4H2O | 1 | 28 | 27.3 | 126,000 | 0.014 | 6.22 (11) |
| 12 | DMC-(OAc/OtBu)(0.95/0.08) | 0 | 30 | 31.9 | 137,000 | 0.013 | 7.15 (1.75) |
| 13 | DMC-(OAc/OtBu)(0.68/0.31) | 0 | 29 | 29.6 | 132,000 | 0.010 | 7.06 (4.02) |
| 14 | DMC-(OAc/OtBu)(0.42/0.57) | 4 | 28 | 28.2 | 130,000 | 0.007 | 6.48 (10.7) |
| 15 | DMC-(OAc/OtBu)(0.31/0.71) | heating | 24 | 24.9 | 134,000 | 0.005 | 6.10 (9.12) |
| 16 | DMC-(OAc/OtBu/Cl)(0.68/0.38/0.06) | 0 | 29 | 29.9 | 133,000 | 0.010 | 6.94 (4.17) |
| 17 | DMC-(OAc/OtBu/Cl)(0.74/0.24/0.10) | 1 | 29 | 30.1 | 134,000 | 0.008 | 6.30 (3.08) |
| 18 | DMC-(OAc/OtBu/Cl)(0.59/0.38/0.15) | 0 | 29 | 30.0 | 134,000 | 0.007 | 6.56 (3.32) |
| 19 | DMC-(OAc/OtBu/Cl)(0.41/0.43/0.28) | 1 | 28 | 29.2 | 134,000 | 0.005 | 6.28 (3.73) |
| 20 | DMC-(OAc/OtBu/Cl)(0.34/0.39/0.39) | 2 | 31 | 31.0 | 129,000 | 0.006 | 6.58 (3.42) |
| Entry | Catalyst | Init. Time, min | Polymer Yield, g | TOF, [PO]∙ [Zn]−1∙h−1; [CO2]∙ [Zn]−1∙h−1 | Cyclic Carbonate, wt% 2 | FCO2, mol% 3 | Mn, kDa (ÐM) |
|---|---|---|---|---|---|---|---|
| 1 | Benchmark DMC | 36 | 25.4 | 19,900; 2980 | 2.5 | 15 | 5760 (3.34) |
| 2 | [DMC-Cl][MeOCMe2OH] | 54 | 5.6 | 4070; 1060 | 25 | 26 | 1420 (3.04) |
| 3 | [DMC-Cl][tBuOH] | 25 | 3.1 | 2280; 547 | 16.2 | 24 | 1120 (1.78) |
| 4 | DMC-OCMe2OMe | 191 | 3.4 | 2640; 422 | 9.2 | 16 | 1270 (1.55) |
| 5 | DMC-OtBu | 111 | 25.9 | 20,200; 3030 | 1.3 | 15 | 6250 (4.04) |
| 6 | DMC-(OCMe2OMe/Cl)(0.5/0.5) | 42 | 6.7 | 4930; 1180 | 16 | 24 | 1630 (2.07) |
| 7 | DMC-(OtBu/Cl)(0.5/0.5) | 37 | 21.7 | 16,500; 3130 | 2.4 | 19 | 5450 (8.81) |
| 8 | DMC-OAc (60 °C, evacuation) | 39 | 18.7 | 14,800; 1920 | 4.9 | 13 | 3960 (1.76) |
| 9 | Zn2[Co(CN)6](OAc)·4H2O | 45 | 6.8 | 4850; 1410 | 7.4 | 29 | 1960 (1.73) |
| 10 | DMC-(OAc/OtBu)(0.95/0.08) | 13 | 26.4 | 18,500; 5910 | 3.5 | 32 | 5420 (1.43) |
| 11 | DMC-(OAc/OtBu)(0.68/0.31) | 15 | 28.4 | 20,800; 5190 | 4.0 | 25 | 3380 (5.39) |
| 12 | DMC-(OAc/OtBu)(0.42/0.57) | 31 | 19.7 | 14,400; 3600 | 3.4 | 25 | 5970 (4.20) |
| 13 | DMC-(OAc/OtBu)(0.31/0.71) | 49 | 19.3 | 11,500; 3040 | 2.4 | 21 | 4810 (9.32) |
| 14 | DMC-(OAc/OtBu/Cl)(0.68/0.38/0.06) | 21 | 24.0 | 17,800; 4090 | 2.7 | 23 | 5510 (5.41) |
| 15 | DMC-(OAc/OtBu/Cl)(0.74/0.24/0.10) | 17 | 25.8 | 19,000; 4560 | 1.1 | 24 | 5790 (3.33) |
| 16 | DMC-(OAc/OtBu/Cl)(0.59/0.38/0.15) | 15 | 26.3 | 19,500; 4480 | 1.8 | 23 | 5830 (2.63) |
| 17 | DMC-(OAc/OtBu/Cl)(0.41/0.43/0.28) | 22 | 25.6 | 19,100; 4200 | 2.8 | 22 | 6070 (2.71) |
| 18 | DMC-(OAc/OtBu/Cl)(0.34/0.39/0.39) | 14 | 23.0 | 17,500; 3320 | 3.2 | 19 | 5510 (3.28) |
| 19 4 | DMC-(OAc/OtBu)(0.95/0.08) | 20 | 18.2 | 12,000; 5040 | 3.7 | 42 | 4290 (1.43) |
| 20 5 | DMC-(OAc/OtBu)(0.95/0.08) | 7 | 22.2 | 15,400; 5100 | 3.5 | 33 | 4960 (1.86) |
| 21 6 | DMC-(OAc/OtBu)(0.95/0.08) | 13 | 25.3 | 34,100; 10,900 | 3.4 | 32 | 5420 (1.43) |
| 22 7 | DMC-(OAc/OtBu)(0.95/0.08) | 6 | 5.4 | 3480; 1600 | 3.2 | 46 | 35.50 (7.17) |
| Catalyst | TON 2 | TOF 3 | FCU 4, mol% | WPC 5, wt% | SCO2 6, % | SPO 6, % | RPEC 7, % | Mw, kDa | ÐM |
|---|---|---|---|---|---|---|---|---|---|
| Ni2[Fe(CN)6] | 93 | 4 | 14.9 | 1.4 | 94.2 | 99.1 | 92.4 | 3.6 | 5.4 |
| Co2[Fe(CN)6] | 223 | 9 | 29.3 | 5.4 | 88.2 | 96.2 | 67.0 | 20.2 | 4.0 |
| KFe[Fe(CN)6] | 170 | 7 | 13.5 | 19.8 | 46.6 | 86.6 | 85.3 | 14.0 | 5.1 |
| Zn2[Fe(CN)6] | 178 | 7 | 19.8 | 7.5 | 76.7 | 95.0 | 83.8 | 3.4 | 4.4 |
| Ni3[Co(CN)6]2 | 86 | 4 | 22.3 | 0.4 | 98.9 | 99.7 | 79.5 | 11.8 | 10.5 |
| Co3[Co(CN)6]2 | 544 | 23 | 20.0 | 4.2 | 87.4 | 97.2 | 71.9 | 68.6 | 4.1 |
| Fe3[Co(CN)6]2 | 428 | 18 | 16.3 | 8.4 | 73.6 | 94.5 | 75.1 | 85.4 | 6.3 |
| Ni3[Fe(CN)6]2 | 84 | 4 | 24.0 | 0.6 | 98.4 | 99.6 | 73.9 | 11.7 | 15.8 |
| Co3[Fe(CN)6]2 | 296 | 12 | 33.5 | 13.3 | 75.4 | 90.2 | 66.0 | 50.0 | 5.9 |
| Fe4[Fe(CN)6]3 | 162 | 7 | 17.3 | 43.1 | 26.2 | 67.2 | 83.3 | 6.0 | 8.4 |
| Zn3[Co(CN)6]2 | 1279 | 53 | 9.7 | 8.9 | 61.9 | 94.4 | 86.6 | 8.3 | 2.5 |
| Entry | Oxirane | Cyclic Carbonate | Time, h | Conv., % | Yield, % | TON 2 |
|---|---|---|---|---|---|---|
| 1 |  | 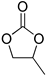 | 6 | 82 | 82 | 3075 |
| 2 |  | 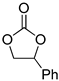 | 6 | 93 | 92 | 3486 |
| 3 | 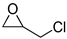 | 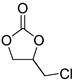 | 6 | 95 | 91 | 3563 |
| 4 |  | 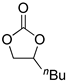 | 6 | 86 | 83 | 3225 |
| 5 |  | 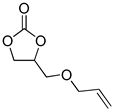 | 4 | 92 | 91 | 3450 |
| 6 |  | 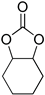 | 12 | 47 | 46 | 1763 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.E.; Ivchenko, P.V. Synthesis, Structure, and Actual Applications of Double Metal Cyanide Catalysts. Int. J. Mol. Sci. 2024, 25, 10695. https://doi.org/10.3390/ijms251910695
Nifant’ev IE, Ivchenko PV. Synthesis, Structure, and Actual Applications of Double Metal Cyanide Catalysts. International Journal of Molecular Sciences. 2024; 25(19):10695. https://doi.org/10.3390/ijms251910695
Chicago/Turabian StyleNifant’ev, Ilya E., and Pavel V. Ivchenko. 2024. "Synthesis, Structure, and Actual Applications of Double Metal Cyanide Catalysts" International Journal of Molecular Sciences 25, no. 19: 10695. https://doi.org/10.3390/ijms251910695
APA StyleNifant’ev, I. E., & Ivchenko, P. V. (2024). Synthesis, Structure, and Actual Applications of Double Metal Cyanide Catalysts. International Journal of Molecular Sciences, 25(19), 10695. https://doi.org/10.3390/ijms251910695









