Effects of Tooth Desensitizers on Streptococcus mutans Biofilm Formation Using a Modified Robbins Device Flow Cell System
Abstract
:1. Introduction
2. Results
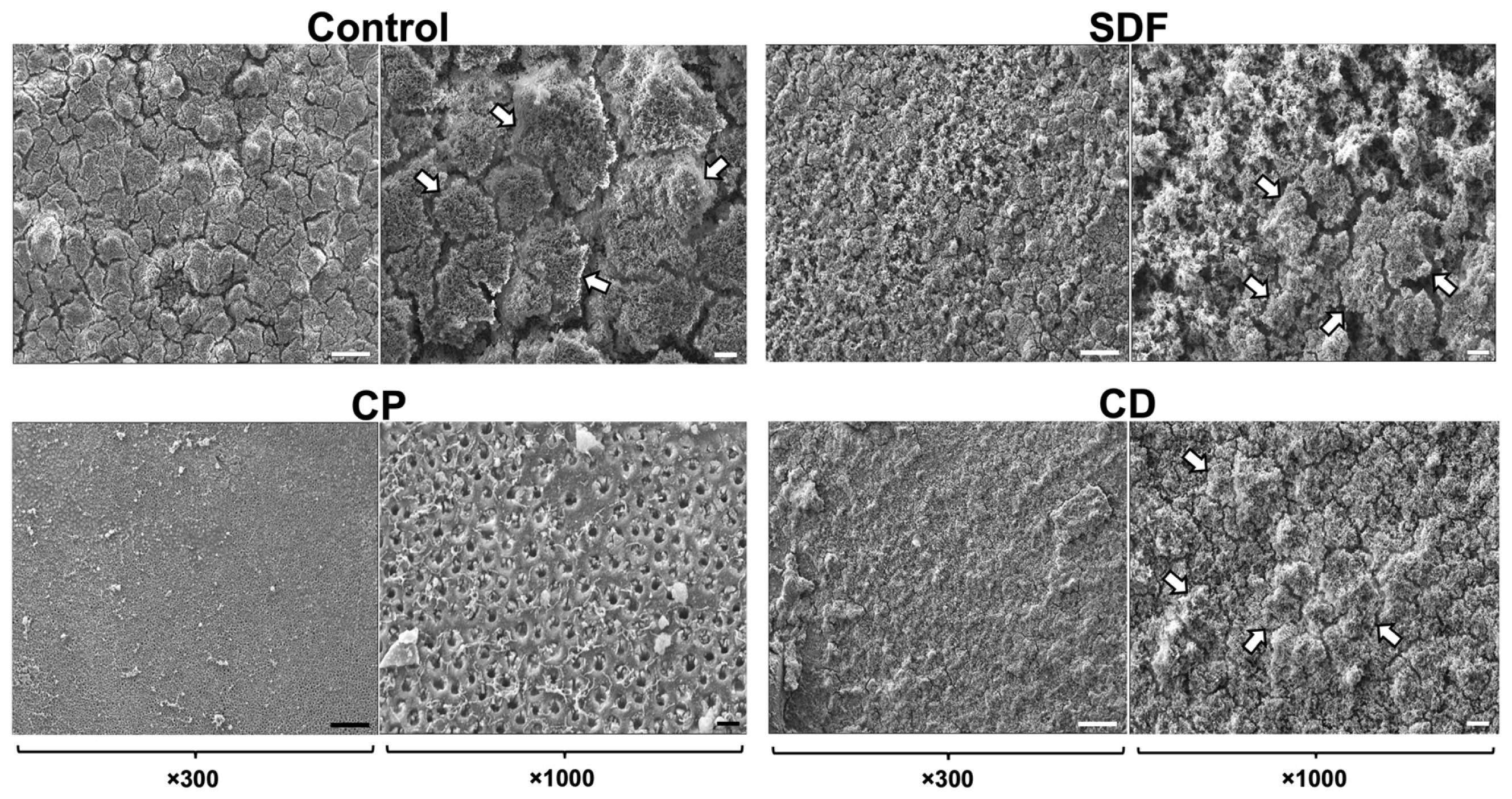
2.1. Scanning Electron Microscopy Observation
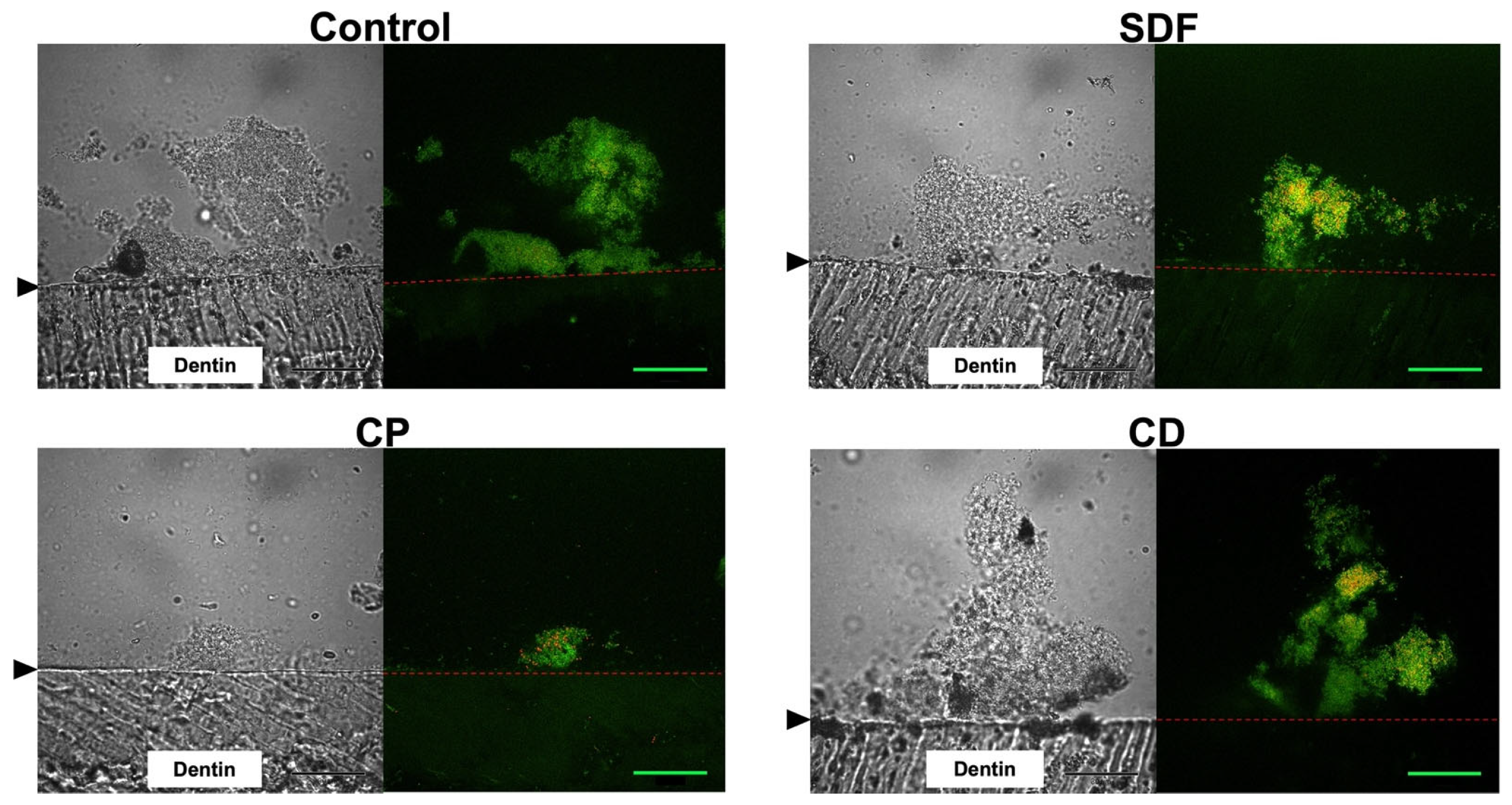
2.2. Confocal Laser Scanning Microscopy Observation
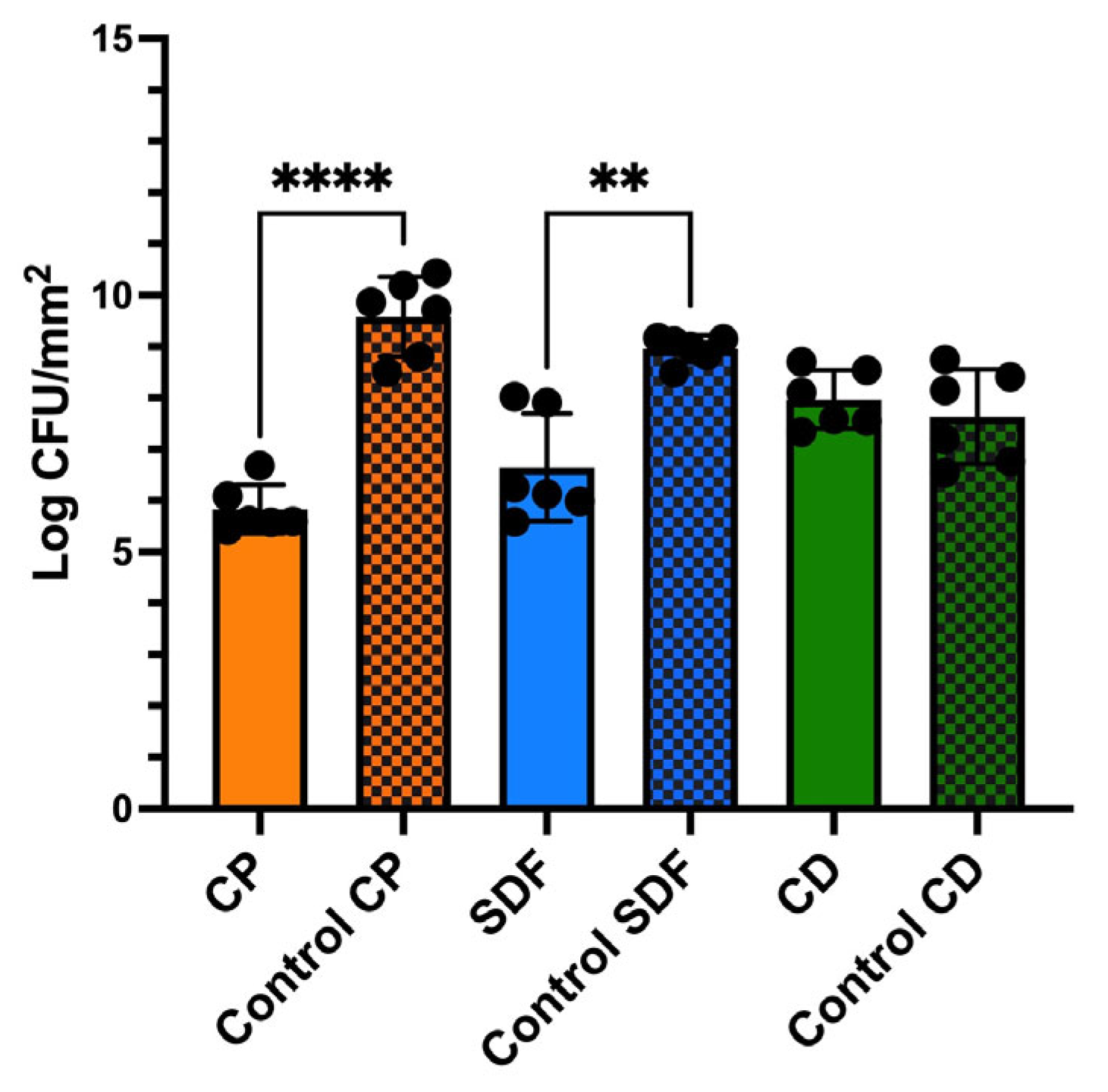
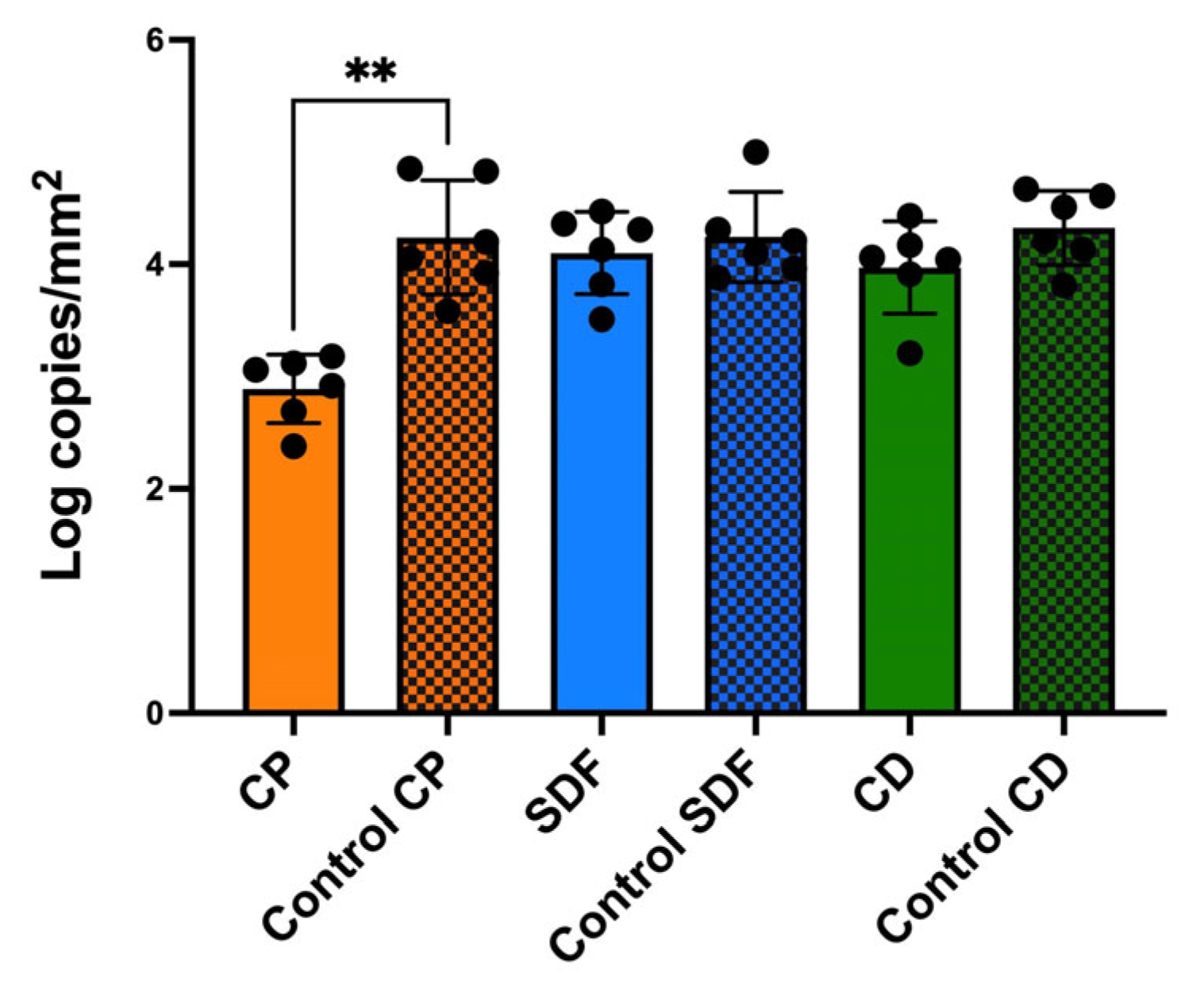
2.3. Viable and Total Cell Counts of S. mutans Biofilm
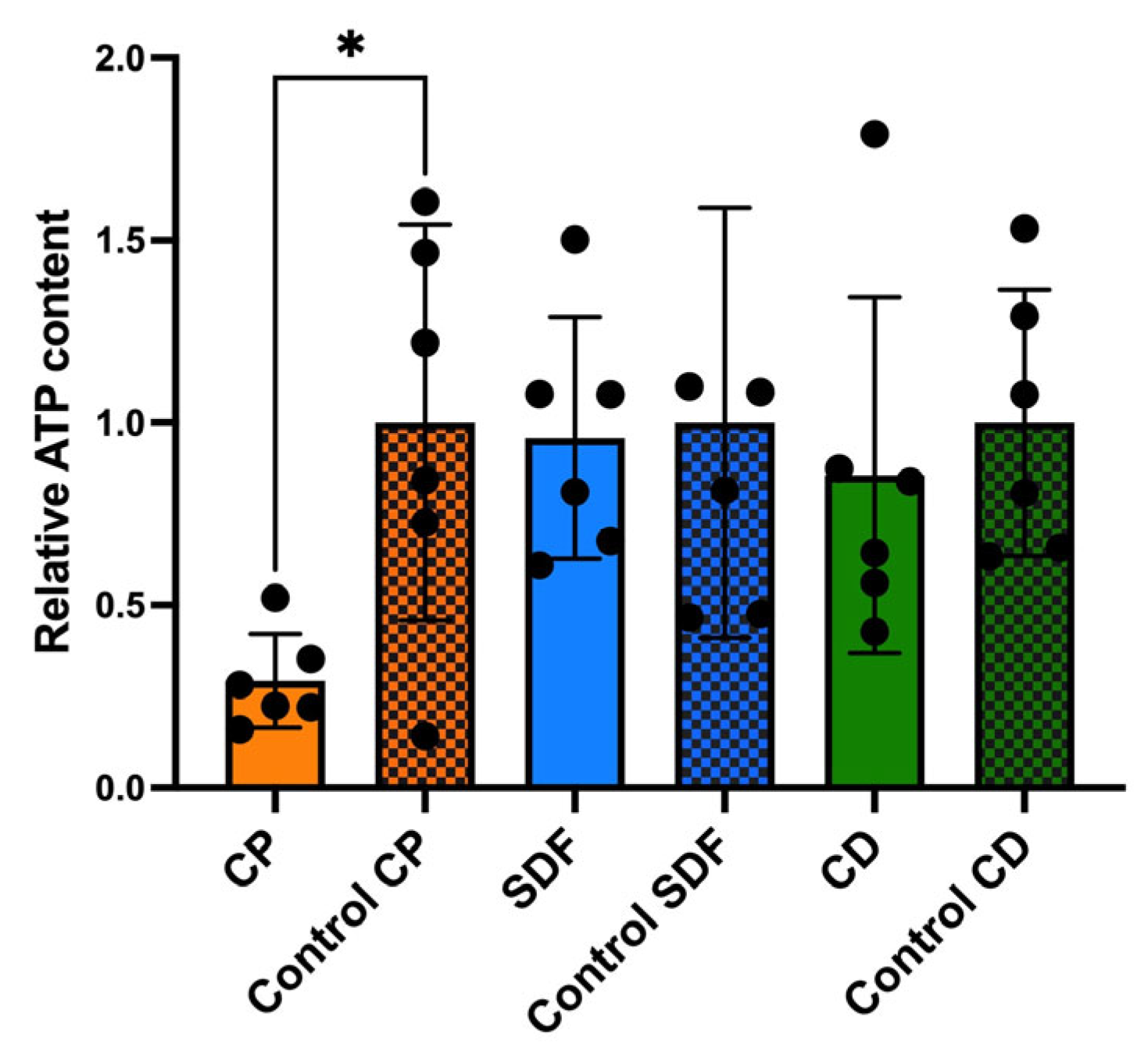
2.4. Relative Adenosine Triphosphate Bioluminescence Content
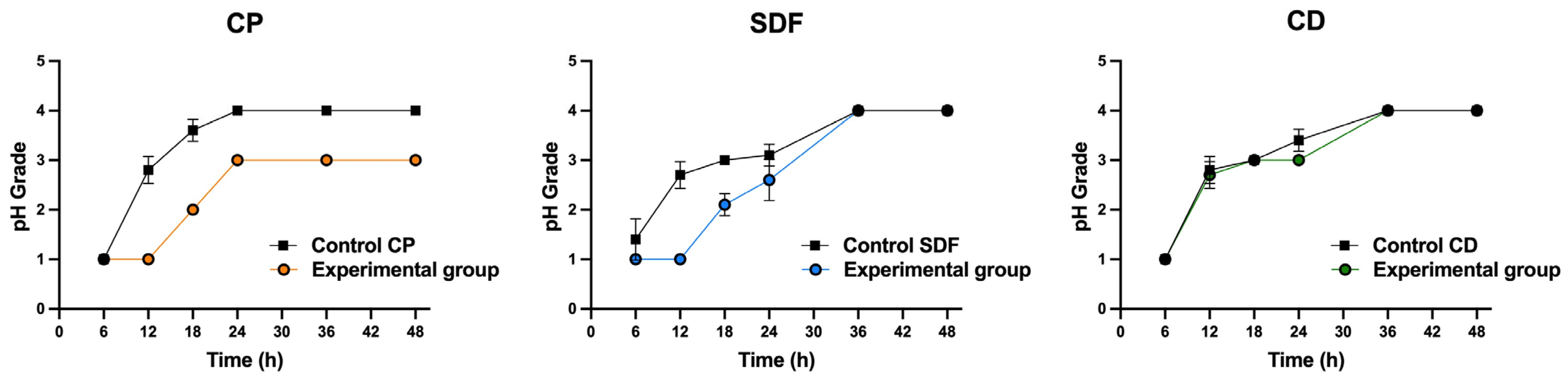
2.5. Acid Production
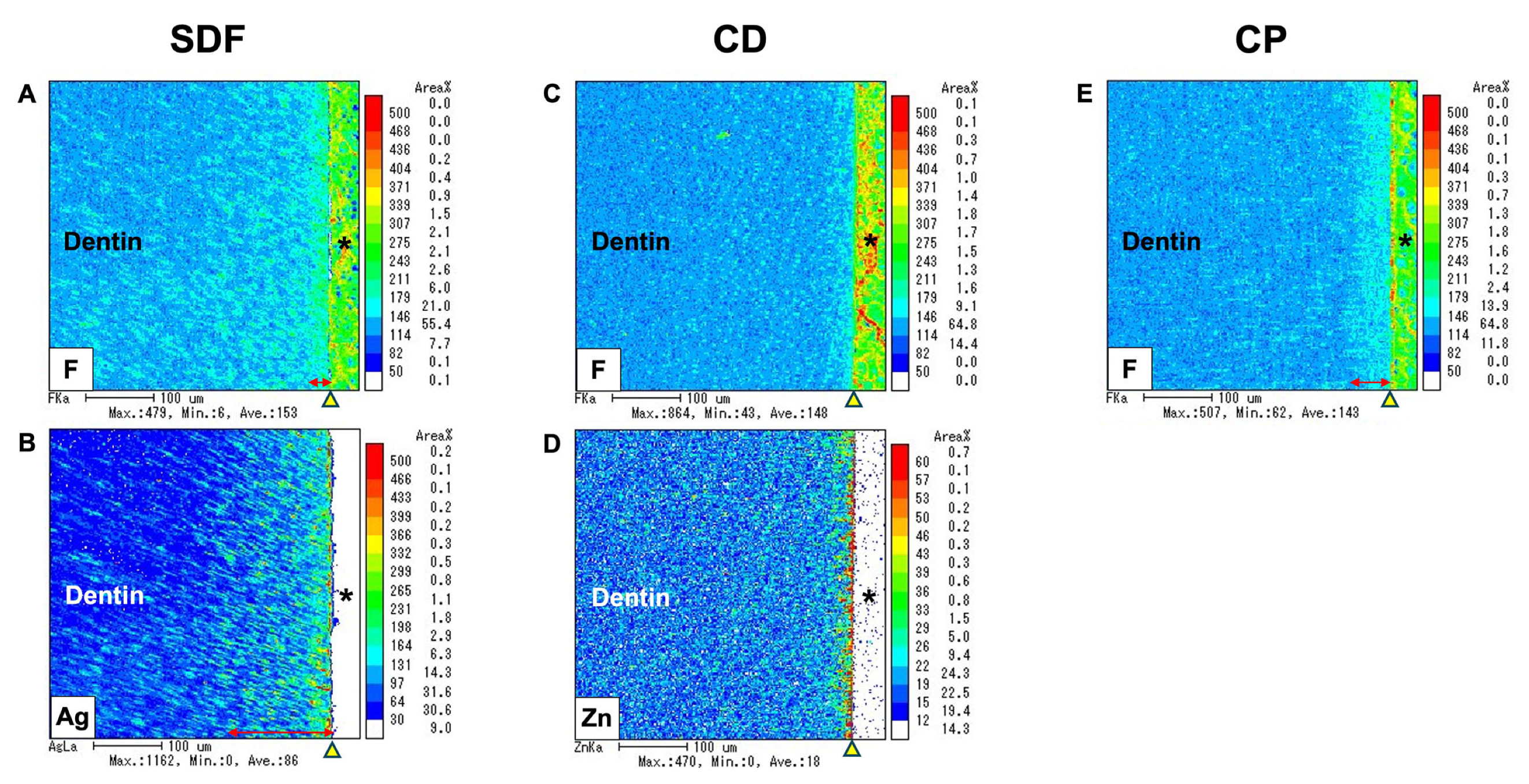
2.6. Electron Probe Microanalyzer
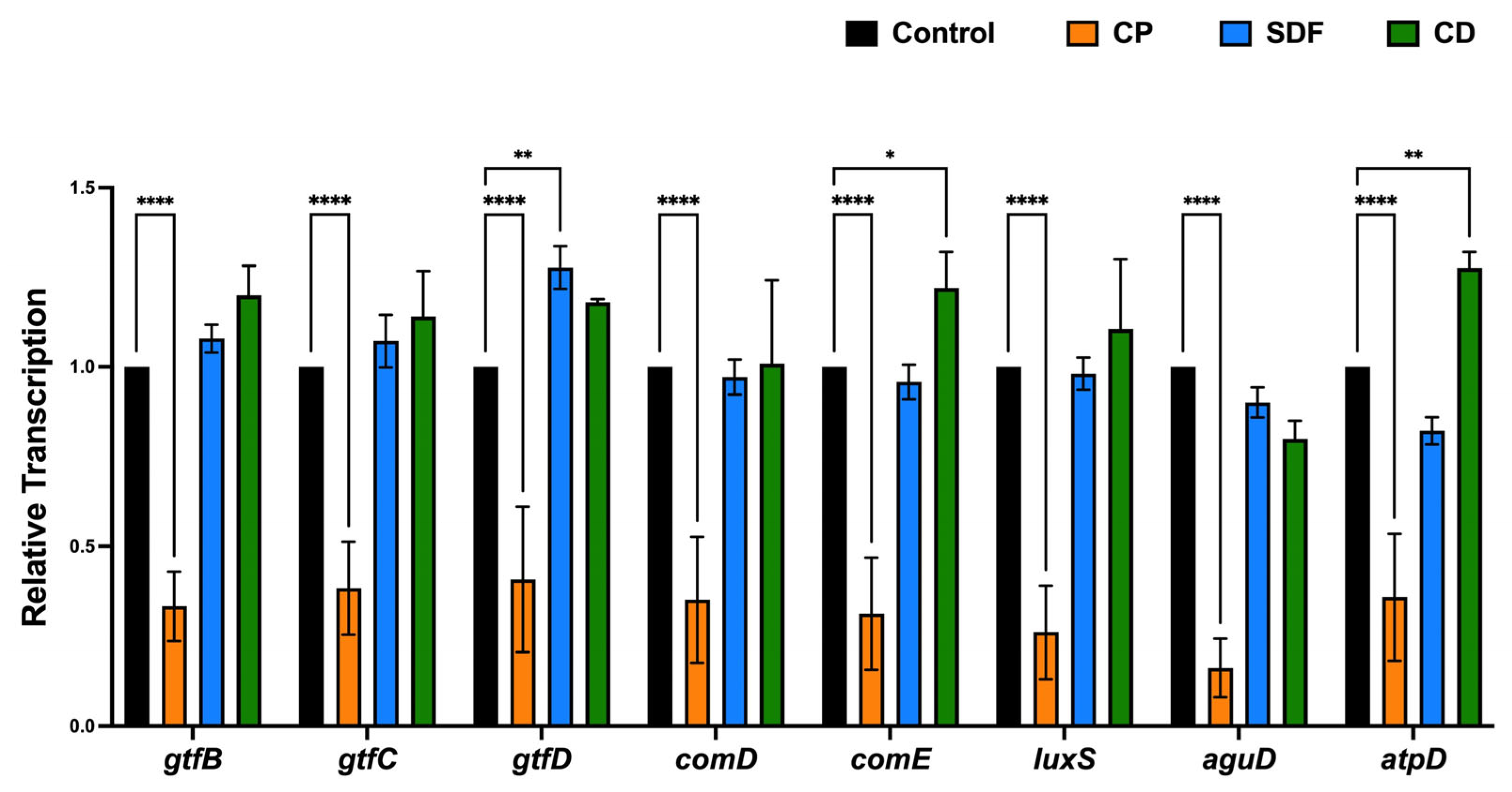
2.7. Gene Expression Analysis
3. Discussion
4. Materials and Methods
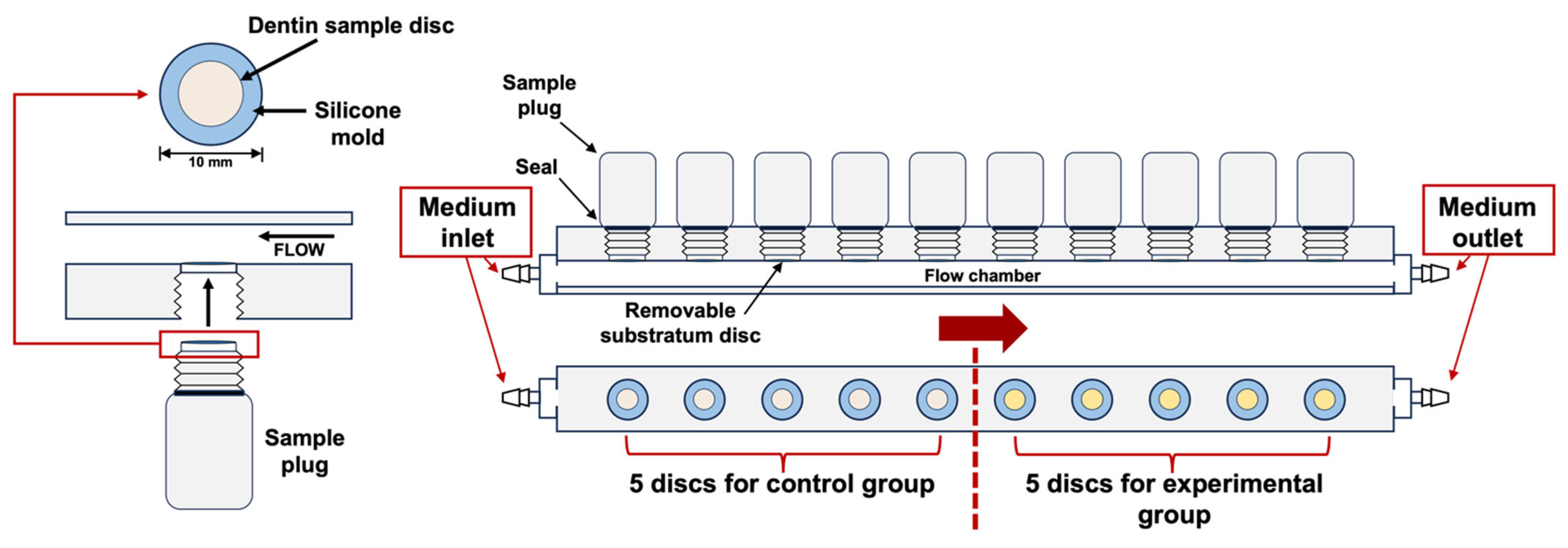
4.1. Specimen Preparation
4.2. Bacteria and Biofilm Formation
4.3. Morphological Observation Using Scanning Electron Microscopy
4.4. Fluorescent Staining and CLSM Observation
4.5. Viable and Total Cell Counting
4.6. Adenosine Triphosphate Bioluminescence Assay
4.7. Acid Production Testing
4.8. EPMA
4.9. Investigation of Gene Expression Related to Bacterial Adhesion
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addy, M. Dentine hypersensitivity: New perspectives on an old problem. Int. Dent. J. 2002, 52, 367–375. [Google Scholar] [CrossRef]
- Wolff, M.S. Dentin hypersensitivity, the biofilm and remineralization: What is the connection? Adv. Dent. Res. 2009, 21, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Splieth, C.H.; Tachou, A. Epidemiology of dentin hypersensitivity. Clin. Oral Investig. 2013, 17, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Michelich, V.J.; Schuster, G.S.; Pashley, D.H. Bacterial penetration of human dentin in vitro. J. Dent. Res. 1980, 59, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Sumitani, M.; Takeuchi, H.; Shimahara, T.; Tsubakimoto, K.; Tsutsui, M. Salivary, serum, and microbial components of human carious dentin. J. Dent. Res. 1972, 51, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, P.A.; De Boever, J.A.; Loesche, W.J. Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans. A reservoir of periodontopathic bacteria. J. Periodontol. 1988, 59, 222–230. [Google Scholar] [CrossRef]
- Love, R.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef]
- Bergenholtz, G. Effect of bacterial products on inflammatory reactions in the dental pulp. Eur. J. Oral Sci. 1977, 85, 122–129. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F.; Abdelsayed, R.A.; Lio, S.G.; Rôças, I.N. Bacterial invasion of pulp blood vessels in teeth with symptomatic irreversible pulpitis. J. Endod. 2021, 47, 1854–1864. [Google Scholar] [CrossRef]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants. Mater. Res. Express. 2020, 7, 015417. [Google Scholar] [CrossRef]
- Minkiewicz-Zochniak, A.; Jarzynka, S.; Iwańska, A.; Strom, K.; Iwańczyk, B.; Bartel, M.; Mazur, M.; Pietruczuk-Padzik, A.; Konieczna, M.; Augustynowicz-Kopeć, E.; et al. Biofilm formation on dental implant biomaterials by Staphylococcus aureus strains isolated from patients with cystic fibrosis. Materials 2021, 14, 2030. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-N.; Kim, J.-B.; Jeong, S.-H. Remineralization effects when using different methods to apply fluoride varnish in vitro. J. Dent. Sci. 2018, 13, 360–366. [Google Scholar] [CrossRef]
- Eyüboğlu, G.B.; Naiboğlu, P. Clinical efficacy of different dentin desensitizers. Oper. Dent. 2020, 45, E317–E333. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, T.; Tanaka, K.; Iijima, Y. Inhibition of dentine demineralization by zinc oxide: In vitro and in situ studies. Dent. Mater. 2006, 21, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Andrea, B.; Carolina, M.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic action of zinc hydroxyapatite on remineralization of enamel and dentin: A review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Mneimne, M.; Hill, R.; Al-Jawad, M.; Lynch, R.; Anderson, P. Physical chemical effects of zinc on in vitro enamel demineralization. J. Dent. 2014, 42, 1096–1104. [Google Scholar] [CrossRef]
- Matsuura, T.; Mae, M.; Ohira, M.; Mihara, Y.; Yamashita, Y.; Sugimoto, K.; Yamada, S.; Yoshimura, A. The efficacy of a novel zinc-containing desensitizer CAREDYNE shield for cervical dentin hypersensitivity: A pilot randomized controlled trial. BMC Oral Health 2022, 22, 294. [Google Scholar] [CrossRef]
- Matsuura, T.; Mae, M.; Ohira, M.; Yamashita, Y.; Nakazono, A.; Sugimoto, K.; Yanagiguchi, K.; Yamada, S. The efficacy of the novel zinc-containing desensitizer CAREDYNE shield on dentin hypersensitivity: A study protocol for a pilot randomized controlled trial. Trials 2020, 21, 464. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Shimada, Y.; Shinno, Y.; Ono, S.; Yamaji, K.; Ohara, N.; Sadr, A.; Sumi, Y.; Tagami, J.; Yoshiyama, M. Assessment of demineralization inhibition effects of dentin desensitizers using swept-source optical coherence tomography. Materials 2021, 14, 1876. [Google Scholar] [CrossRef]
- Piovesan, É.T.A.; Alves, J.B.; Ribeiro, C.; Massignan, C.; Bezerra, A.C.B.; Leal, S.C. Is silver diamine fluoride effective in reducing dentin hypersensitivity? A systematic review. J. Dent. Res. Dent. Clin. Dent. Prospect 2023, 17, 63–70. [Google Scholar] [CrossRef]
- Contreras, V.; Toro, M.J.; Elías-Boneta, A.R.; Encarnación-Burgos, A. Effectiveness of silver diamine fluoride in caries prevention and arrest: A systematic literature review. Gen. Dent. 2017, 65, 22–29. [Google Scholar] [PubMed]
- Ruff, R.R.; Barry Godín, T.J.; Niederman, R. Noninferiority of silver diamine fluoride vs sealants for reducing dental caries prevalence and incidence: A randomized clinical trial. JAMA Pediatr. 2024, 178, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Owais, A.I.; Lu, G.; Keratithamkul, K.; Kanellis, M.J.; Haes, A.J. Silver diamine fluoride chemical mechanisms of action as a caries arresting and preventing agent. J. Calif. Dent. Assoc. 2018, 46, 113–120. [Google Scholar] [CrossRef]
- Trieu, A.; Mohamed, A.; Lynch, E. Silver diamine fluoride versus sodium fluoride for arresting dentine caries in children: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 2115. [Google Scholar] [CrossRef]
- Gao, S.S.; Chen, K.J.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Arresting early childhood caries using silver and fluoride products—A randomised trial. J. Dent. 2020, 103, 103522. [Google Scholar] [CrossRef]
- Linton, C.J.; Sherriff, A.; Millar, M.R. Use of a modified robbins device to directly compare the adhesion of Staphylococcus epidermidis rp62a to surfaces. J. Appl. Microbiol. 1999, 86, 194–202. [Google Scholar] [CrossRef]
- Luo, T.L.; Vanek, M.E.; Gonzalez-Cabezas, C.; Marrs, C.F.; Foxman, B.; Rickard, A.H. In vitro model systems for exploring oral biofilms: From single-species populations to complex multi-species communities. J. Appl. Microbiol. 2022, 132, 855–871. [Google Scholar] [CrossRef]
- Noiri, Y.; Okami, Y.; Narimatsu, M.; Takahashi, Y.; Kawahara, T.; Ebisu, S. Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J. Periodontol. 2003, 74, 1647–1651. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Rayner, J.C.; Stoodley, P.; Lappin-Scott, H.M. Establishment of experimental biofilms using the modified robbins device and flow cells. In Environmental Monitoring of Bacteria; Edwards, C., Ed.; Springer: Cham, Switzerland, 1999; pp. 307–319. [Google Scholar]
- Coenye, T.; Prijck, K.; Wever, B.; Nelis, H.J. Use of the modified robbins device to study the in vitro biofilm removal efficacy of nitradinetm, a novel disinfecting formula for the mantainance of oral medical devices. J. Appl. Microbiol. 2008, 105, 733–740. [Google Scholar] [CrossRef]
- Erkmen Almaz, M. Antibacterial activity of fluoride varnishes containing different agents in children with severe early childhood caries: A randomised controlled trial. Clin. Oral Investig. 2020, 24, 2129–2136. [Google Scholar] [CrossRef]
- Han, Y. Effects of brief sodium fluoride treatments on the growth of early and mature cariogenic biofilms. Sci. Rep. 2021, 11, 18290. [Google Scholar] [CrossRef] [PubMed]
- Damé-Teixeira, N.; Deng, D.; Do, T. Streptococcus mutans transcriptome in the presence of sodium fluoride and sucrose. Arch. Oral Biol. 2019, 102, 186–192. [Google Scholar] [CrossRef]
- Pandit, S.; Kim, J.-E.; Jung, K.-H.; Chang, K.-W.; Jeon, J.-G. Effect of sodium fluoride on the virulence factors and composition of Streptococcus mutans biofilms. Arch. Oral Biol. 2011, 56, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Pessan, J.P.; Honório, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral Sci. 2011, 22, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Vinson, L.A.; Gilbert, P.R.; Sanders, B.J.; Moser, E.; Gregory, R.L. Silver diamine fluoride and potassium iodide disruption of in vitro Streptococcus mutans biofilm. J. Dent. Child. 2018, 85, 120–124. [Google Scholar]
- Takahashi, M.; Matin, K.; Matsui, N.; Shimizu, M.; Tsuda, Y.; Uchinuma, S.; Hiraishi, N.; Nikaido, T.; Tagami, J. Effects of silver diamine fluoride preparations on biofilm formation of Streptococcus mutans. Dent. Mater. J. 2021, 40, 911–917. [Google Scholar] [CrossRef]
- Kumagai, T.; Kashiwamura, H.; Katsumata, M.; Ozaki, M. Verification of antibacterial activity to enamel surfaces of new type of surface coating. Pediatr. Dent. J. 2021, 31, 86–91. [Google Scholar] [CrossRef]
- Abdullah, N.; Al-Marzooq, F.; Id, S.; Abd Rahman, N.; Kg, A.R.; Ngo, H.; Samaranayake, L. The antibacterial efficacy of silver diamine fluoride (sdf) is not modulated by potassium iodide (ki) supplements: A study on in-situ plaque biofilms using viability real-time pcr with propidium monoazide. PLoS ONE 2020, 15, e0241519. [Google Scholar] [CrossRef]
- Lynch, R.; Churchley, D.; Butler, A.; Kearns, S.; Thomas, G.V.; Badrock, T.C.; Cooper, L.; Higham, S. Effects of zinc and fluoride on the remineralisation of artificial carious lesions under simulated plaque fluid conditions. Caries Res. 2011, 45, 313–322. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Rabieh, S.; Janal, M.N.; Rasamimari, S.; Bromage, T.G. Silver and fluoride content and short-term stability of 38% silver diamine fluoride. J. Am. Dent. Assoc. 2019, 150, 140–146. [Google Scholar] [CrossRef]
- Chen, K.J.; Gao, S.; Duangthip, D.; Lo, E.; Chu, C.-H. Randomized clinical trial on sodium fluoride with tricalcium phosphate. J. Dent. Res. 2021, 100, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Effect of silver nitrate and sodium fluoride with tri-calcium phosphate on Streptococcus mutans and demineralised dentine. Int. J. Mol. Sci. 2018, 19, 1288. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Lee, H.-C.; Lee, J.-Y.; Jin, B.-H. Remineralization ability of fluoride varnish containing tricalcium phosphate by time. J. Korean. Acad. Oral Health 2017, 41, 3–8. [Google Scholar] [CrossRef]
- Elkassas, D.; Arafa, A. Remineralizing efficacy of different calcium-phosphate and fluoride based delivery vehicles on artificial caries like enamel lesions. J. Dent. 2014, 42, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Yamamoto, H.; Naito, K.; Kanda, H.; Takahashi, Y.; Hayashi, M. Analyzing the anti-caries effect of fluoride varnish containing tricalcium phosphate using pixe/pige. Dent. Mater. J. 2023, 42, 591–597. [Google Scholar] [CrossRef]
- Wang, Y.; Hoffmann, J.P.; Baker, S.M.; Bentrup, K.H.Z.; Wimley, W.C.; Fuselier, J.A.; Bitoun, J.P.; Morici, L.A. Inhibition of Streptococcus mutans biofilms with bacterial-derived outer membrane vesicles. BMC Microbiol. 2021, 21, 234. [Google Scholar] [CrossRef]
- Ham, S.Y.; Kim, H.S.; Cha, E.; Lim, T.; Byun, Y.; Park, H.D. Raffinose inhibits Streptococcus mutans biofilm formation by targeting glucosyltransferase. Microbiol. Spectr. 2022, 10, e0207621. [Google Scholar] [CrossRef]
- Kornsombut, N.; Takenaka, S.; Sotozono, M.; Nagata, R.; Ida, T.; Manuschai, J.; Saito, R.; Takahashi, R.; Noiri, Y. Antibiofilm properties and demineralization suppression in early enamel lesions using dental coating materials. Antibiotics 2024, 13, 106. [Google Scholar] [CrossRef]
- Takenaka, S.; Oda, M.; Domon, H.; Ohsumi, T.; Suzuki, Y.; Ohshima, H.; Yamamoto, H.; Terao, Y.; Noiri, Y. Vizantin inhibits bacterial adhesion without affecting bacterial growth and causes Streptococcus mutans biofilm to detach by altering its internal architecture. Biochem. Biophys. Res. Commun. 2016, 480, 173–179. [Google Scholar] [CrossRef]
- Naksagoon, T.; Takenaka, S.; Nagata, R.; Sotozono, M.; Ohsumi, T.; Ida, T.; Edanami, N.; Maeda, T.; Noiri, Y. A repeated state of acidification enhances the anticariogenic biofilm activity of glass ionomer cement containing fluoro-zinc-silicate fillers. Antibiotics 2021, 10, 977. [Google Scholar] [CrossRef]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of clearfil protect bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Takenaka, S.; Wakamatsu, R.; Ohsumi, T.; Domon, H.; Ohshima, H.; Terao, Y.; Noiri, Y. A horizontal sequential cutting method to estimate the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J. Endod. 2019, 45, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin. Oral Investig. 2020, 24, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Asahi, Y.; Noiri, Y.; Igarashi, J.; Suga, H.; Azakami, H.; Ebisu, S. Synergistic effects of antibiotics and an n-acyl homoserine lactone analog on porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2012, 112, 404–411. [Google Scholar] [CrossRef]
- Rodis, O.M.M.; Ji, Y.; Matsumura, S.; Shimono, T.; Okazaki, Y. Comparison of plaque samples and saliva samples using the cat21 test® (cariostat method). Pediatr. Dent. J. 2005, 15, 6–9. [Google Scholar] [CrossRef]
- Sakaue, Y.; Takenaka, S.; Ohsumi, T.; Domon, H.; Terao, Y.; Noiri, Y. The effect of chlorhexidine on dental calculus formation: An in vitro study. BMC Oral Health 2018, 18, 52. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takenaka, S.; Oda, M.; Domon, H.; Hiyoshi, T.; Sasagawa, K.; Ohsumi, T.; Hayashi, N.; Okamoto, Y.; Yamamoto, H.; et al. Sulfated vizantin causes detachment of biofilms composed mainly of the genus Streptococcus without affecting bacterial growth and viability. BMC Microbiol. 2020, 20, 361. [Google Scholar] [CrossRef]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents. 2012, 39, 390–395. [Google Scholar] [CrossRef]
- Jeon, J.-G.; Klein, M.I.; Xiao, J.; Gregoire, S.; Rosalen, P.L.; Koo, H. Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiol. 2009, 9, 228. [Google Scholar] [CrossRef]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory effects of ethyl gallate on Streptococcus mutans biofilm formation by optical profilometry and gene expression analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef]
| Desensitizer | Composition | Application Procedure | Manufacturer |
|---|---|---|---|
| Saforide (SDF) | Silver diamine fluoride (SDF); 38% SDF consists of silver and fluoride ions stabilized by ammonia | Clean and dry the dentin surface. Apply SDF using a microbrush or cotton pellet for at least 1 min. Allow it to air dry and avoid rinsing post-application. Protective measures include the isolation of soft tissues with petroleum jelly. Reapply biannually for optimal results. | Bee Brand Medico Dental Co., Ltd., Osaka, Japan Lot number: 104TA |
| Caredyne Shield (CD) | Fluoro-zinc-silicate glass | Clean the area and isolate with cotton rolls. Mix two equal proportions of solution A and B and apply to the dentin surface using a microbrush for 20 s. Rinse with water after application. | GC Corporation, Tokyo, Japan Lot number: 2210261 |
| Clinpro White Varnish (CP) | 5% sodium fluoride (NaF) and tricalcium phosphate (TCP) | Clean and dry the teeth. Apply the varnish using a brush or applicator directly onto the dentin. The product is saliva-activated, adhering to both dry and moist teeth. | 3M Dental, St. Paul, MN, USA Lot number: NF27309 |
| Gene Name | Nucleotide Sequence | Reference |
|---|---|---|
| 16s rRNA | F: 5′-CCATGTGTAGCGGTGAAATGC-3′ R: 5′-TCATCGTTTACGGCGTGGAC-3′ | [59] |
| gtfB | F: 5′-AGCCGAAAGTTGGTATCGTCC-3′ R: 5′-TGACGCTGTGTTTCTTGGCTC-3′ | [59] |
| gtfC | F: 5′-TTCCGTCCCTTATTGATGACATG-3′ R: 5′-AATTGAAGCGGACTGGTTGCT-3′ | [59] |
| gtfD | F:5′-ACAGCAGACAGCAGCCAAGA-3′ R: 5′-ACTGGGTTTGCTGCGTTTG-3′ | [59] |
| comD | F: 5′-TTCCTGCAAACTCGATCATATAGG-3′ R: 5′-TGCCAGTTCTGACTTGTTTAGGC-3′ | [59] |
| comE | F: 5′-TTCCTCTGATTGACCATTCTTCTG-3′ R: 5′-GAGTTTATGCCCCTCACTTTTCAG-3′ | [59] |
| luxS | F: 5′-CCAGGGACATCTTTCCATGAGAT-3′ R: 5′-ACGGGATGATTGACTGTTCCC-3′ | [59] |
| aguD | F: 5′-ATCCCGTGAGTGATAGTATTTG-3′ R: 5′-CAAGCCACCAACAAGTAAGG-3′ | [60] |
| atpD | F: 5′-ACTGGGTTTGCTGCGTTTG-3′ R: 5′-CCAGGCGGTTCATTCATCTGAC-3′ | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornsombut, N.; Takenaka, S.; Manuschai, J.; Sotozono, M.; Nagata, R.; Ida, T.; Sato, R.; Saito, R.; Takahashi, R.; Sato, D.; et al. Effects of Tooth Desensitizers on Streptococcus mutans Biofilm Formation Using a Modified Robbins Device Flow Cell System. Int. J. Mol. Sci. 2024, 25, 10703. https://doi.org/10.3390/ijms251910703
Kornsombut N, Takenaka S, Manuschai J, Sotozono M, Nagata R, Ida T, Sato R, Saito R, Takahashi R, Sato D, et al. Effects of Tooth Desensitizers on Streptococcus mutans Biofilm Formation Using a Modified Robbins Device Flow Cell System. International Journal of Molecular Sciences. 2024; 25(19):10703. https://doi.org/10.3390/ijms251910703
Chicago/Turabian StyleKornsombut, Niraya, Shoji Takenaka, Jutharat Manuschai, Maki Sotozono, Ryoko Nagata, Takako Ida, Risako Sato, Rui Saito, Ryouhei Takahashi, Daichi Sato, and et al. 2024. "Effects of Tooth Desensitizers on Streptococcus mutans Biofilm Formation Using a Modified Robbins Device Flow Cell System" International Journal of Molecular Sciences 25, no. 19: 10703. https://doi.org/10.3390/ijms251910703
APA StyleKornsombut, N., Takenaka, S., Manuschai, J., Sotozono, M., Nagata, R., Ida, T., Sato, R., Saito, R., Takahashi, R., Sato, D., & Noiri, Y. (2024). Effects of Tooth Desensitizers on Streptococcus mutans Biofilm Formation Using a Modified Robbins Device Flow Cell System. International Journal of Molecular Sciences, 25(19), 10703. https://doi.org/10.3390/ijms251910703






