Metabolic Rate and Oxidative Stress as a Risk Factors in the Development of Colorectal Cancer
Abstract
1. Introduction
2. Results and Discussion
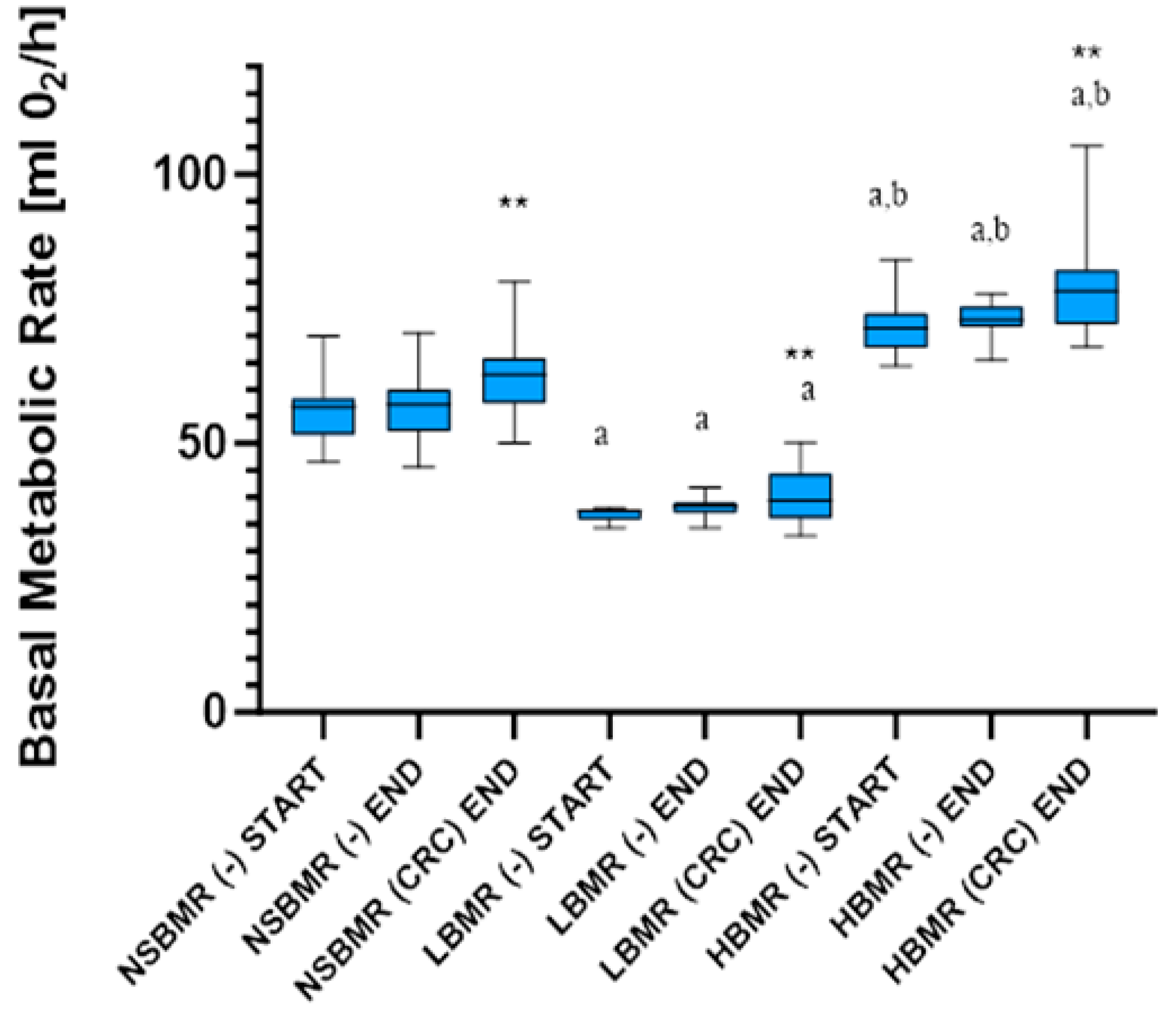
2.1. BMR Values and Body Mass
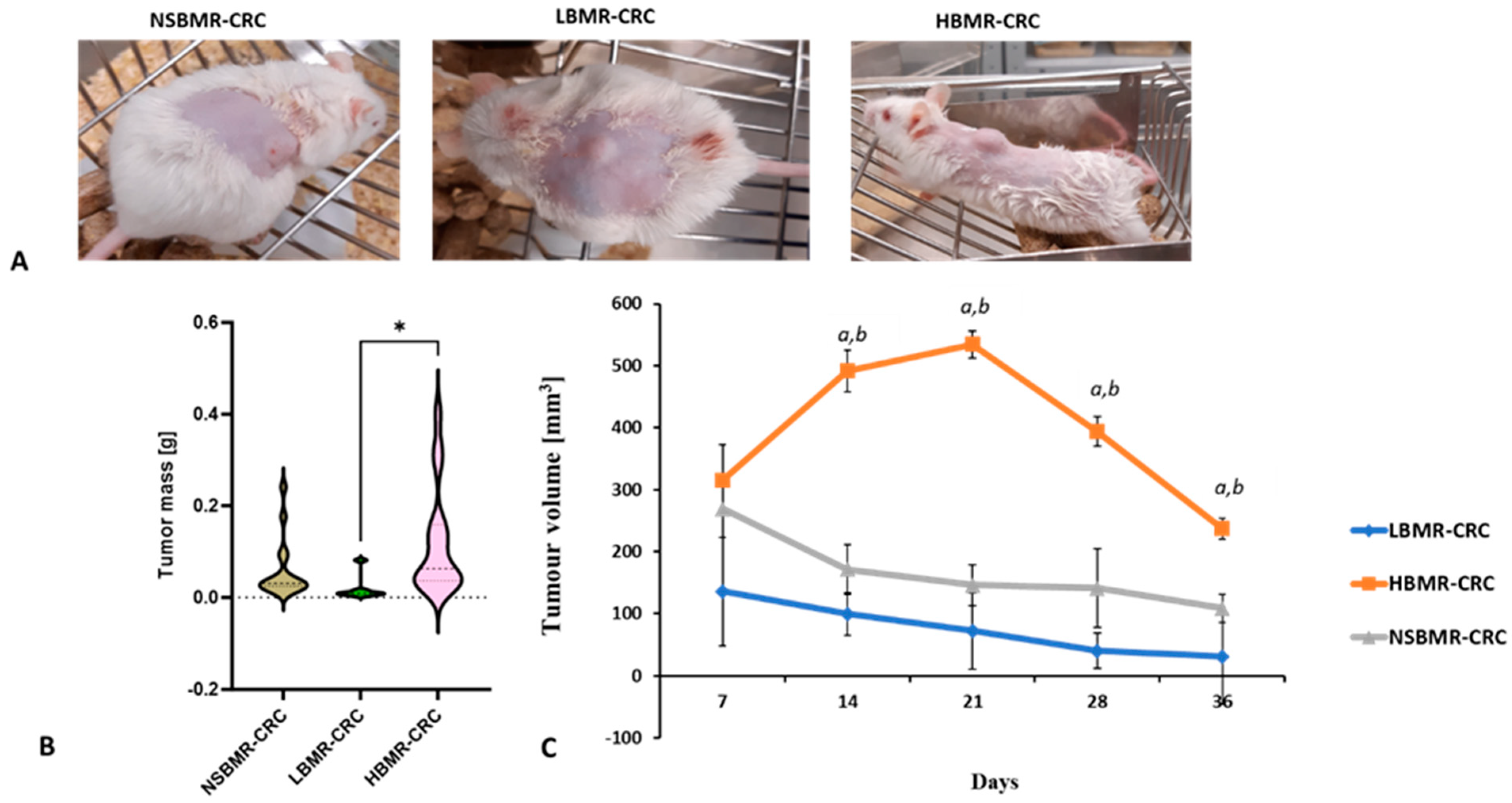
2.2. Tumor Growth in Relation to BMR Values
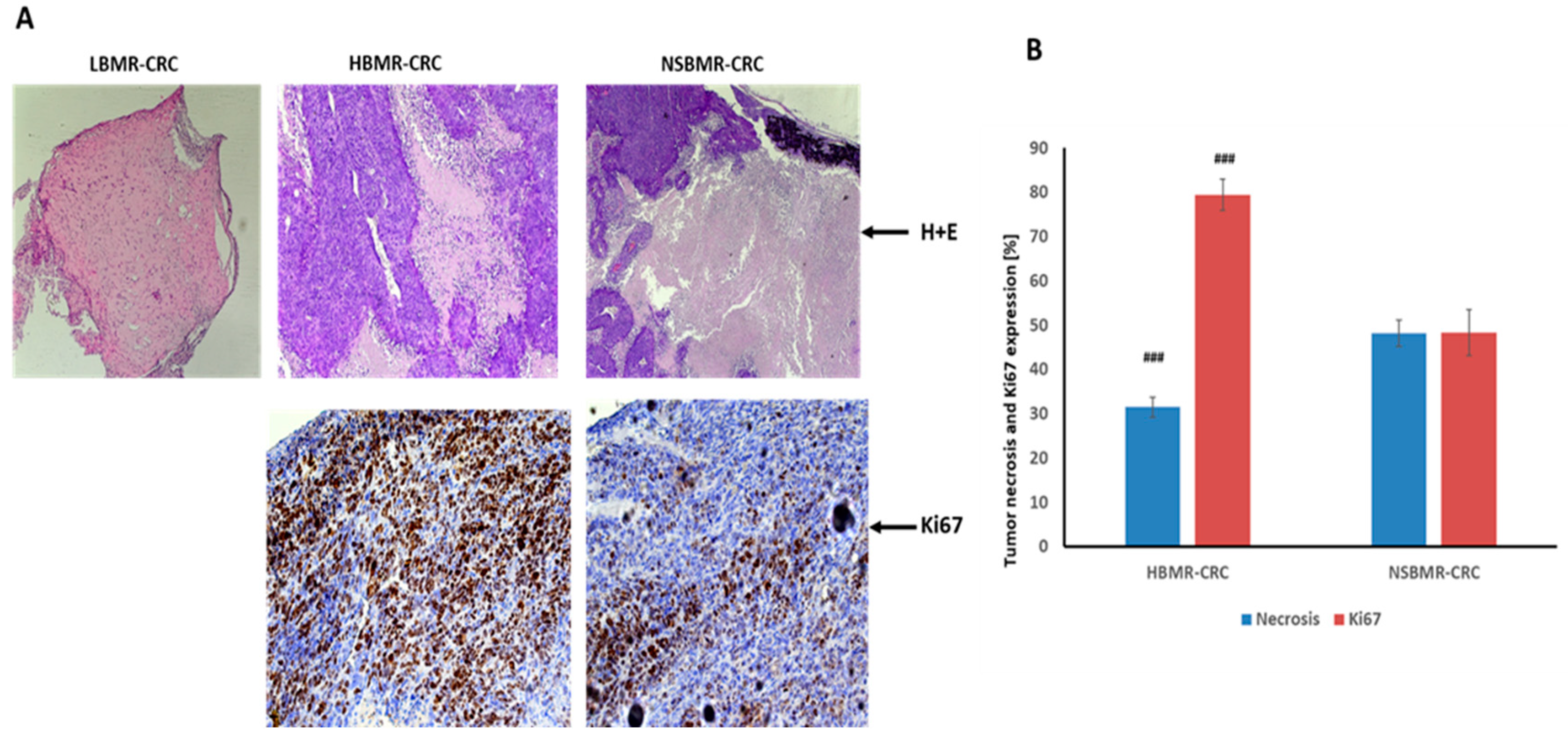
2.3. Effect of BMR on CRC Development and Ki67 Expression
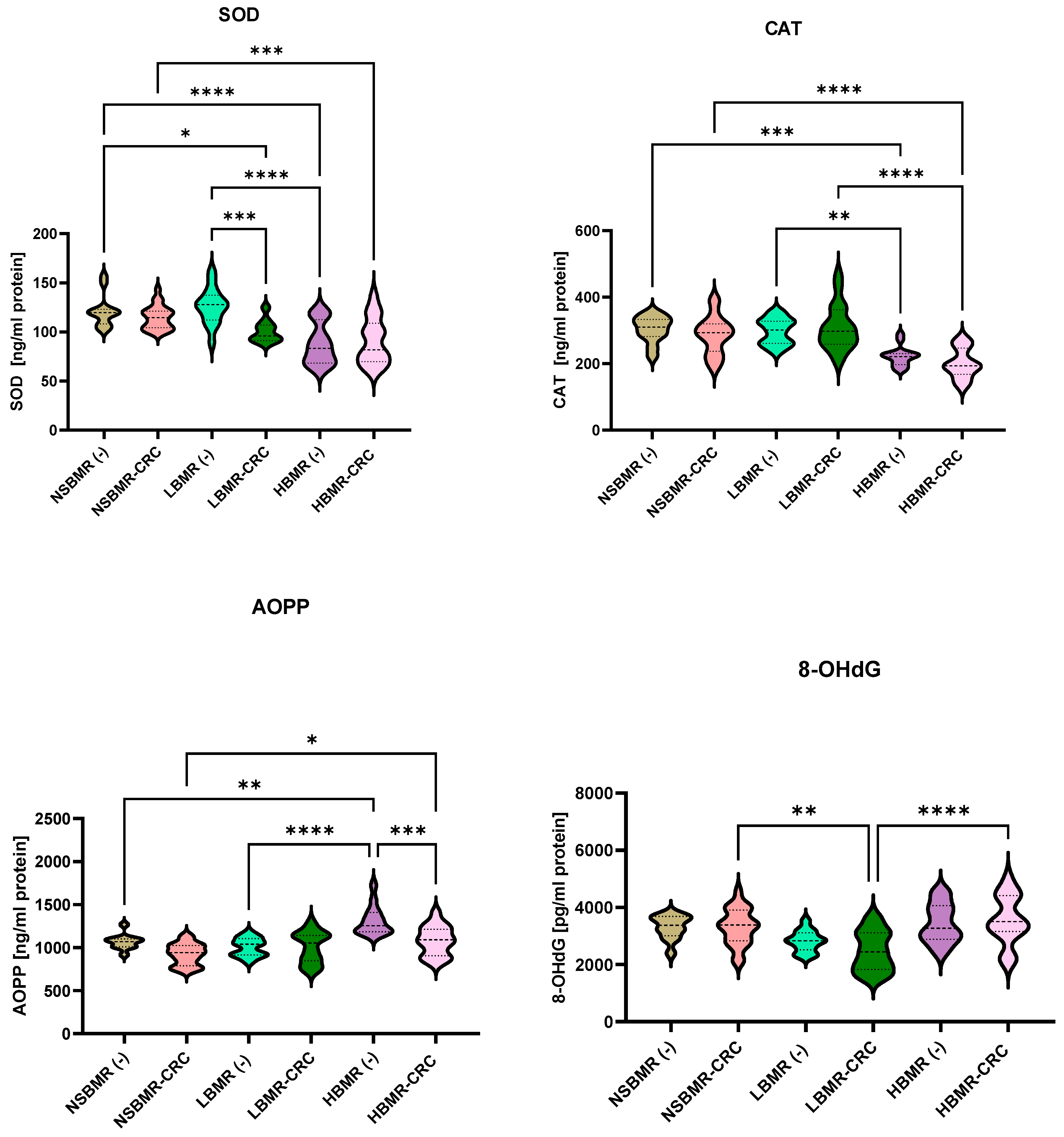
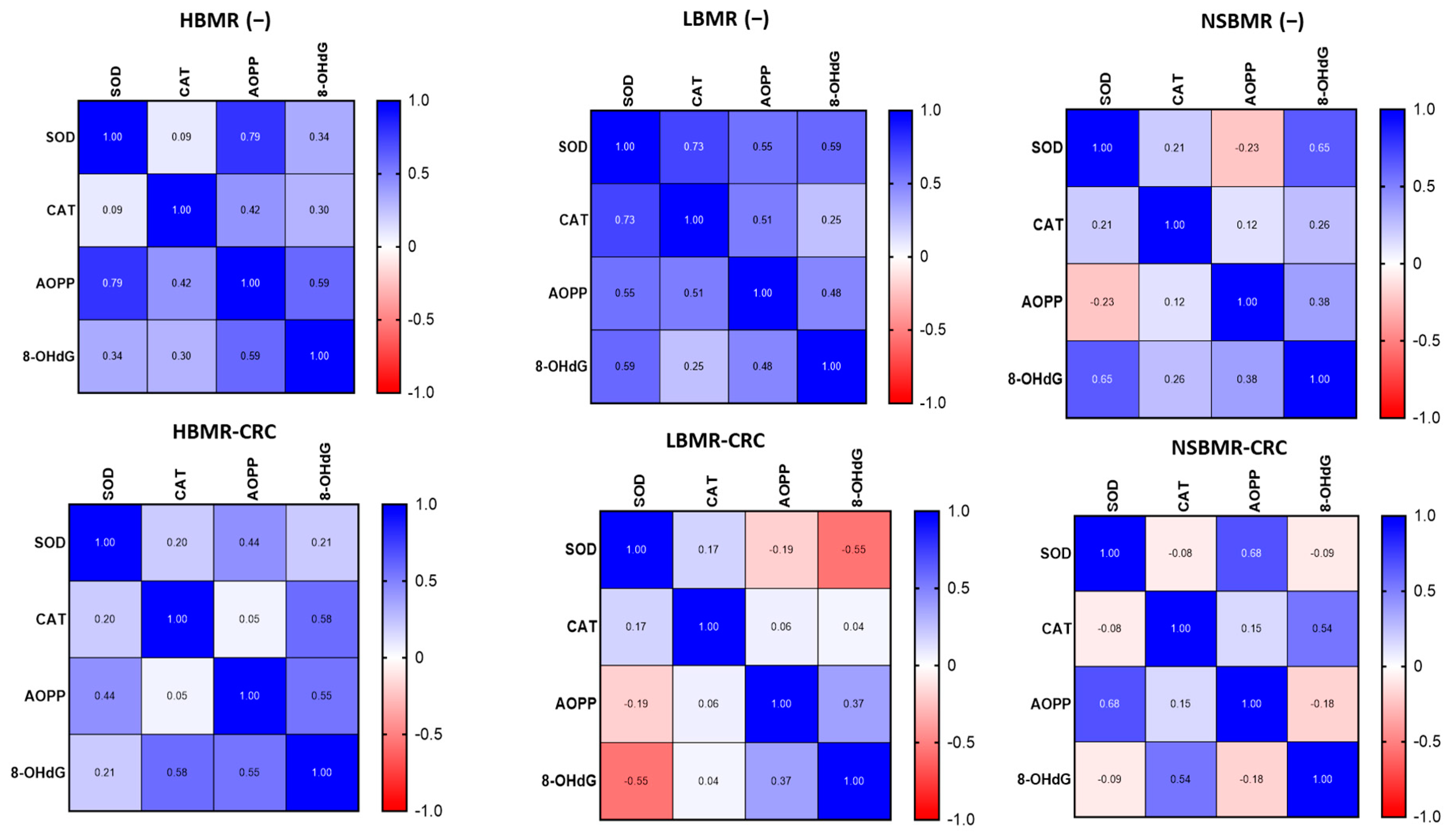
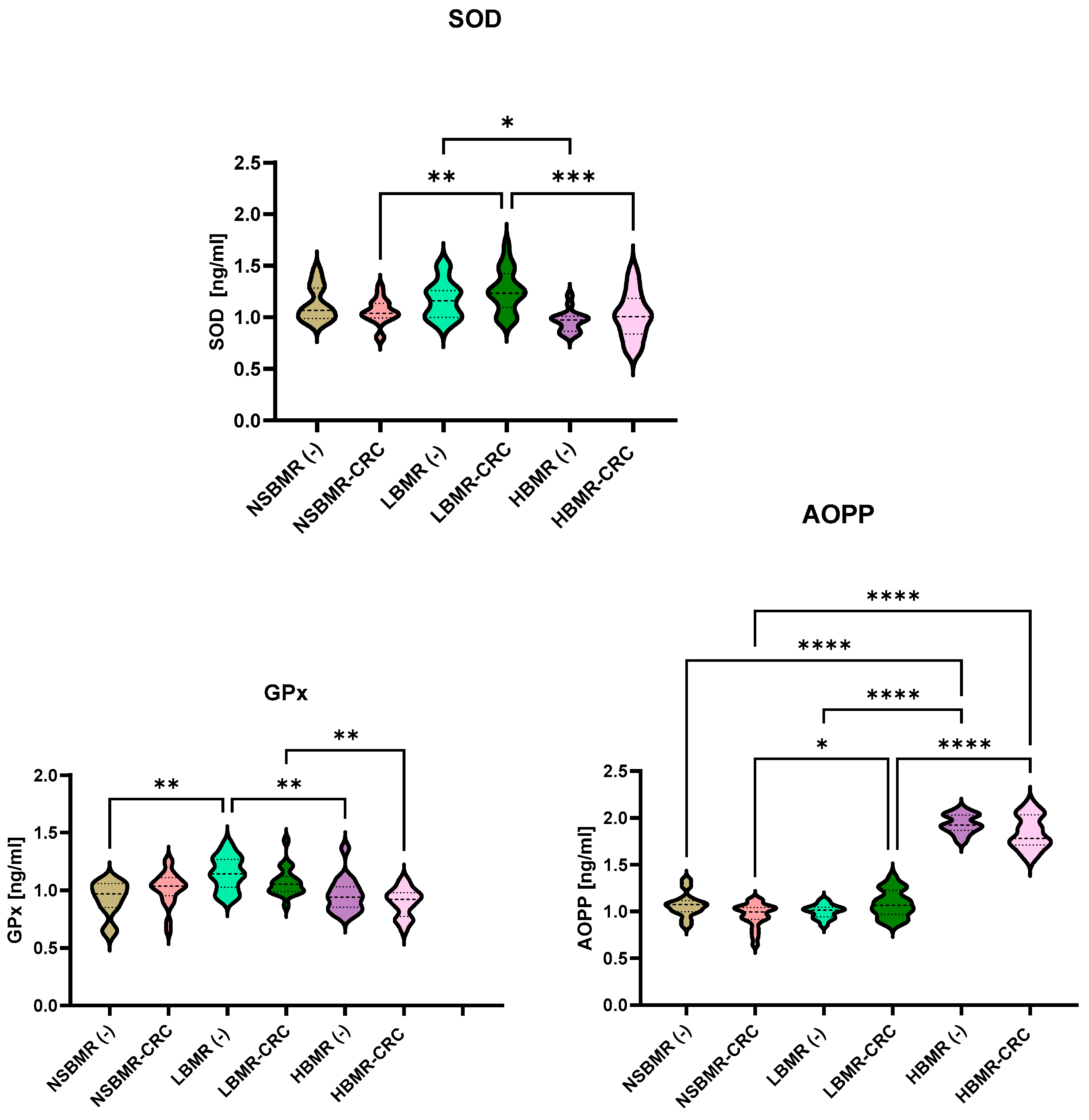
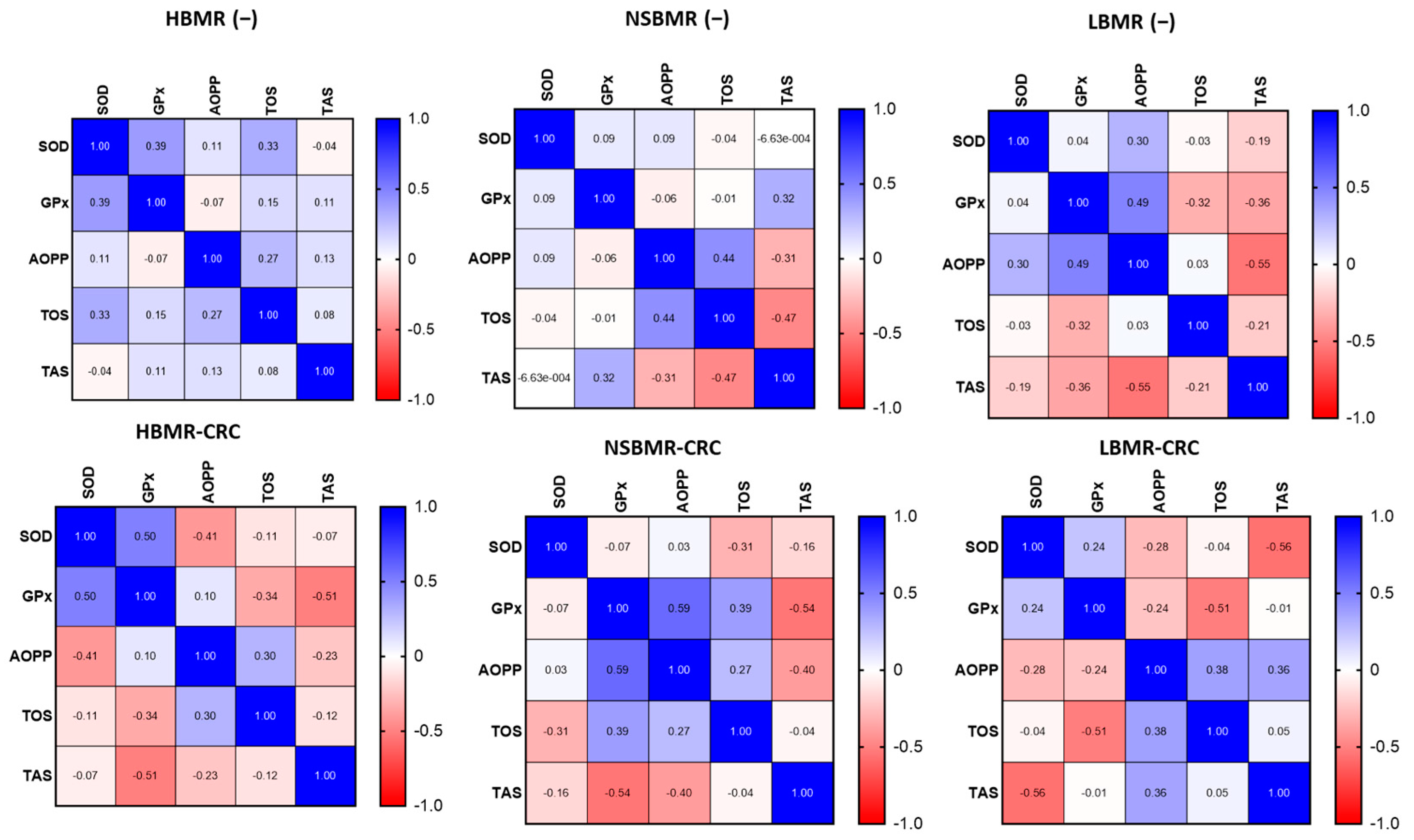
2.4. Oxidative and Antioxidant Status in Experimental Animals
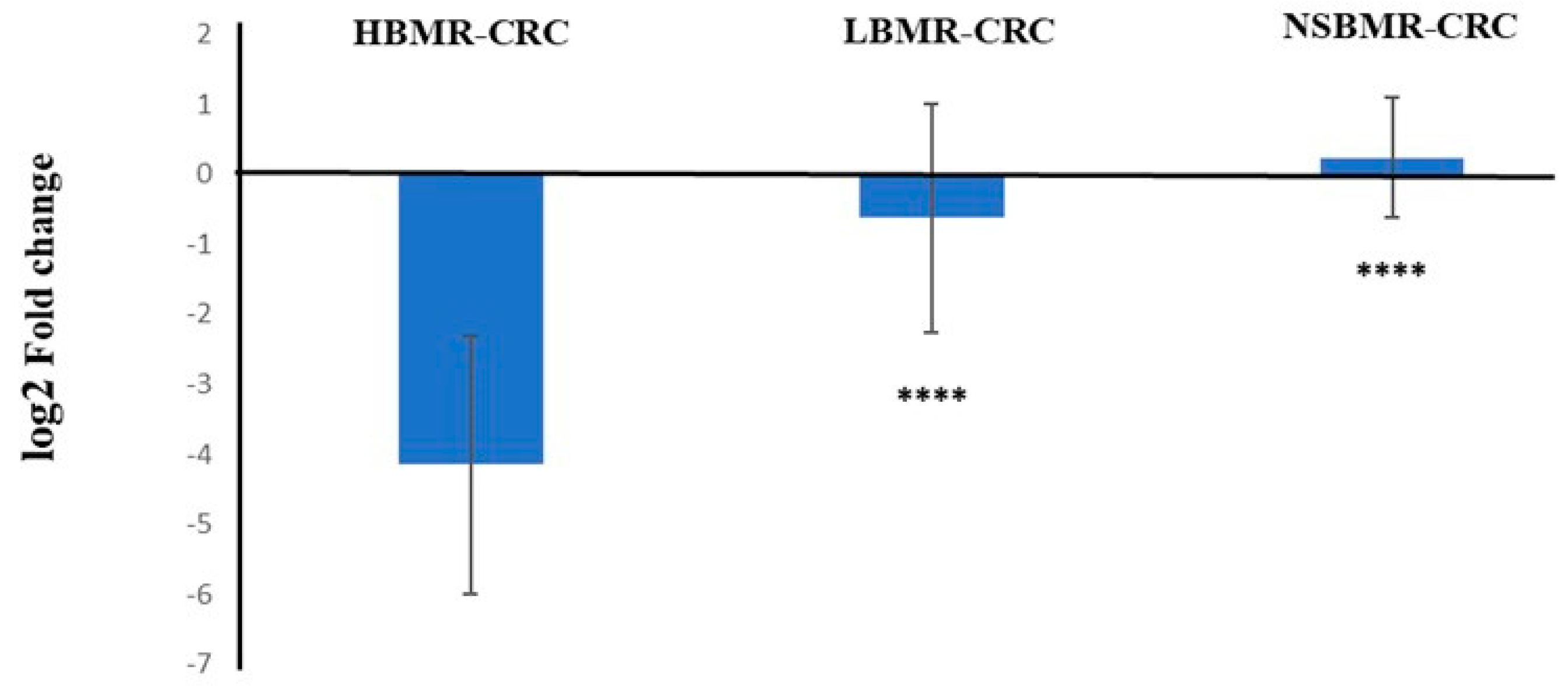
2.5. Keap1 Expression in the Liver of Experimental Animals
3. Materials and Methods
3.1. Animals and Experiment Design
3.2. Basal Metabolic Rate Measurements
3.3. Cell Culture and Induction of Colon Cancer
3.4. Histopathological and Immunohistochemical Analysis
3.5. Preparation of Homogenates
3.6. Biochemical Assays
3.7. Keap1 Expression
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stec, R.; Bodnar, L.; Szczylik, C. Feasibility and Efficacy of Capecitabine and FOLFIRI in Patients Aged 65 Years and Older with Advanced Colorectal Cancer: A Retrospective Analysis. J. Cancer Res. Clin. Oncol. 2010, 136, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zheng, Z.; Wang, Y.; Yang, L.; Zhou, Z. Genetically Predicted Thyroid Function and Risk of Colorectal Cancer: A Bidirectional Mendelian Randomization Study. J. Cancer Res. Clin. Oncol. 2023, 149, 14015–14024. [Google Scholar] [CrossRef]
- Kliemann, N.; Huybrechts, I.; Murphy, N.; Gunter, M. Basal Metabolic Rate and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition. Rev. Épidémiologie Santé Publique 2018, 66, S252. [Google Scholar] [CrossRef]
- Sawicka, D.; Maciak, S.; Kozłowska, H.; Kasacka, I.; Kloza, M.; Sadowska, A.; Sokołowska, E.; Konarzewski, M.; Car, H. Functional and Structural Changes in Aorta of Mice Divergently Selected for Basal Metabolic Rate. J. Comp. Physiol. B 2020, 190, 101–112. [Google Scholar] [CrossRef]
- Maciak, S.; Sawicka, D.; Sadowska, A.; Prokopiuk, S.; Buczyńska, S.; Bartoszewicz, M.; Niklińska, G.; Konarzewski, M.; Car, H. Low Basal Metabolic Rate as a Risk Factor for Development of Insulin Resistance and Type 2 Diabetes. BMJ Open Diab. Res. Care 2020, 8, e001381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, R.; Yang, G.; Wang, Y. Cancer Stem Cell Biomarkers and Related Signalling Pathways. J. Drug Target. 2024, 32, 33–44. [Google Scholar] [CrossRef]
- Ashrafian, H.; Ahmed, K.; Rowland, S.P.; Patel, V.M.; Gooderham, N.J.; Holmes, E.; Darzi, A.; Athanasiou, T. Metabolic Surgery and Cancer: Protective Effects of Bariatric Procedures. Cancer 2011, 117, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Else, P.L. Basal Metabolic Rate: History, Composition, Regulation, and Usefulness. Physiol. Biochem. Zool. 2004, 77, 869–876. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial Metabolism and Cancer. Cell Res 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell. 2008, 13, 472–482. [Google Scholar] [CrossRef]
- Tong, L.; Chuang, C.-C.; Wu, S.; Zuo, L. Reactive Oxygen Species in Redox Cancer Therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef]
- Perše, M. Oxidative Stress in the Pathogenesis of Colorectal Cancer: Cause or Consequence? BioMed Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef]
- Maciak, S. Cell Size, Body Size and Peto’s Paradox. BMC Ecol. Evo. 2022, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Rokavec, M.; Öner, M.G.; Li, H.; Jackstadt, R.; Jiang, L.; Lodygin, D.; Kaller, M.; Horst, D.; Ziegler, P.K.; Schwitalla, S.; et al. IL-6R/STAT3/miR-34a Feedback Loop Promotes EMT-Mediated Colorectal Cancer Invasion and Metastasis. J. Clin. Investig. 2014, 124, 1853–1867. [Google Scholar] [CrossRef]
- Miao, C.; Ren, Y.; Chen, M.; Wang, Z.; Wang, T. Microcystin-LR Promotes Migration and Invasion of Colorectal Cancer through Matrix Metalloproteinase-13 up-Regulation: MC-LR promotes migration and invasion of colorectal cancer cell. Mol. Carcinog. 2016, 55, 514–524. [Google Scholar] [CrossRef]
- Rhee, S.H.; Im, E.; Pothoulakis, C. Toll-Like Receptor 5 Engagement Modulates Tumor Development and Growth in a Mouse Xenograft Model of Human Colon Cancer. Gastroenterology 2008, 135, 518–528.e3. [Google Scholar] [CrossRef]
- Sawicka, D.; Hryniewicka, A.; Gohal, S.; Sadowska, A.; Pryczynicz, A.; Guzińska-Ustymowicz, K.; Sokołowska, E.; Morzycki, J.W.; Car, H. Establishment of In Vitro and In Vivo Anticolorectal Cancer Efficacy of Lithocholic Acid-Based Imidazolium Salts. Int. J. Mol. Sci. 2022, 23, 7019. [Google Scholar] [CrossRef]
- Sadowska, J.; Gębczyński, A.K.; Konarzewski, M. Metabolic Risk Factors in Mice Divergently Selected for BMR Fed High Fat and High Carb Diets. PLoS ONE 2017, 12, e0172892. [Google Scholar] [CrossRef]
- Maciak, S.; Bonda-Ostaszewska, E.; Czarnołęski, M.; Konarzewski, M.; Kozłowski, J. Mice Divergently Selected for High and Low Basal Metabolic Rates Evolved Different Cell Size and Organ Mass. J. Evol. Biol. 2014, 27, 478–487. [Google Scholar] [CrossRef]
- Brzęk, P.; Gębczyński, A.K.; Książek, A.; Konarzewski, M. Effect of Calorie Restriction on Spontaneous Physical Activity and Body Mass in Mice Divergently Selected for Basal Metabolic Rate (BMR). Physiol. Behav. 2016, 161, 116–122. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced Oxidation Protein Products Induce Microglia-Mediated Neuroinflammation via MAPKs-NF-κB Signaling Pathway and Pyroptosis after Secondary Spinal Cord Injury. J. Neuroinflammation 2020, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Kamendulis, L.M. The Role of Oxidative Stress in Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.-H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Goto, A.; Nakatochi, M.; Narita, A.; Yamaji, T.; Sawada, N.; Katagiri, R.; Iwagami, M.; Hanyuda, A.; Hachiya, T.; et al. Body Mass Index and Colorectal Cancer Risk: A Mendelian Randomization Study. Cancer Sci. 2021, 112, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Hong, P.; Mao, D.; Zhuo, Q.; Chen, X.; Yang, X. Basal metabolic rate of overweight and obese adults in Beijing. Wei Sheng Yan Jiu 2016, 45, 739–748. [Google Scholar] [PubMed]
- Kodama, M.; Oshikawa, K.; Shimizu, H.; Yoshioka, S.; Takahashi, M.; Izumi, Y.; Bamba, T.; Tateishi, C.; Tomonaga, T.; Matsumoto, M.; et al. A Shift in Glutamine Nitrogen Metabolism Contributes to the Malignant Progression of Cancer. Nat. Commun. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Isa, A. Dysregulated Metabolism in Cancer. Biomed. Res. Ther. 2022, 9, 5201–5208. [Google Scholar] [CrossRef]
- Stearns, S.C. Evolutionary Medicine: Its Scope, Interest and Potential. Proc. R. Soc. B. 2012, 279, 4305–4321. [Google Scholar] [CrossRef]
- Książek, A.; Konarzewski, M.; Chadzińska, M.; Cichoń, M. Costs of Immune Response in Cold-Stressed Laboratory Mice Selected for High and Low Basal Metabolism Rates. Proc. R. Soc. Lond. B 2003, 270, 2025–2031. [Google Scholar] [CrossRef]
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive Immunity Maintains Occult Cancer in an Equilibrium State. Nature 2007, 450, 903–907. [Google Scholar] [CrossRef]
- Fujita, M.; Imadome, K.; Somasundaram, V.; Kawanishi, M.; Karasawa, K.; Wink, D.A. Metabolic Characterization of Aggressive Breast Cancer Cells Exhibiting Invasive Phenotype: Impact of Non-Cytotoxic Doses of 2-DG on Diminishing Invasiveness. BMC Cancer 2020, 20, 929. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Korzonek-Szlacheta, I.; Zubelewicz-Szkodzińska, B.; Gąsior, M. Płytki Krwi—Ogniwo Łączące Zakrzepicę Ze Stanem Zapalnym. Folia Cardiol. 2018, 13, 303–308. [Google Scholar] [CrossRef][Green Version]
- Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Harbaum, L.; Schlemmer, A.; Rehak, P.; Langner, C. Tumor Necrosis Is a New Promising Prognostic Factor in Colorectal Cancer. Hum. Pathol. 2010, 41, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.H.; Roxburgh, C.S.D.; Anderson, J.H.; McKee, R.F.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. Prognostic Value of Tumour Necrosis and Host Inflammatory Responses in Colorectal Cancer. Br. J. Surg. 2012, 99, 287–294. [Google Scholar] [CrossRef]
- Li, P.; Xiao, Z.-T.; Braciak, T.A.; Ou, Q.-J.; Chen, G.; Oduncu, F.S. Association between Ki67 Index and Clinicopathological Features in Colorectal Cancer. Oncol. Res. Treat. 2016, 39, 696–702. [Google Scholar] [CrossRef]
- Melling, N.; Kowitz, C.M.; Simon, R.; Bokemeyer, C.; Terracciano, L.; Sauter, G.; Izbicki, J.R.; Marx, A.H. High Ki67 Expression Is an Independent Good Prognostic Marker in Colorectal Cancer. J. Clin. Pathol. 2016, 69, 209–214. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Kozłowski, J.; Konarzewski, M.; Czarnoleski, M. Coevolution of Body Size and Metabolic Rate in Vertebrates: A Life-history Perspective. Biol. Rev. 2020, 95, 1393–1417. [Google Scholar] [CrossRef]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Mehramiz, M.; Rezayi, M.; Ferns, G.A.; Khazaei, M.; Avan, A. Reactive Oxygen Species in Colorectal Cancer: The Therapeutic Impact and Its Potential Roles in Tumor Progression via Perturbation of Cellular and Physiological Dysregulated Pathways. J. Cell. Physiol. 2019, 234, 10072–10079. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, H.; Ma, X.; Zhou, X.; Gan, L.; Liu, Y.; Wang, Z. Isoliquiritigenin Enhances Radiosensitivity of HepG2 Cells via Disturbance of Redox Status. Cell Biochem. Biophys 2013, 65, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, R.; Torrens-Mas, M.; Pons, D.G.; Company, M.M.; Falcó, E.; Fernández, T.; Ibarra De La Rosa, J.M.; Sastre-Serra, J.; Oliver, J.; Roca, P. Non-Tumor Adjacent Tissue of Advanced Stage from CRC Shows Activated Antioxidant Response. Free. Radic. Biol. Med. 2018, 126, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Malafa, M.; Margenthaler, J.; Webb, B.; Neitzel, L.; Christophersen, M. MnSOD Expression Is Increased in Metastatic Gastric Cancer. J. Surg. Res. 2000, 88, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Yasuda, M.; Honda, M.; Inutsuka, S.; Korenaga, D. Fas Ligand Expression Is Correlated with Metastasis in Colorectal Carcinoma. Oncology 2003, 65, 83–88. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative Stress Inhibits Distant Metastasis by Human Melanoma Cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting Antioxidants for Cancer Therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, Y.; Luo, Y.; Wang, N.-X.; Xiao, J.-H. Role of Nrf2 in Cell Senescence Regulation. Mol. Cell. Biochem. 2021, 476, 247–259. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; Fu, W.; Wang, X.; Zhou, L.; Xu, X.; Huang, F.; Wu, Y. Downregulation of Keap1 Contributes to Poor Prognosis and Axitinib Resistance of Renal Cell Carcinoma via Upregulation of Nrf2 Expression. Int. J. Mol. Med. 2019, 43, 2044–2054. [Google Scholar] [CrossRef]
- Frank, R.; Scheffler, M.; Merkelbach-Bruse, S.; Ihle, M.A.; Kron, A.; Rauer, M.; Ueckeroth, F.; König, K.; Michels, S.; Fischer, R.; et al. Clinical and Pathological Characteristics of KEAP1- and NFE2L2 -Mutated Non–Small Cell Lung Carcinoma (NSCLC). Clin. Cancer Res. 2018, 24, 3087–3096. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox Regulation by NRF2 in Aging and Disease. Free. Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.A.; Forman, H.J. Does Bach1 & C-Myc Dependent Redox Dysregulation of Nrf2 & Adaptive Homeostasis Decrease Cancer Risk in Ageing? Free. Radic. Biol. Med. 2019, 134, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.P.; Pessa, H.K.J.; Caldez, M.J.; Fuhrer, T.; Diril, M.K.; Sauer, U.; Kaldis, P.; Björklund, M. Identification of Transcriptional and Metabolic Programs Related to Mammalian Cell Size. Curr. Biol. 2014, 24, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M.; D’Orazi, G. NRF2 in Cancer: Cross-Talk with Oncogenic Pathways and Involvement in Gammaherpesvirus-Driven Carcinogenesis. Int. J. Mol. Sci. 2022, 24, 595. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Guo, W.; Zhu, X.; Ahuja, N.; Fu, T. MAPT Promoter CpG Island Hypermethylation Is Associated with Poor Prognosis in Patients with Stage II Colorectal Cancer. Cancer Manag. Res. 2019, 11, 7337–7343. [Google Scholar] [CrossRef]
- Wani, Z.A.; Guru, S.K.; Rao, A.V.S.; Sharma, S.; Mahajan, G.; Behl, A.; Kumar, A.; Sharma, P.R.; Kamal, A.; Bhushan, S.; et al. A Novel Quinazolinone Chalcone Derivative Induces Mitochondrial Dependent Apoptosis and Inhibits PI3K/Akt/mTOR Signaling Pathway in Human Colon Cancer HCT-116 Cells. Food Chem. Toxicol. 2016, 87, 1–11. [Google Scholar] [CrossRef]
- Książek, A.; Konarzewski, M.; Łapo, I.B. Anatomic and Energetic Correlates of Divergent Selection for Basal Metabolic Rate in Laboratory Mice. Physiol. Biochem. Zool. 2004, 77, 890–899. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Parameters | Line Type | GLM Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| NSBMR | LBMR | HBMR | Line Type | |||||
| (−) | CRC | (−) | CRC | (−) | CRC | F2,98 | p | |
| Body Mass [g] | 39.35 ± 4.41 | 38.30 ± 3.26 | 34.36 ± 2.04 | 34.09 ± 3.18 | 34.30 ± 2.55 | 35.26 ± 2.32 | 23.50 | <0.001 |
| Body Mass END [g] | 40.42 ± 4.93 | 38.84 ± 2.74 | 37.13 ± 2.59 | 33.63 ± 3.25 | 35.93 ± 1.98 | 36.94 ± 3.02 | 16.63 | <0.001 |
| BMR [mL O2/h] | 52.88 ± 5.99 | - | 38.21 ± 2.36 a | - | 71.01 ± 4.71 a,b | - | 717.02 | <0.001 (+) |
| BMR END [mL O2/h] | 53.15 ± 5.02 | 62.10 ± 6.28 | 37.64 ± 1.85 a | 42.26 ± 4.61 a | 72.15 ± 3.92 a,b | 76.85 ± 6.17 a,b | 670.65 | <0.001 (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka, D.; Maciak, S.; Sadowska, A.; Sokołowska, E.; Gohal, S.; Guzińska-Ustymowicz, K.; Niemirowicz-Laskowska, K.; Car, H. Metabolic Rate and Oxidative Stress as a Risk Factors in the Development of Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 10713. https://doi.org/10.3390/ijms251910713
Sawicka D, Maciak S, Sadowska A, Sokołowska E, Gohal S, Guzińska-Ustymowicz K, Niemirowicz-Laskowska K, Car H. Metabolic Rate and Oxidative Stress as a Risk Factors in the Development of Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(19):10713. https://doi.org/10.3390/ijms251910713
Chicago/Turabian StyleSawicka, Diana, Sebastian Maciak, Anna Sadowska, Emilia Sokołowska, Sylwia Gohal, Katarzyna Guzińska-Ustymowicz, Katarzyna Niemirowicz-Laskowska, and Halina Car. 2024. "Metabolic Rate and Oxidative Stress as a Risk Factors in the Development of Colorectal Cancer" International Journal of Molecular Sciences 25, no. 19: 10713. https://doi.org/10.3390/ijms251910713
APA StyleSawicka, D., Maciak, S., Sadowska, A., Sokołowska, E., Gohal, S., Guzińska-Ustymowicz, K., Niemirowicz-Laskowska, K., & Car, H. (2024). Metabolic Rate and Oxidative Stress as a Risk Factors in the Development of Colorectal Cancer. International Journal of Molecular Sciences, 25(19), 10713. https://doi.org/10.3390/ijms251910713







