Global Transcriptomic Analysis of Topical Sodium Alginate Protection against Peptic Damage in an In Vitro Model of Treatment-Resistant Gastroesophageal Reflux Disease
Abstract
:1. Introduction
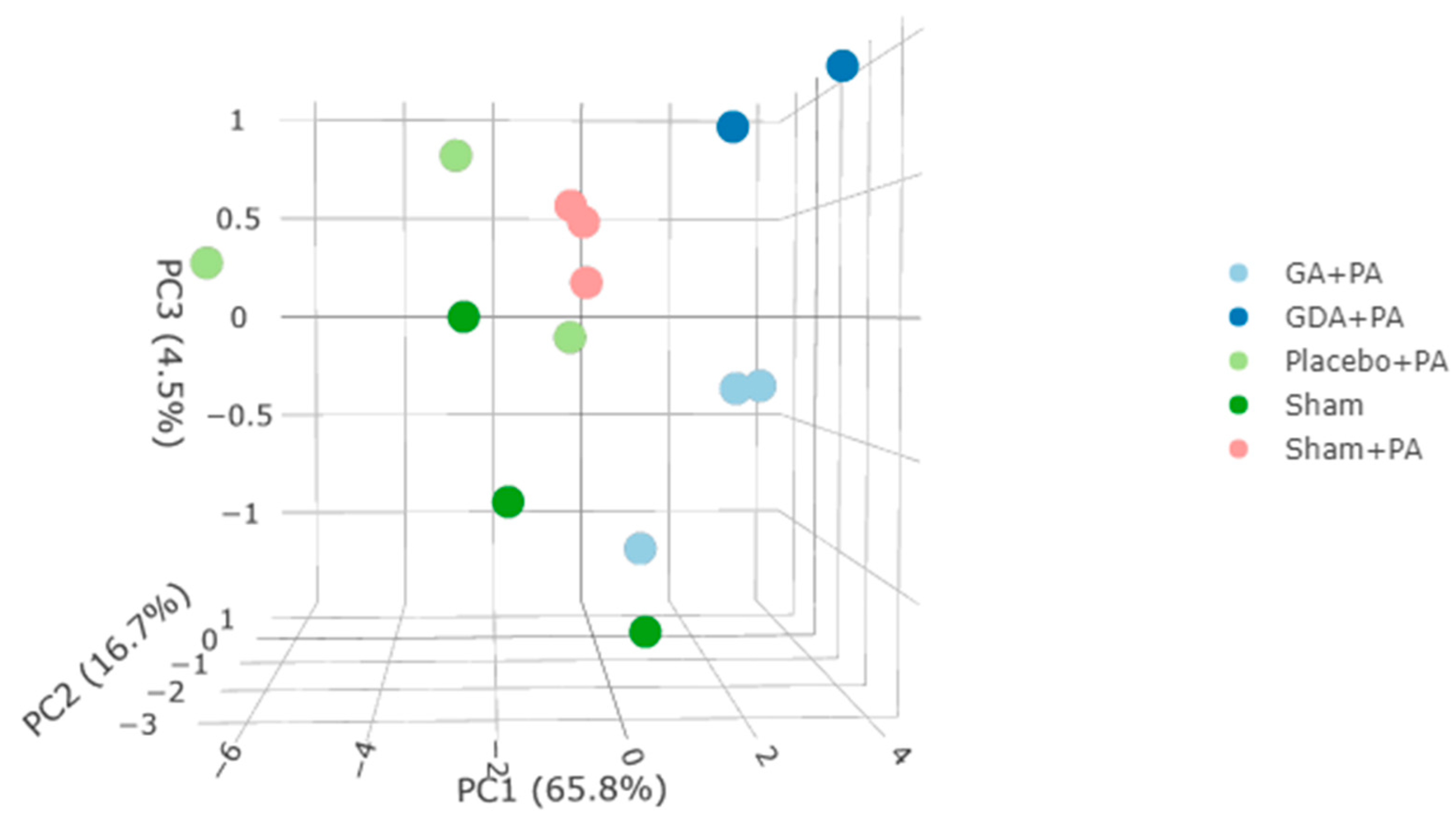
2. Results
3. Discussion
3.1. Genes Differentially Expressed by PA Treatment
3.2. Alginate Rescue of PA-Induced DE Genes Relative to Placebo or Sham
3.3. Limitations and Summary
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Sequencing and Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Zerbib, F.; Bredenoord, A.J.; Fass, R.; Kahrilas, P.J.; Roman, S.; Savarino, E.; Sifrim, D.; Vaezi, M.; Yadlapati, R.; Gyawali, C.P. ESNM/ANMS consensus paper: Diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2021, 33, e14075. [Google Scholar] [CrossRef]
- Yadlapati, R.; Gyawali, C.P.; Pandolfino, J.E.; Participants, C.G.C.C. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 984–994.e981. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.U.; Conway, B.R.; Ghori, M.U. Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Ergun, P.; Kipcak, S.; Bor, S. Epigenetic Alterations from Barrett’s Esophagus to Esophageal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7817. [Google Scholar] [CrossRef]
- Delshad, S.D.; Almario, C.V.; Chey, W.D.; Spiegel, B.M.R. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology 2020, 158, 1250–1261.e1252. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Gyawali, C.P. Refractory Gastroesophageal Reflux Disease: Diagnosis and Management. J. Neurogastroenterol. Motil. 2024, 30, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Fass, R.; Vaezi, M. Untangling Nonerosive Reflux Disease From Functional Heartburn. Clin. Gastroenterol. Hepatol. 2021, 19, 1314–1326. [Google Scholar] [CrossRef]
- Ergun, P.; Kipcak, S.; Dettmar, P.W.; Fisher, J.; Woodcock, A.D.; Bor, S. Pepsin and pH of Gastric Juice in Patients with Gastrointestinal Reflux Disease and Subgroups. J. Clin. Gastroenterol. 2022, 56, 512–517. [Google Scholar] [CrossRef]
- Castellana, C.; Pecere, S.; Furnari, M.; Telese, A.; Matteo, M.V.; Haidry, R.; Eusebi, L.H. Side effects of long-term use of proton pump inhibitors: Practical considerations. Pol. Arch. Intern. Med. 2021, 131, 541–549. [Google Scholar] [CrossRef]
- Fossmark, R.; Martinsen, T.C.; Waldum, H.L. Adverse Effects of Proton Pump Inhibitors-Evidence and Plausibility. Int. J. Mol. Sci. 2019, 20, 5203. [Google Scholar] [CrossRef]
- Ergun, P.; Kipcak, S.; Selvi Gunel, N.; Yildirim Sozmen, E.; Bor, S. Inflammatory responses in esophageal mucosa before and after laparoscopic antireflux surgery. World J. Gastrointest. Surg. 2024, 16, 871–881. [Google Scholar] [CrossRef]
- Pauwels, A.; Boecxstaens, V.; Andrews, C.N.; Attwood, S.E.; Berrisford, R.; Bisschops, R.; Boeckxstaens, G.E.; Bor, S.; Bredenoord, A.J.; Cicala, M.; et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut 2019, 68, 1928–1941. [Google Scholar] [CrossRef] [PubMed]
- Pessaux, P.; Arnaud, J.P.; Delattre, J.F.; Meyer, C.; Baulieux, J.; Mosnier, H. Laparoscopic antireflux surgery: Five-year results and beyond in 1340 patients. Arch Surg 2005, 140, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Leiman, D.A.; Riff, B.P.; Morgan, S.; Metz, D.C.; Falk, G.W.; French, B.; Umscheid, C.A.; Lewis, J.D. Alginate therapy is effective treatment for GERD symptoms: A systematic review and meta-analysis. Dis. Esophagus 2017, 30, 1–9. [Google Scholar] [CrossRef]
- Samuels, T.L.; Blaine-Sauer, S.; Yan, K.; Plehhova, K.; Coyle, C.; Johnston, N. Topical Alginate Protection against Pepsin-Mediated Esophageal Damage: E-Cadherin Proteolysis and Matrix Metalloproteinase Induction. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Strugala, V.; Avis, J.; Jolliffe, I.G.; Johnstone, L.M.; Dettmar, P.W. The role of an alginate suspension on pepsin and bile acids—Key aggressors in the gastric refluxate. Does this have implications for the treatment of gastro-oesophageal reflux disease? J. Pharm. Pharmacol. 2009, 61, 1021–1028. [Google Scholar] [CrossRef]
- Sweis, R.; Kaufman, E.; Anggiansah, A.; Wong, T.; Dettmar, P.; Fried, M.; Schwizer, W.; Avvari, R.K.; Pal, A.; Fox, M. Post-prandial reflux suppression by a raft-forming alginate (Gaviscon Advance) compared to a simple antacid documented by magnetic resonance imaging and pH-impedance monitoring: Mechanistic assessment in healthy volunteers and randomised, controlled, double-blind study in reflux patients. Aliment. Pharmacol. Ther. 2013, 37, 1093–1102. [Google Scholar] [CrossRef]
- Samuels, T.L.; Yan, K.; Patel, N.; Plehhova, K.; Coyle, C.; Hurley, B.P.; Johnston, N. Alginates for Protection Against Pepsin-Acid Induced Aerodigestive Epithelial Barrier Disruption. Laryngoscope 2022, 132, 2327–2334. [Google Scholar] [CrossRef]
- Giannini, E.G.; Zentilin, P.; Dulbecco, P.; Iiritano, E.; Bilardi, C.; Savarino, E.; Mansi, C.; Savarino, V. A comparison between sodium alginate and magaldrate anhydrous in the treatment of patients with gastroesophageal reflux symptoms. Dig. Dis. Sci. 2006, 51, 1904–1909. [Google Scholar] [CrossRef]
- Wilkinson, J.; Wade, A.; Thomas, S.J.; Jenner, B.; Hodgkinson, V.; Coyle, C. Randomized clinical trial: A double-blind, placebo-controlled study to assess the clinical efficacy and safety of alginate-antacid (Gaviscon Double Action) chewable tablets in patients with gastro-oesophageal reflux disease. Eur. J. Gastroenterol. Hepatol. 2019, 31, 86–93. [Google Scholar] [CrossRef]
- Rohof, W.O.; Bennink, R.J.; Smout, A.J.; Thomas, E.; Boeckxstaens, G.E. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin. Gastroenterol. Hepatol. 2013, 11, 1585–1591; quiz e1590. [Google Scholar] [CrossRef]
- Sonmez, S.; Coyle, C.; Sifrim, D.; Woodland, P. Duration of adhesion of swallowed alginates to distal oesophageal mucosa: Implications for topical therapy of oesophageal diseases. Aliment. Pharmacol. Ther. 2020, 52, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Woodland, P.; Batista-Lima, F.; Lee, C.; Preston, S.L.; Dettmar, P.; Sifrim, D. Topical protection of human esophageal mucosal integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G975–G980. [Google Scholar] [CrossRef]
- Ranaldo, N.; Losurdo, G.; Iannone, A.; Principi, M.; Barone, M.; De Carne, M.; Ierardi, E.; Di Leo, A. Tailored therapy guided by multichannel intraluminal impedance pH monitoring for refractory non-erosive reflux disease. Cell Death Dis. 2017, 8, e3040. [Google Scholar] [CrossRef] [PubMed]
- Sadatomi, D.; Kono, T.; Mogami, S.; Fujitsuka, N. Weak acids induce PGE(2) production in human oesophageal cells: Novel mechanisms underlying GERD symptoms. Sci. Rep. 2020, 10, 20775. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, V.; Arora, A.S.; Murray, J.A. Weakly acidic reflux. Dis. Esophagus 2011, 24, 56–62. [Google Scholar] [CrossRef]
- Tamhankar, A.P.; Peters, J.H.; Portale, G.; Hsieh, C.C.; Hagen, J.A.; Bremner, C.G.; DeMeester, T.R. Omeprazole does not reduce gastroesophageal reflux: New insights using multichannel intraluminal impedance technology. J. Gastrointest. Surg. 2004, 8, 890–897; discussion 897–898. [Google Scholar] [CrossRef]
- Zikos, T.A.; Clarke, J.O. Non-acid Reflux: When It Matters and Approach to Management. Curr. Gastroenterol. Rep. 2020, 22, 43. [Google Scholar] [CrossRef]
- Hvid-Jensen, F.; Pedersen, L.; Funch-Jensen, P.; Drewes, A.M. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett’s oesophagus: A nationwide study of 9883 patients. Aliment Pharmacol Ther 2014, 39, 984–991. [Google Scholar] [CrossRef]
- Sharma, P.; Yadlapati, R. Pathophysiology and treatment options for gastroesophageal reflux disease: Looking beyond acid. Ann. N. Y Acad. Sci. 2021, 1486, 3–14. [Google Scholar] [CrossRef]
- Samuels, T.L.; Altman, K.W.; Gould, J.C.; Kindel, T.; Bosler, M.; MacKinnon, A.; Hagen, C.E.; Johnston, N. Esophageal pepsin and proton pump synthesis in barrett’s esophagus and esophageal adenocarcinoma. Laryngoscope 2019, 129, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Blaine-Sauer, S.; Samuels, T.L.; Yan, K.; Johnston, N. The Protease Inhibitor Amprenavir Protects against Pepsin-Induced Esophageal Epithelial Barrier Disruption and Cancer-Associated Changes. Int. J. Mol. Sci. 2023, 24, 6765. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Bobin, F. Variability and accuracy of multiple saliva pepsin measurements in laryngopharyngeal reflux patients. J. Otolaryngol. Head. Neck Surg. 2023, 52, 66. [Google Scholar] [CrossRef]
- Piper, D.W.; Fenton, B.H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6, 506–508. [Google Scholar] [CrossRef]
- Johnston, N.; Dettmar, P.W.; Bishwokarma, B.; Lively, M.O.; Koufman, J.A. Activity/stability of human pepsin: Implications for reflux attributed laryngeal disease. Laryngoscope 2007, 117, 1036–1039. [Google Scholar] [CrossRef]
- Johnston, N.; Wells, C.W.; Samuels, T.L.; Blumin, J.H. Rationale for targeting pepsin in the treatment of reflux disease. Ann. Otol. Rhinol. Laryngol. 2010, 119, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.; Hoekzema, C.; Gould, J.; Goldblatt, M.; Frelich, M.; Bosler, M.; Lee, S.H.; Johnston, N. Local Synthesis of Pepsin in Barrett’s Esophagus and the Role of Pepsin in Esophageal Adenocarcinoma. Ann. Otol. Rhinol. Laryngol. 2015, 124, 893–902. [Google Scholar] [CrossRef]
- Stabenau, K.A.; Samuels, T.L.; Lam, T.K.; Mathison, A.J.; Wells, C.; Altman, K.W.; Battle, M.A.; Johnston, N. Pepsinogen/Proton Pump Co-Expression in Barrett’s Esophageal Cells Induces Cancer-Associated Changes. Laryngoscope 2023, 133, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Yamato, M.; Nishio, H.; Takeuchi, K. Essential role of pepsin in pathogenesis of acid reflux esophagitis in rats. Dig. Dis. Sci. 2006, 51, 303–309. [Google Scholar] [CrossRef]
- Ergun, P.; Capanoglu, D.; Kipcak, S.; Bor, S. Response of Esophageal Epithelium to Acute and Chronic Stress in Rabbits. Bull. Exp. Biol. Med. 2021, 171, 582–587. [Google Scholar] [CrossRef]
- Rettura, F.; Bronzini, F.; Campigotto, M.; Lambiase, C.; Pancetti, A.; Berti, G.; Marchi, S.; de Bortoli, N.; Zerbib, F.; Savarino, E.; et al. Refractory Gastroesophageal Reflux Disease: A Management Update. Front Med. 2021, 8, 765061. [Google Scholar] [CrossRef] [PubMed]
- Reimer, C.; Lodrup, A.B.; Smith, G.; Wilkinson, J.; Bytzer, P. Randomised clinical trial: Alginate (Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment. Pharmacol. Ther. 2016, 43, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ang, D.; Pauwels, A.; De Santis, A.; Vanuytsel, T.; Tack, J. Management of refractory typical GERD symptoms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ergun, P.; Kipcak, S.; Gunel, N.S.; Bor, S.; Sozmen, E.Y. Roles of Cytokines in Pathological and Physiological Gastroesophageal Reflux Exposure. J. Neurogastroenterol. Motil. 2023, 30, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.; Huo, X.; Mittal, V.; Schuler, C.M.; Carmack, S.W.; Zhang, H.Y.; Zhang, X.; Yu, C.; Hormi-Carver, K.; Genta, R.M.; et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009, 137, 1776–1784. [Google Scholar] [CrossRef]
- Dunbar, K.B.; Agoston, A.T.; Odze, R.D.; Huo, X.; Pham, T.H.; Cipher, D.J.; Castell, D.O.; Genta, R.M.; Souza, R.F.; Spechler, S.J. Association of Acute Gastroesophageal Reflux Disease With Esophageal Histologic Changes. JAMA 2016, 315, 2104–2112. [Google Scholar] [CrossRef]
- Orlando, R.C. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best. Pr. Res. Clin. Gastroenterol. 2010, 24, 873–882. [Google Scholar] [CrossRef]
- Samuels, T.L.; Johnston, N. Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngol. Head. Neck Surg. 2009, 141, 559–563. [Google Scholar] [CrossRef]
- Tan, J.J.; Dai, Y.F.; Wang, F.; Lv, Z.H.; Huang, L.J.; Peng, L.Y.; Li, X.P. Pepsin-mediated inflammation in laryngopharyngeal reflux via the ROS/NLRP3/IL-1beta signaling pathway. Cytokine 2024, 178, 156568. [Google Scholar] [CrossRef]
- McCann, A.J.; Samuels, T.L.; Blumin, J.H.; Johnston, N. The role of pepsin in epithelia-mesenchymal transition in idiopathic subglottic stenosis. Laryngoscope 2020, 130, 154–158. [Google Scholar] [CrossRef]
- Korbut, E.; Janmaat, V.T.; Wierdak, M.; Hankus, J.; Wojcik, D.; Surmiak, M.; Magierowska, K.; Brzozowski, T.; Peppelenbosch, M.P.; Magierowski, M. Molecular Profile of Barrett’s Esophagus and Gastroesophageal Reflux Disease in the Development of Translational Physiological and Pharmacological Studies. Int. J. Mol. Sci. 2020, 21, 6436. [Google Scholar] [CrossRef]
- Tan, J.J.; Wang, L.; Mo, T.T.; Wang, J.; Wang, M.G.; Li, X.P. Pepsin promotes IL-8 signaling-induced epithelial-mesenchymal transition in laryngeal carcinoma. Cancer Cell Int. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Fadista, J.; Yakimov, V.; Vosa, U.; Hansen, C.S.; Kasela, S.; Skotte, L.; Geller, F.; Courraud, J.; Esko, T.; Kukuskina, V.; et al. Genetic regulation of spermine oxidase activity and cancer risk: A Mendelian randomization study. Sci. Rep. 2021, 11, 17463. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Roh, S.; Hong, I.; Kim, H.; Ahn, T.S.; Kang, D.H.; Lee, M.S.; Baek, M.J.; Kwak, H.J.; et al. Expression of Spermine Oxidase Is Associated with Colorectal Carcinogenesis and Prognosis of Patients. Biomedicines 2022, 10, 626. [Google Scholar] [CrossRef]
- Gobert, A.P.; Al-Greene, N.T.; Singh, K.; Coburn, L.A.; Sierra, J.C.; Verriere, T.G.; Luis, P.B.; Schneider, C.; Asim, M.; Allaman, M.M.; et al. Distinct Immunomodulatory Effects of Spermine Oxidase in Colitis Induced by Epithelial Injury or Infection. Front. Immunol. 2018, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Asim, M.; Romero-Gallo, J.; Barry, D.P.; Hoge, S.; de Sablet, T.; Delgado, A.G.; Wroblewski, L.E.; Piazuelo, M.B.; Yan, F.; et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 2011, 141, 1696–1708.e2. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Sierra, J.C.; Piazuelo, M.B.; Mera, R.M.; Chaturvedi, R.; Bravo, L.E.; Correa, P.; Schneider, B.G.; Wilson, K.T.; Casero, R.A. Epigenetic silencing of miR-124 prevents spermine oxidase regulation: Implications for Helicobacter pylori-induced gastric cancer. Oncogene 2016, 35, 5480–5488. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Chen, M.C.; Chen, K.C.; Chang, G.C.; Lin, H.; Wu, C.C.; Kao, W.H.; Teng, C.J.; Hsu, S.L.; Yang, T.Y. RAGE acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dis. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.R.; Cui, M.; Sun, B.L.; Yu, J.; Wang, H.M. Tissue-specific expression profiling of receptor for advanced glycation end products and its soluble forms in esophageal and lung cancer. Genet. Test. Mol. Biomark. 2010, 14, 355–361. [Google Scholar] [CrossRef]
- Faruqui, T.; Khan, M.S.; Akhter, Y.; Khan, S.; Rafi, Z.; Saeed, M.; Han, I.; Choi, E.H.; Yadav, D.K. RAGE Inhibitors for Targeted Therapy of Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2022, 24, 266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Beeraka, N.M.; Xie, L.; Dong, L.; Liu, J.; Wang, L. Co-expression of High-mobility group box 1 protein (HMGB1) and receptor for advanced glycation end products (RAGE) in the prognosis of esophageal squamous cell carcinoma. Discov. Oncol. 2022, 13, 64. [Google Scholar] [CrossRef]
- Mao, W.; Zhou, J.; Hu, J.; Zhao, K.; Fu, Z.; Wang, J.; Mao, K. A pan-cancer analysis of FAT atypical cadherin 4 (FAT4) in human tumors. Front. Public Health 2022, 10, 969070. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wan, X.; Bai, Y. Identification of Down-Expressed CRNN Associated with Cancer Progression and Poor Prognosis in Laryngeal Squamous Cell Carcinoma. Front Biosci. 2024, 29, 125. [Google Scholar] [CrossRef]
- Kaur, R.; Chauhan, A.; Bhat, S.A.; Chatterjee, D.; Ghoshal, S.; Pal, A. Gene of the month: Cornulin. J. Clin. Pathol. 2022, 75, 289–291. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Wang, K.; Zhou, L.; Jiang, M.; Gao, Y.; Yang, R.; Yan, S.; Zhang, W.; Lu, B.; et al. Spatial transcriptomics analysis of esophageal squamous precancerous lesions and their progression to esophageal cancer. Nat. Commun. 2023, 14, 4779. [Google Scholar] [CrossRef]
- Visser, E.; Franken, I.A.; Brosens, L.A.A.; de Leng, W.W.J.; Strengman, E.; Offerhaus, J.A.; Ruurda, J.P.; van Hillegersberg, R. Targeted next-generation sequencing of commonly mutated genes in esophageal adenocarcinoma patients with long-term survival. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Onursal, C.; Dick, E.; Angelidis, I.; Schiller, H.B.; Staab-Weijnitz, C.A. Collagen Biosynthesis, Processing, and Maturation in Lung Ageing. Front Med. 2021, 8, 593874. [Google Scholar] [CrossRef]
- Abu-Romman, A.; Scholand, K.K.; Govindarajan, G.; Yu, Z.; Pal-Ghosh, S.; Stepp, M.A.; de Paiva, C.S. Age-Related Differences in the Mouse Corneal Epithelial Transcriptome and Their Impact on Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 2024, 65, 21. [Google Scholar] [CrossRef] [PubMed]
- Deraman, M.A.; Abdul Hafidz, M.I.; Lawenko, R.M.; Ma, Z.F.; Wong, M.S.; Coyle, C.; Lee, Y.Y. Randomised clinical trial: The effectiveness of Gaviscon Advance vs non-alginate antacid in suppression of acid pocket and post-prandial reflux in obese individuals after late-night supper. Aliment. Pharmacol. Ther. 2020, 51, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Sheppard, D. TGF-beta signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, X.Q.; Li, P. The Role of TGF-beta and Its Receptors in Gastrointestinal Cancers. Transl. Oncol. 2019, 12, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.R.; Xiang, X.; Mishra, L. TGF-beta Signaling in Liver, Pancreas, and Gastrointestinal Diseases and Cancer. Gastroenterology 2021, 161, 434–452.e415. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-beta Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, M.U.; Yatoo, A.M.; Arafah, A.; Khan, A.; Rashid, S.; Majid, S.; Ali, A.; Ali, M.N. TGF-beta signaling pathway: Therapeutic targeting and potential for anti-cancer immunity. Eur. J. Pharmacol. 2023, 947, 175678. [Google Scholar] [CrossRef]
- Lee, S.W.; Lien, H.C.; Lin, C.C.; Wen, M.C.; Chang, C.S. Low Expression of Transforming Growth Factor beta in the Epithelium of Barrett’s Esophagus. Gastroenterol. Res. 2018, 11, 189–194. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Liu, T.; Zhang, S.; Lu, J.; Fan, W.; Lin, L.; Xiang, T.; Yang, J.; Zhao, X.; et al. Epithelial cells activate fibroblasts to promote esophageal cancer development. Cancer Cell 2023, 41, 903–918.e908. [Google Scholar] [CrossRef]
- Tambunting, L.; Kelleher, D.; Duggan, S.P. The Immune Underpinnings of Barrett’s-Associated Adenocarcinogenesis: A Retrial of Nefarious Immunologic Co-Conspirators. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1297–1315. [Google Scholar] [CrossRef]
- Doukas, P.G.; Vageli, D.P.; Sasaki, C.T.; Judson, B.L. Pepsin Promotes Activation of Epidermal Growth Factor Receptor and Downstream Oncogenic Pathways, at Slightly Acidic and Neutral pH, in Exposed Hypopharyngeal Cells. Int. J. Mol. Sci. 2021, 22, 4275. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Zhang, S.S.; Zhong, J.T.; Zhou, S.H. Pepsin and Laryngeal and Hypopharyngeal Carcinomas. Clin. Exp. Otorhinolaryngol. 2021, 14, 159–168. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Kametani, F.; Cui, X.; Igarashi, Y.; Huo, J.; Miyahara, H.; Mori, M.; Higuchi, K. Curcumin promotes AApoAII amyloidosis and peroxisome proliferation in mice by activating the PPARalpha signaling pathway. Elife 2021, 10, 63538. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, F.; Lin, Y.; Han, Y.; Yang, L.; Tan, H. Peroxisome proliferator-activated receptors as therapeutic target for cancer. J. Cell Mol. Med. 2023, 28, e17931. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef] [PubMed]
- Nickkho-Amiry, M.; McVey, R.; Holland, C. Peroxisome proliferator-activated receptors modulate proliferation and angiogenesis in human endometrial carcinoma. Mol. Cancer Res. 2012, 10, 441–453. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Ding, C.; Wang, J.; Ma, Y.; Li, Z.; Zhang, X.; Wang, P. Proteomic Profiling Change and Its Implies in the Early Mycosis Fungoides (MF) Using Isobaric Tags for Relative and Absolute Quantification (iTRAQ). Biomed. Res. Int. 2020, 2020, 9237381. [Google Scholar] [CrossRef]
- Wong, Y.L.; Ramanathan, A.; Yuen, K.M.; Mustafa, W.M.W.; Abraham, M.T.; Tay, K.K.; Rahman, Z.A.A.; Chen, Y. Comparative sera proteomics analysis of differentially expressed proteins in oral squamous cell carcinoma. PeerJ 2021, 9, e11548. [Google Scholar] [CrossRef]
- Si, X.; Xu, F.; Xu, F.; Wei, M.; Ge, Y.; Chenge, S. CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway. Biomed. Pharmacother. 2020, 123, 109717. [Google Scholar] [CrossRef]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Liu, Z.; Zhang, L.; Fang, X.; Sun, J.; Zhang, Z.; Sun, Y. Sodium Alginate Prevents Non-Alcoholic Fatty Liver Disease by Modulating the Gut-Liver Axis in High-Fat Diet-Fed Rats. Nutrients 2022, 14, 4846. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, S.; Horibe, S.; Sasaki, N.; Tanahashi, T.; Mizuno, S.; Hamaguchi, T.; Rikitake, Y. Inhibitory Effects of Sodium Alginate on Hepatic Steatosis in Mice Induced by a Methionine- and Choline-deficient Diet. Mar. Drugs 2019, 17, 104. [Google Scholar] [CrossRef]
- Woodland, P.; Lee, C.; Duraisamy, Y.; Farre, R.; Dettmar, P.; Sifrim, D. Assessment and protection of esophageal mucosal integrity in patients with heartburn without esophagitis. Am. J. Gastroenterol. 2013, 108, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shan, C.; Zhang, H.; Kong, P.; Zhang, L.; Wang, Y.; Hu, X.; Cheng, X. FAT1 downregulation enhances stemness and cisplatin resistance in esophageal squamous cell carcinoma. Mol. Cell Biochem. 2022, 477, 2689–2702. [Google Scholar] [CrossRef]
- Huang, F.Y.; Wong, D.K.; Mak, L.Y.; Cheung, T.T.; Zhang, S.S.; Chau, H.T.; Hui, R.W.; Seto, W.K.; Yuen, M.F. FAT4 loss initiates hepatocarcinogenesis through the switching of canonical to noncanonical WNT signaling pathways. Hepatol. Commun. 2023, 7, e0338. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Zhou, L.; Ma, Z.; Zhang, J.; Wu, Y.; Shao, Y.; Yang, Y. FAT1 and PTPN14 Regulate the Malignant Progression and Chemotherapy Resistance of Esophageal Cancer through the Hippo Signaling Pathway. Anal. Cell Pathol. 2021, 2021, 9290372. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Ma, Y.; Teng, J.; Wang, Y.; Cui, Y.; Dong, Y.; Shao, S.; Zhan, Q.; Liu, X. FAT1, a direct transcriptional target of E2F1, suppresses cell proliferation, migration and invasion in esophageal squamous cell carcinoma. Chin. J. Cancer Res. 2019, 31, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.K.; Ye, Y.J.; Liu, T.D.; Peng, S.Z.; Zhang, R.S. Fat1 inhibits cell proliferation via ERK signaling pathway in esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi 2021, 43, 523–527. [Google Scholar] [CrossRef]
- Takaki, W.; Konishi, H.; Shoda, K.; Arita, T.; Kataoka, S.; Shibamoto, J.; Furuke, H.; Takabatake, K.; Shimizu, H.; Komatsu, S.; et al. Significance of Circular FAT1 as a Prognostic Factor and Tumor Suppressor for Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2021, 28, 8508–8518. [Google Scholar] [CrossRef]
- Wang, L.; Li, K.; Wang, C.; Shi, X.; Yang, H. miR-107 regulates growth and metastasis of gastric cancer cells via activation of the PI3K-AKT signaling pathway by down-regulating FAT4. Cancer Med. 2019, 8, 5264–5273. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Z.; Liu, Y.; Guo, L.; Teng, L.; Guo, L.; Liang, L.; Wang, J.; Gao, J.; Li, R.; et al. Genomic characterization reveals distinct mutation landscapes and therapeutic implications in neuroendocrine carcinomas of the gastrointestinal tract. Cancer Commun. 2022, 42, 1367–1386. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer. Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Tsiambas, E.; Mastronikolis, N.; P, P.F.; Kyrodimos, E.; Chrysovergis, A.; Papanikolaou, V.; Mastronikolis, S.; Peschos, D.; Roukas, D.; Ragos, V. c-Jun/c-Fos complex in laryngeal squamous cell carcinoma. J. BUON 2020, 25, 618–620. [Google Scholar]
- Im, N.R.; Kim, B.; Jung, K.Y.; Baek, S.K. Matrix metalloproteinase-7 induces E-cadherin cleavage in acid-exposed primary human pharyngeal epithelial cells via the ROS/ERK/c-Jun pathway. J. Mol. Med. 2022, 100, 313–322. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Sun, X.; Liu, M.; Zhu, M.; Yuan, Y.; Zhang, J.; Dong, Y.; Hu, K.; Ma, S.; et al. JUNB mediates oxaliplatin resistance via the MAPK signaling pathway in gastric cancer by chromatin accessibility and transcriptomic analysis. Acta Biochim. Biophys. Sin. 2023, 55, 1784–1796. [Google Scholar] [CrossRef]
- Roumeliotou, A.; Strati, A.; Chamchougia, F.; Xagara, A.; Tserpeli, V.; Smilkou, S.; Lagopodi, E.; Christopoulou, A.; Kontopodis, E.; Drositis, I.; et al. Comprehensive Analysis of CXCR4, JUNB, and PD-L1 Expression in Circulating Tumor Cells (CTCs) from Prostate Cancer Patients. Cells 2024, 13, 782. [Google Scholar] [CrossRef]
- Ren, F.J.; Cai, X.Y.; Yao, Y.; Fang, G.Y. JunB: A paradigm for Jun family in immune response and cancer. Front. Cell Infect. Microbiol. 2023, 13, 1222265. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Han, B.; Chen, Y.; Bai, X.; Yu, S.; Liu, M. Huanglian ointment alleviates eczema by maintaining the balance of c-Jun and JunB and inhibiting AGE-RAGE-mediated pro-inflammation signaling pathway. Phytomedicine 2022, 105, 154372. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Cheng, Y.; Ren, H.; Hu, Y.; Zhang, Y.; Su, H.; Zou, Z.; Wang, Q.; Liu, Z.; et al. MZT2A promotes NSCLC viability and invasion by increasing Akt phosphorylation via the MOZART2 domain. Cancer Sci. 2021, 112, 2210–2222. [Google Scholar] [CrossRef]
- Wieczorek, M.; Huang, T.L.; Urnavicius, L.; Hsia, K.C.; Kapoor, T.M. MZT Proteins Form Multi-Faceted Structural Modules in the gamma-Tubulin Ring Complex. Cell Rep. 2020, 31, 107791. [Google Scholar] [CrossRef]
- Heitor da Silva Maues, J.; Ferreira Ribeiro, H.; de Maria Maues Sacramento, R.; Maia de Sousa, R.; Pereira de Tommaso, R.; Dourado Kovacs Machado Costa, B.; Cardoso Soares, P.; Pimentel Assumpcao, P.; de Fatima Aquino Moreira-Nunes, C.; Mario Rodriguez Burbano, R. Downregulated genes by silencing MYC pathway identified with RNA-SEQ analysis as potential prognostic biomarkers in gastric adenocarcinoma. Aging 2020, 12, 24651–24670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lin, Q.; Zheng, H.; Rao, Y.; Ji, T. The pro-invasive factor COL6A2 serves as a novel prognostic marker of glioma. Front. Oncol. 2022, 12, 897042. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, Y.C.; Wang, W.; Wang, Y.Q.; Liu, Y.; Yang, Z.; Zhao, M.M.; Feng, N.; Wang, Y.H.; Zhang, X.W.; et al. Deoxyhypusine hydroxylase as a novel pharmacological target for ischemic stroke via inducing a unique post-translational hypusination modification. Pharmacol. Res. 2022, 176, 106046. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, J.; Karatas, O.; Ittmann, M. MEX3D is an oncogenic driver in prostate cancer. Prostate 2021, 81, 1202–1213. [Google Scholar] [CrossRef]
- Wu, R.; Sun, J.Y.; Zhao, L.L.; Fan, Z.N.; Yang, C. Systematic Identification of Key Functional Modules and Genes in Gastric Cancer. Biomed. Res. Int. 2020, 2020, 8853348. [Google Scholar] [CrossRef]
- Gilman, K.E.; Limesand, K.H. The complex role of prostaglandin E(2)-EP receptor signaling in wound healing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R287–R296. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 2021, 11, 8836–8854. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Qu, Y.X.; Wang, B.; Shen, P.F.; Xu, J.D.; Chen, Y.X. COX-2/PGE2 facilitates fracture healing by activating the Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9721–9728. [Google Scholar] [CrossRef]
- Haworth, R.; Oakley, K.; McCormack, N.; Pilling, A. Differential expression of COX-1 and COX-2 in the gastrointestinal tract of the rat. Toxicol. Pathol. 2005, 33, 239–245. [Google Scholar] [CrossRef]
- Wallace, J.L.; Devchand, P.R. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br. J. Pharmacol. 2005, 145, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, N.; Yang, X.; Xin, X.; Jia, C.H.; Li, S.; Lu, Q.; Jiang, T.; Wang, T. Machine Learning and Novel Biomarkers Associated with Immune Infiltration for the Diagnosis of Esophageal Squamous Cell Carcinoma. J. Oncol. 2022, 2022, 6732780. [Google Scholar] [CrossRef] [PubMed]
- Funato, K.; Smith, R.C.; Saito, Y.; Tabar, V. Dissecting the impact of regional identity and the oncogenic role of human-specific NOTCH2NL in an hESC model of H3.3G34R-mutant glioma. Cell Stem Cell 2021, 28, 894–905.e897. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, Q.; Ai, B.; Fang, Y.; Wang, Z.; Wang, J. Prognostic Value and Clinical Significance of LIPH in Breast Cancer. Int. J. Gen. Med. 2021, 14, 7613–7623. [Google Scholar] [CrossRef]
- Kim, K.; Choi, E.Y.; Ahn, H.M.; Kim, D.G.; Kim, Y.J. Hemoglobin Subunit Theta 1 Promotes Proliferation by Reducing Reactive Oxygen Species in Lung Adenocarcinoma. Cancers 2023, 15, 5504. [Google Scholar] [CrossRef]
- Samuels, T.L.; Zimmermann, M.T.; Zeighami, A.; Demos, W.; Southwood, J.E.; Blumin, J.H.; Bock, J.M.; Johnston, N. RNA Sequencing Reveals Cancer-Associated Changes in Laryngeal Cells Exposed to Non-Acid Pepsin. Laryngoscope 2021, 131, 121–129. [Google Scholar] [CrossRef]

| Sham + PA vs. Sham + Sham | ||
| Top Canonical Pathways | p-Value | Overlap |
| Keratinization | 7.01 × 10−7 | 2.8% 6/214 |
| Collagen Degradation | 3.74 × 10−3 | 3.3% 2/61 |
| Melatonin Degradation II | 5.95 × 10−3 | 25.0% 1/4 |
| Top Diseases | p-Value Range | Number of Molecules |
| Gastrointestinal Disease | 4.78 × 10−2–2.28 × 10−8 | 33 |
| Organismal Injury and Abnormalities | 4.95 × 10−2–2.28 × 10−8 | 36 |
| Cancer | 4.73 × 10−2–4.19 × 10−5 | 36 |
| Molecular and Cellular Functions | p-Value Range | #Number of Molecules |
| Cell Morphology | 4.95 × 10−2–2.67 × 10−5 | 11 |
| Cellular Development | 4.80 × 10−2–1.46 × 10−4 | 21 |
| Cellular Growth and Proliferation | 4.80 × 10−2–1.46 × 10−4 | 20 |
| Cell Death and Survival | 4.89 × 10−2–4.08 × 10−4 | 18 |
| Cellular Assembly and Organization | 4.66 × 10−2–4.45 × 10−4 | 14 |
| Top Networks | Score | |
| Gastrointestinal Disease, Organismal Injury and Abnormalities, Cardiovascular System, Development and Function | 56 | |
| Cancer, Organismal Injury and Abnormalities, Cell Death and Survival | 24 | |
| Top Upstream Regulators | p-Value Range | |
| POU2F3 | 5.91 × 10−6 | |
| SPRR5 | 2.95 × 10−5 | |
| EFNA3 | 3.38 × 10−5 | |
| PAX1 | 3.86 × 10−5 | |
| USP12 | 4.94 × 10−5 | |
| GA + PA vs. Sham + PA | ||
| Top Canonical Pathways | p-Value | Overlap |
| Polyamine Regulation in Colon Cancer | 4.95 × 10−4 | 5.1% 3/59 |
| Collagen Degradation | 5.45 × 10−4 | 4.9% 3/61 |
| Collagen Chain Trimerization | 5.90 × 10−3 | 4.5% 2/44 |
| Top Diseases | p-Value Range | Number of Molecules |
| Organismal Injury and Abnormalities | 4.98 × 10−2–9.96 × 10−5 | 60 |
| Developmental Disorders | 4.34 × 10−2–3.62 × 10−4 | 15 |
| Connective Tissue Disorders | 4.84 × 10−2–3.62 × 10−4 | 11 |
| Molecular and Cellular Functions | p-Value Range | Number of Molecules |
| Cell Death and Survival | 4.98 × 10−2–2.61 × 10−3 | 12 |
| Cell-To-Cell Signaling and Interaction | 3.63 × 10−2–2.61 × 10−3 | 6 |
| Cellular Development | 4.59 × 10−2–2.61 × 10−3 | 12 |
| Cellular Growth and Proliferation | 4.59 × 10−2–2.61 × 10−3 | 8 |
| Cellular Movement | 4.84 × 10−2–2.61 × 10−3 | 8 |
| Top Networks | Score | |
| Cardiovascular System, Development and Function, Organismal Development, Cardiovascular Disease | 53 | |
| Cell Death and Survival, Organismal Injury and Abnormalities, Cell Signaling | 32 | |
| Top Upstream Regulators | p-Value Range | |
| HNRNPK | 2.73 × 10−4 | |
| HIPK1 | 3.24 × 10−4 | |
| N1,N11-diethylnorspermine | 7.00 × 10−4 | |
| TLR5 | 7.22 × 10−4 | |
| Salmonella enterica serotype abortus equilibrium polysaccharide | 8.09 × 10−4 | |
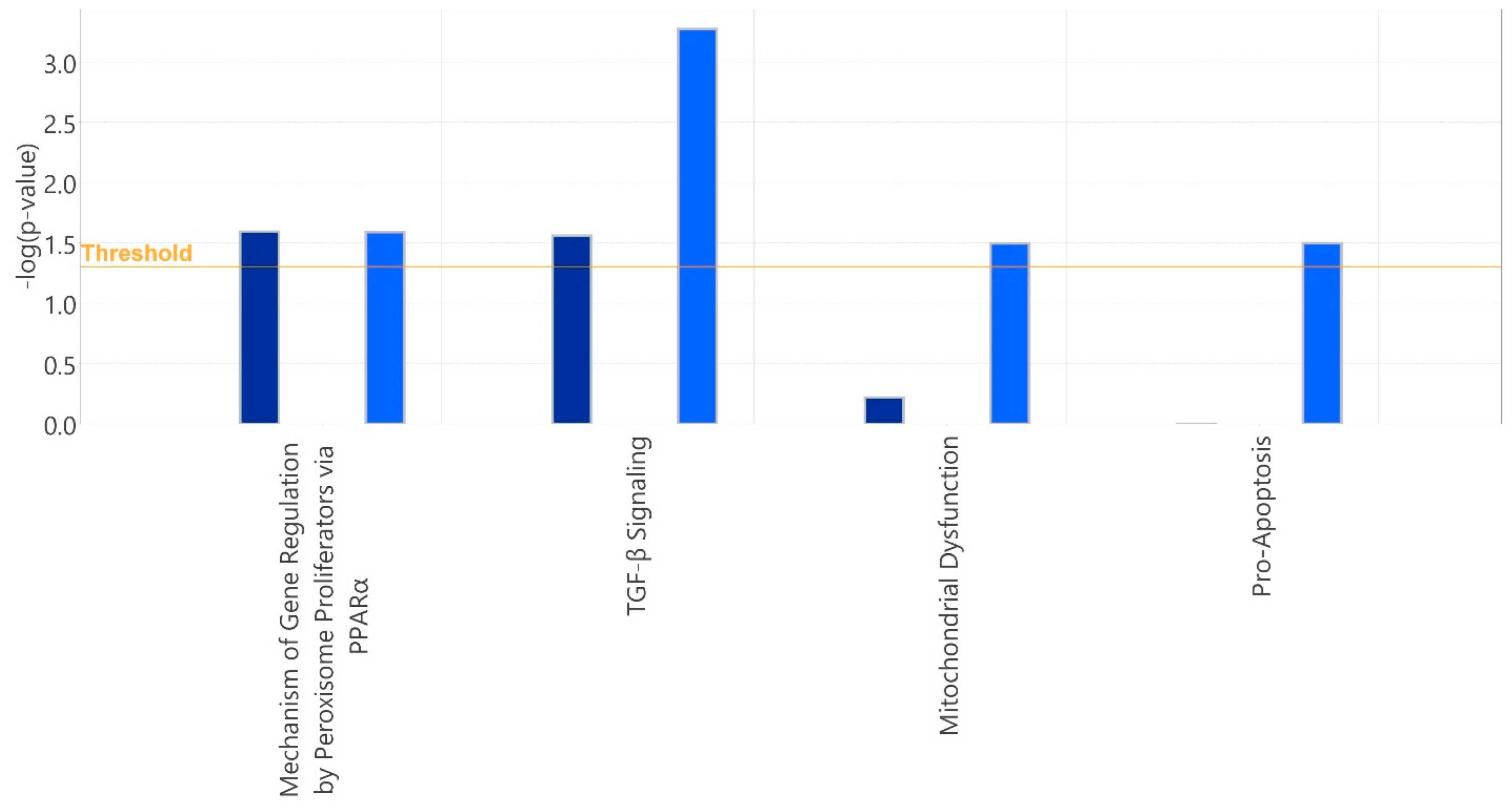
| GDA + PA vs. Sham + PA | ||
| Top Canonical Pathways | p-Value | Overlap |
| Synaptic Adhesion-like Molecules | 3.53 × 10−4 | 14.3% 3/21 |
| TGF- Signaling | 4.62 × 10−4 | 5.2% 5/96 |
| Collagen Degradation | 7.37 × 10−4 | 6.6% 4/61 |
| Top Diseases | p-Value Range | Number of Molecules |
| Cancer | 3.93 × 10−2–1.08 × 10−12 | 156 |
| Endocrine System Disorders | 3.48 × 10−2–1.08 × 10−12 | 138 |
| Organismal Injury and Abnormalities | 3.93 × 10−2–1.08 × 10−12 | 157 |
| Molecular and Cellular Functions | p-Value Range | Number of Molecules |
| Cellular Assembly and Organization | 3.93 × 10−2–2.62 × 10−4 | 29 |
| Gene Expression | 3.93 × 10−2–3.67 × 10−4 | 41 |
| Cellular Movement | 3.93 × 10−2–9.06 × 10−4 | 16 |
| Cell Death and Survival | 3.93 × 10−2–1.37 × 10−3 | 37 |
| Cellular Development | 3.93 × 10−2–1.37 × 10−3 | 59 |
| Top Networks | Score | |
| Cardiovascular System, Development and Function, Cellular Development, Cellular Growth and Proliferation | 35 | |
| Cancer, Organismal Injury and Abnormalities, Developmental Disorders | 35 | |
| Top Upstream Regulators | p-Value Range | |
| SMYD3 | 8.21 × 10−6 | |
| Firre | 1.31 × 10−5 | |
| SPOP | 1.86 × 10−5 | |
| FMN2 | 5.11 × 10−5 | |
| IL2RG | 5.99 × 10−5 | |
| Placebo + PA vs. Sham + PA | ||
| Top Canonical Pathways | p-Value | Overlap |
| Keratinization | 5.89 × 10−10 | 3.3% 7/214 |
| Collagen Degradation | 1.40 × 10−3 | 3.3% 2/61 |
| Wound Healing Signaling | 1.49 × 10−3 | 1.2% 3/252 |
| Top Diseases | p-Value Range | Number of Molecules |
| Gastrointestinal Disease | 2.25 × 10−2–9.24 × 10−10 | 9 |
| Organismal Injury and Abnormalities | 4.89 × 10−2–9.24 × 10−10 | 22 |
| Dermatological Diseases and Conditions | 4.89 × 10−2–3.77 × 10−7 | 10 |
| Molecular and Cellular Functions | p-Value Range | Number of Molecules |
| Cell Morphology | 1.98 × 10−2–5.86 × 10−6 | 3 |
| Cellular Movement | 3.79 × 10−2–1.03 × 10−4 | 4 |
| Cellular Development | 4.63 × 10−2–8.46 × 10−4 | 7 |
| Carbohydrate Metabolism | 9.10 × 10−4–9.10 × 10−4 | 1 |
| Cell Cycle | 4.54 × 10−3–9.10 × 10−4 | 2 |
| Top Networks | Score | |
| Gastrointestinal Disease, Organismal Injury and Abnormalities, Dermatological Diseases and Conditions | 33 | |
| Lipid Metabolism, Molecular Transport, Small Molecule Biochemistry | 23 | |
| Top Upstream Regulators | p-Value Range | |
| POU2F3 | 4.10 × 10−6 | |
| EFNA4 | 6.35 × 10−6 | |
| EFNA3 | 7.30 × 10−6 | |
| PAX1 | 7.80 × 10−6 | |
| SPRR5 | 1.02 × 10−5 | |
| Based on False Discovery Rate | |||||
|---|---|---|---|---|---|
| Upregulated | Downregulated | Upregulated | Downregulated | ||
| Sham + PA vs. Sham + Sham | SAPCD1 | TPGS1 | Placebo + PA vs. Sham + PA | MMP1 | DNAAF4 |
| BEST1 | KRT14 | ZNF837 | TRPC3 | ||
| SMOX | KRT13 | CCDC85A | TIGD3 | ||
| AGER | FAT4 | LIPH | NUTM2E | ||
| TMEM91 | RAPGEF2 | SPRR2E | GPRIN3 | ||
| FSCN3 | POLR2J2 | PDZRN3 | PRAM1 | ||
| RAMP1 | MIURF | NOTCH2NL | ST8SIA4 | ||
| SNX22 | EEA1 | HBQ1 | PADI1 | ||
| SLC52A1 | HERC1 | GRIN3B | GARIN1A | ||
| ACAP1 | LPP | AL121899.2 | ITK | ||
| Upregulated | Downregulated | Upregulated | Downregulated | ||
| GA + PA vs. Sham + PA | MT-ND6 | FBXL15 | GDA + PA vs. Sham + PA | MT-ND6 | FBXL15 |
| FAT4 | PCSK1N | EMP1 | JUN | ||
| RAPGEF2 | MZT2A | FAT4 | MEX3D | ||
| POLR2J2 | SCAND1 | GCNT4 | ATP6C | ||
| HERC1 | TPGS1 | AHNAK | COL6A2 | ||
| LPP | DOHH | POLR2J2 | PCSK1N | ||
| POLR2J3 | CLEC11A | HERC1 | MZT2A | ||
| RP11 | CIMAP1B | POLR2J3 | KLF2 | ||
| NCR3LG1 | HES4 | RP11 | SBNO2 | ||
| FAT1 | PRR7 | UBR4 | SCAND1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergun, P.; Samuels, T.L.; Mathison, A.J.; Plehhova, K.; Coyle, C.; Horvath, L.; Johnston, N. Global Transcriptomic Analysis of Topical Sodium Alginate Protection against Peptic Damage in an In Vitro Model of Treatment-Resistant Gastroesophageal Reflux Disease. Int. J. Mol. Sci. 2024, 25, 10714. https://doi.org/10.3390/ijms251910714
Ergun P, Samuels TL, Mathison AJ, Plehhova K, Coyle C, Horvath L, Johnston N. Global Transcriptomic Analysis of Topical Sodium Alginate Protection against Peptic Damage in an In Vitro Model of Treatment-Resistant Gastroesophageal Reflux Disease. International Journal of Molecular Sciences. 2024; 25(19):10714. https://doi.org/10.3390/ijms251910714
Chicago/Turabian StyleErgun, Pelin, Tina L. Samuels, Angela J. Mathison, Kate Plehhova, Cathal Coyle, Lizzie Horvath, and Nikki Johnston. 2024. "Global Transcriptomic Analysis of Topical Sodium Alginate Protection against Peptic Damage in an In Vitro Model of Treatment-Resistant Gastroesophageal Reflux Disease" International Journal of Molecular Sciences 25, no. 19: 10714. https://doi.org/10.3390/ijms251910714








