Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14
Abstract
1. Introduction
2. Results
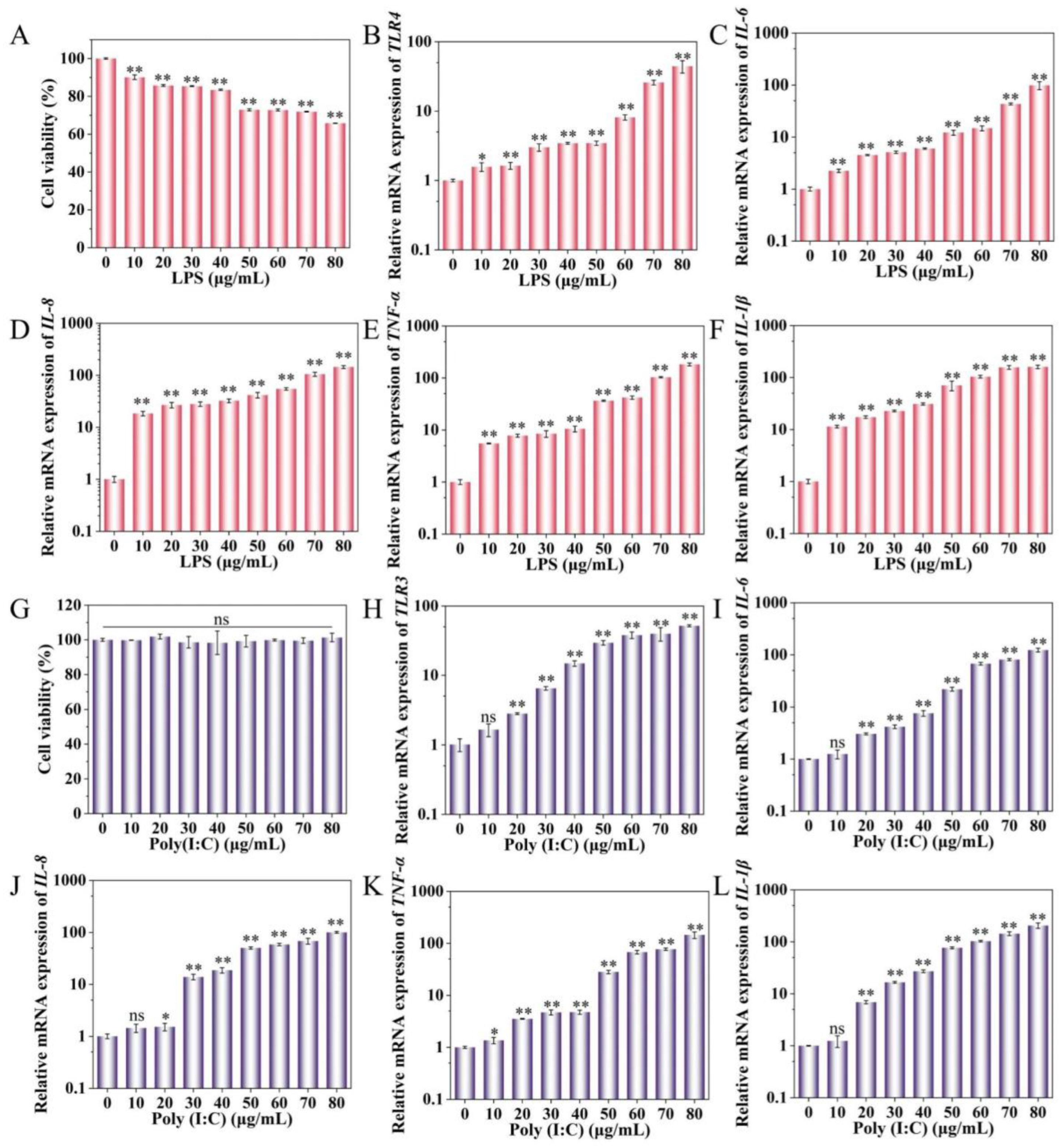
2.1. Effect of LPS and Poly(I:C) on IPEC-J2 Cell Activity and Cytokine Expression
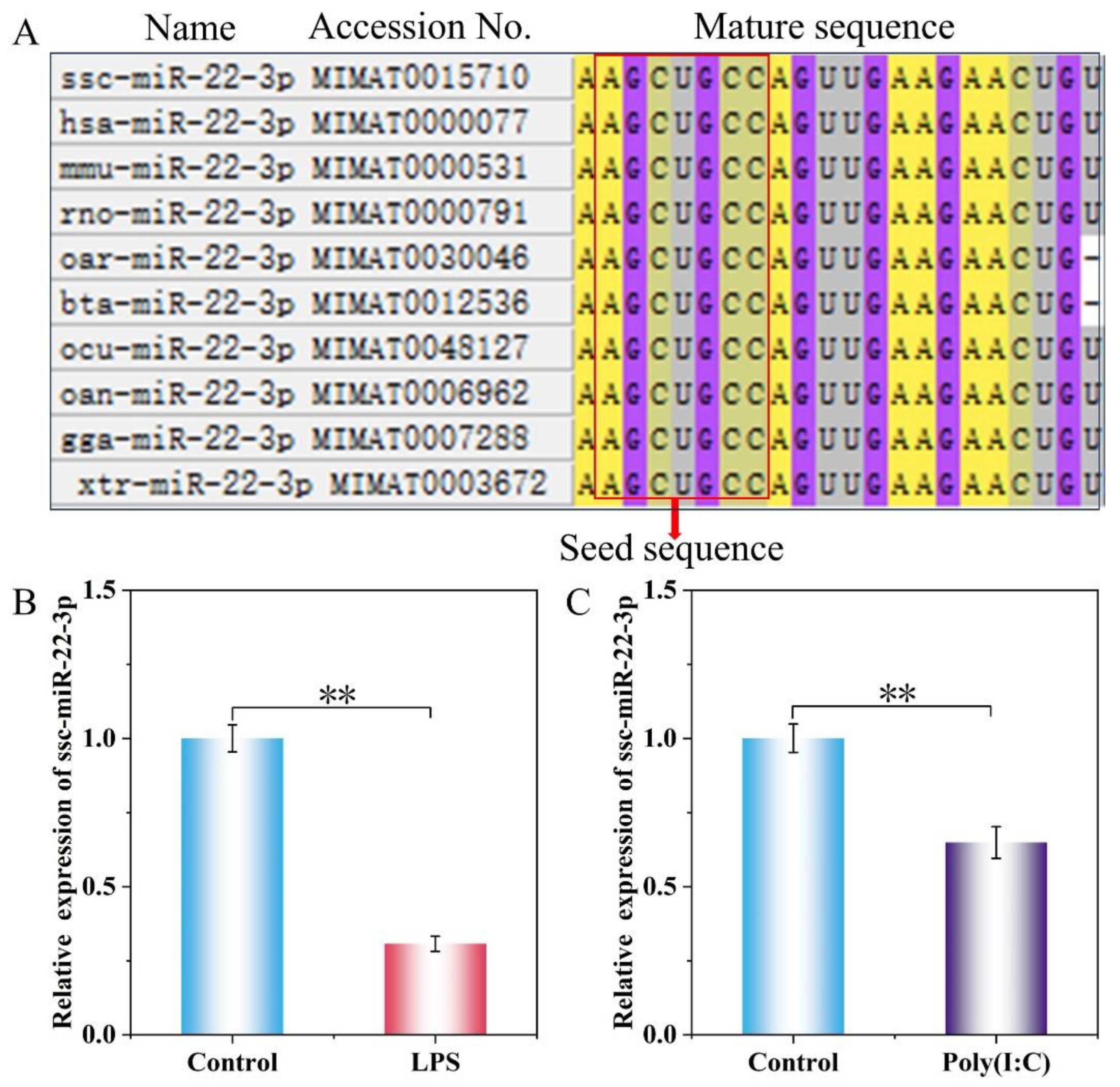
2.2. Expression Levels of ssc-miR-22-3p in LPS and Poly(I:C)-Stimulated IPEC-J2 Cells
2.3. ssc-miR-22-3p Transfection Efficiency Assay
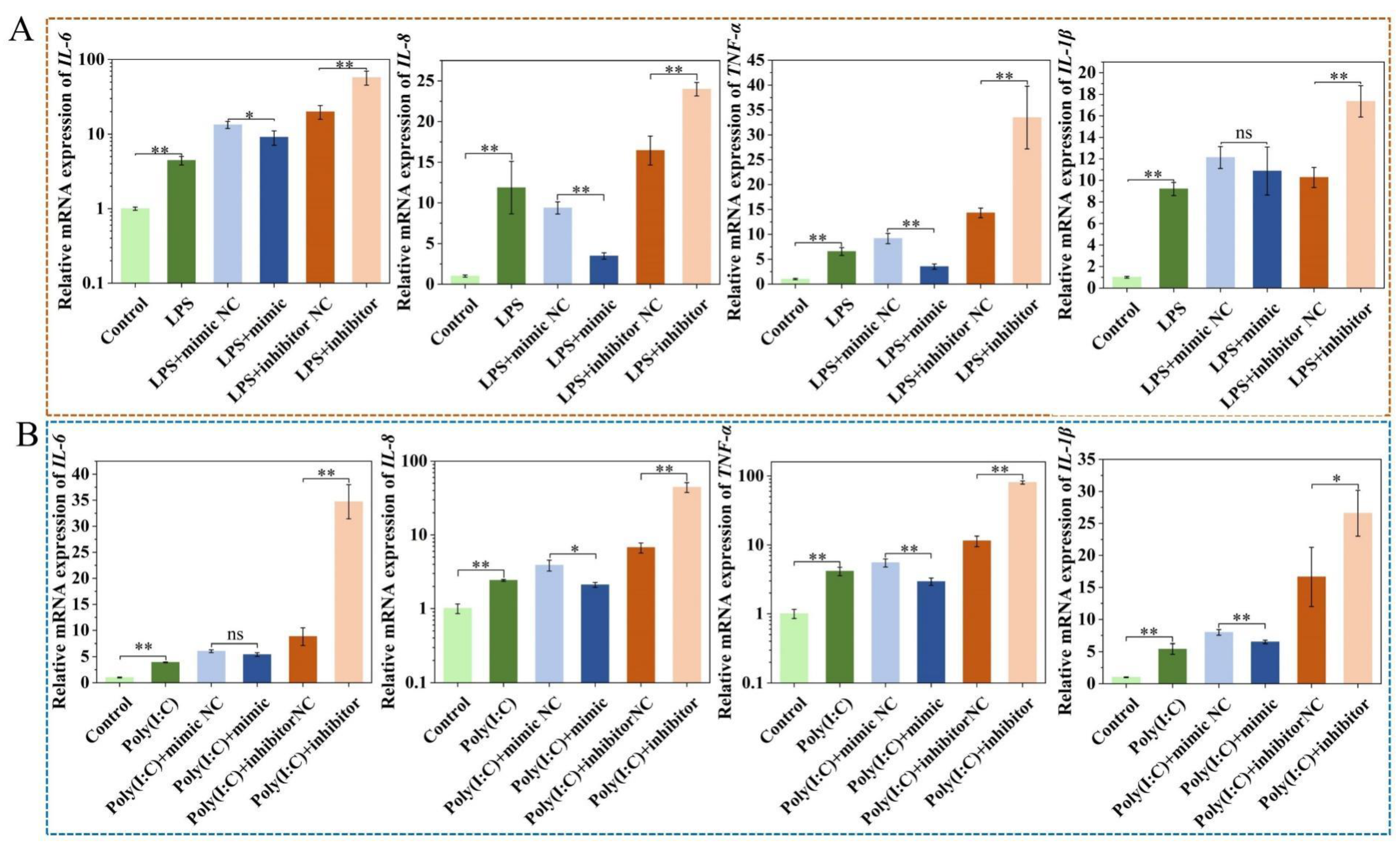
2.4. Upregulation of ssc-miR-22-3p Significantly Inhibits LPS and Poly (I:C)-Induced IPEC-J2 Cell Cytokine Expression
2.5. Effect of ssc-miR-22-3p on LPS and Poly (I:C)-Induced IPEC-J2 Cell Cytotoxicity
2.6. Effect of ssc-miR-22-3p on LPS and Poly (I:C)-Induced IPEC-J2 Cell Viability and Proliferation
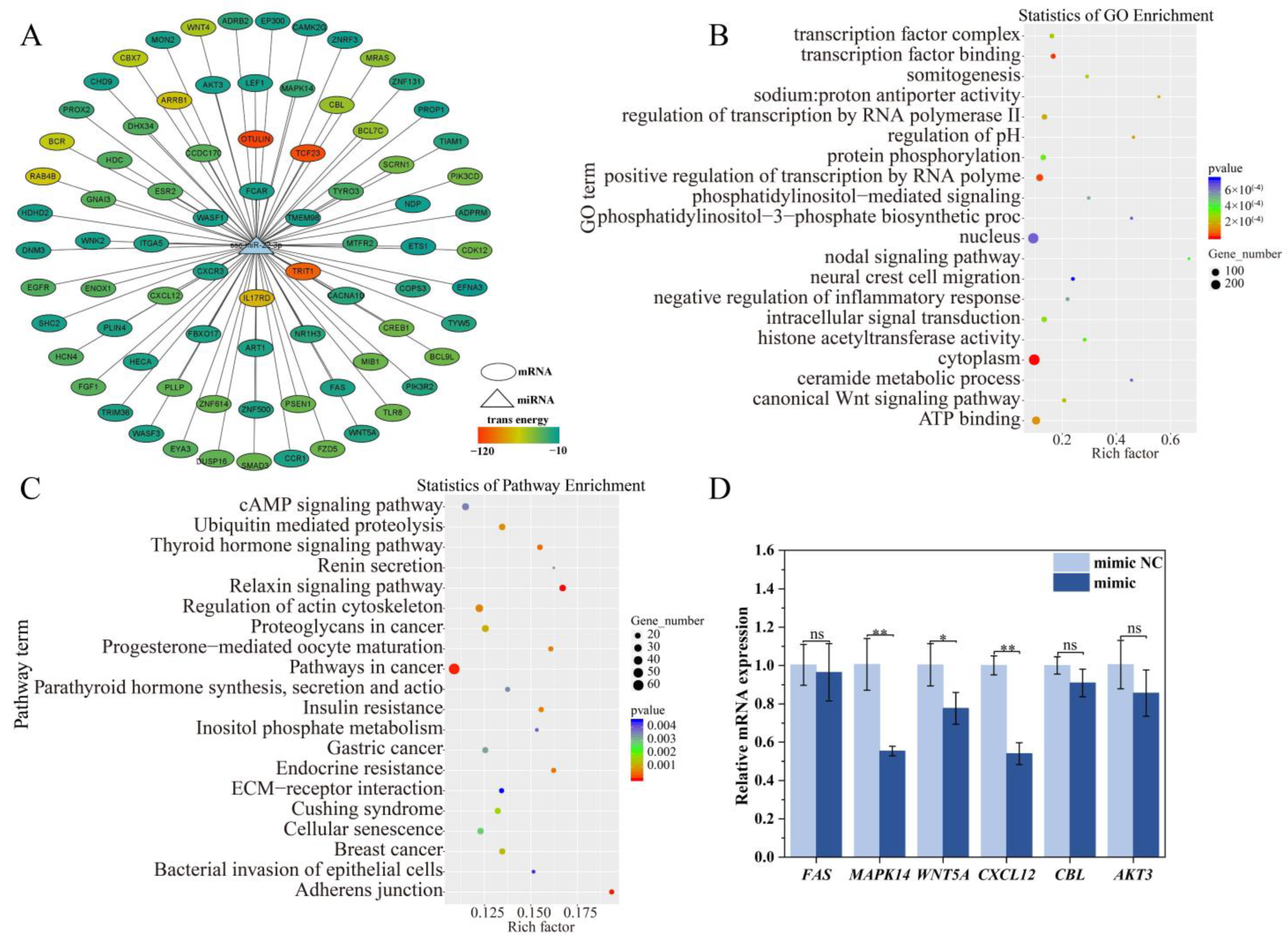
2.7. Target Gene and Functional Enrichment Analysis of ssc-miR-22-3p
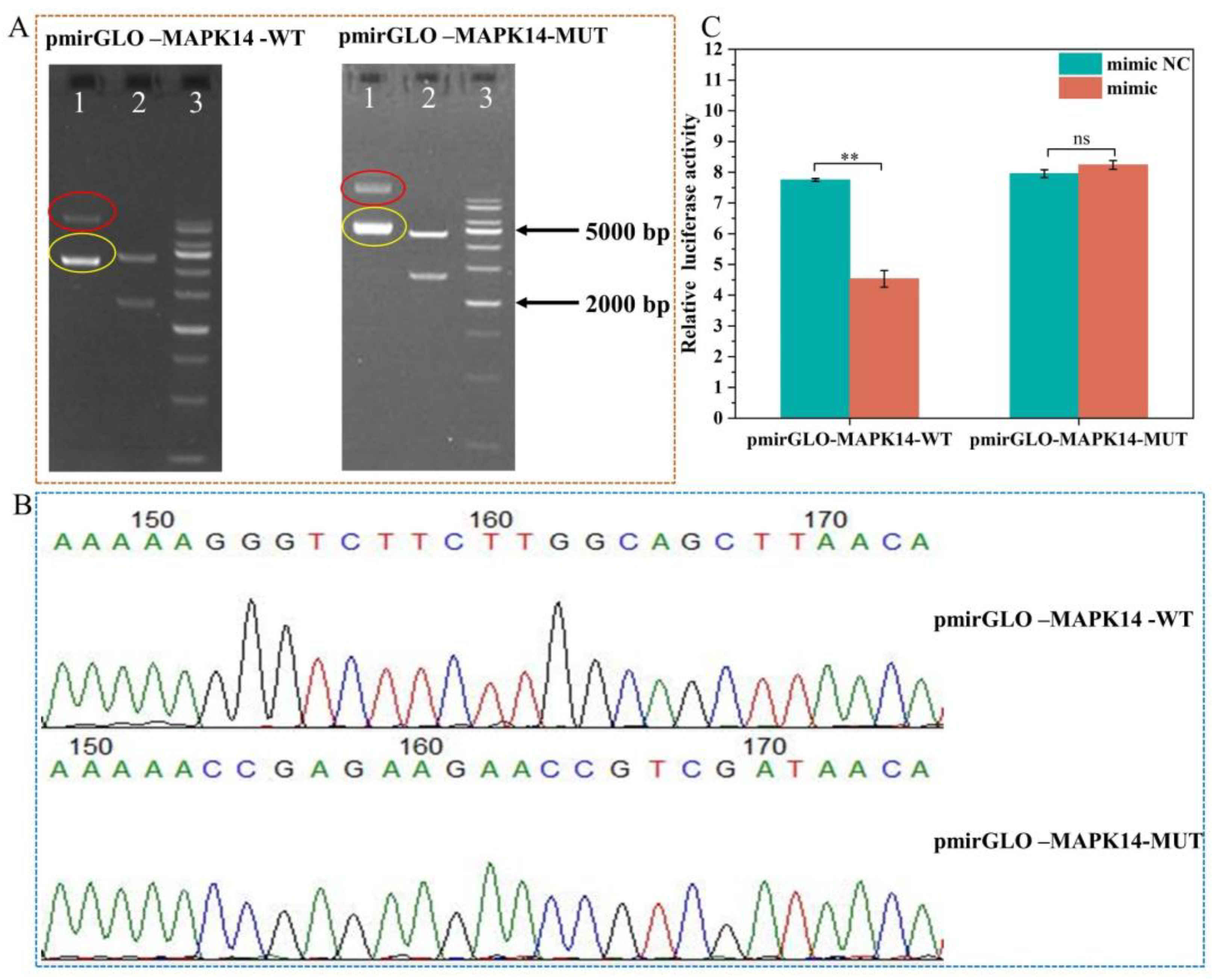
2.8. Validation of ssc-miR-22-3p and MAPK14 Targeting Relationship
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Transfection, and Construction of Cell Injury Models
4.2. Lactate Dehydrogenase (LDH) Activity Assay
4.3. Cell Viability and Proliferation Assays
4.4. Target Gene Prediction and Bioinformatics Analysis of ssc-miR-22-3p
4.5. Screening of Immune-Related Target Genes
4.6. Construction of Wild-Type and Mutant Plasmids in the MAPK14 3′ UTR Region
4.7. Dual Luciferase Reporter Gene Activity Assay
4.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J.; Jia, J.; Zhang, L.; Chen, Q.; Zhang, X.; Sun, W.; Ma, C.; Xu, F.; Zhan, S.; Ma, L.; et al. Jejunal inflammatory cytokines, barrier proteins and microbiome-metabolome responses to early supplementary feeding of Bamei suckling piglets. BMC Microbiol. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Hou, Y.; Yi, D.; Ding, B.; Xie, J.; Zhang, Y.; Chen, H.; Wu, T.; Zhao, D.; et al. N-Acetylcysteine supplementation alleviates intestinal injury in piglets infected by porcine epidemic diarrhea virus. Amino Acids 2017, 49, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Grouls, M.; van der Zande, M.; de Haan, L.; Bouwmeester, H. Responses of increasingly complex intestinal epithelium in vitro models to bacterial toll-like receptor agonists. Toxicol. Vitr. 2022, 79, 105280. [Google Scholar] [CrossRef]
- Finn, K.L.; Kineman, B.D.; Czerkies, L.A.; Carvalho, R.S. Human Milk Bioactives: Future Perspective. Nestle Nutr. Inst. Workshop Ser. 2021, 96, 166–174. [Google Scholar] [CrossRef]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef]
- Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early. Nutrients 2020, 12, 581. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef]
- Wani, S.; Man Law, I.K.; Pothoulakis, C. Role and mechanisms of exosomal miRNAs in IBD pathophysiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G646–G654. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F.A.-O. Exosome-mediated effects and applications in inflammatory bowel disease. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1287–1307. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.A.-O.; Stremmel, W.; Weiskirchen, R.A.-O.; John, S.M.; Schmitz, G.A.-O. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.A.-O. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of microRNA-target recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.F.; Zhou, B.; Liu, G.; Chen, C.-Z. Micromanagement of the immune system by microRNAs. Nat. Rev. Immunol. 2008, 8, 120–130. [Google Scholar] [CrossRef]
- Chen, T.; Xi, Q.Y.; Ye, R.S.; Cheng, X.; Qi, Q.E.; Wang, S.B.; Shu, G.; Wang, L.N.; Zhu, X.T.; Jiang, Q.Y.; et al. Exploration of microRNAs in porcine milk exosomes. BMC Genom. 2014, 15, 100. [Google Scholar] [CrossRef]
- Xie, M.Y.; Chen, T.; Xi, Q.Y.; Hou, L.J.; Luo, J.Y.; Zeng, B.; Li, M.; Sun, J.J.; Zhang, Y.L. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochem. Pharmacol. 2020, 175, 113898. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Jin, X.; Hu, D.; Xia, C.; Xu, H.; Hu, J. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J. Neuroinflammation 2021, 17, 126. [Google Scholar] [CrossRef]
- Izadparast, F.; Riahi-Zajani, B.; Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Protective effect of berberine against LPS-induced injury in the intestine: A review. Cell Cycle 2022, 21, 2365–2378. [Google Scholar] [CrossRef]
- Yi, D.; Hou, Y.; Xiao, H.; Wang, L.; Zhang, Y.; Chen, H.; Wu, T.; Ding, B.; Hu, C.A.; Wu, G. N-Acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways. Amino Acids 2017, 49, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Quach, C.; Helou, D.G.; Li, M.; Hurrell, B.P.; Howard, E.; Shafiei-Jahani, P.; Soroosh, P.; Ou, J.J.; Razani, B.; Rehan, V.; et al. Enhancing autophagy in CD11c+ antigen-presenting cells as a therapeutic strategy for acute respiratory distress syndrome. Cell Rep. 2023, 42, 112990. [Google Scholar] [CrossRef]
- Bianchi, F.A.-O.; Pretto, S.; Tagliabue, E.A.-O.; Balsari, A.A.-O.; Sfondrini, L.A.-O. Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol. Ther. 2017, 18, 747–756. [Google Scholar] [CrossRef]
- Foster, C.G.; Landowski, L.M.; Sutherland, B.A.; Howells, D.W. Differences in fatigue-like behavior in the lipopolysaccharide and poly I:C inflammatory animal models. Physiol. Behav. 2021, 232, 113347. [Google Scholar] [CrossRef]
- Li, J.; Shang, X.; Zhang, S.; Yang, Q.A.-O.; Yan, Z.A.-O.; Wang, P.; Gao, X.; Gun, S.; Huang, X. Breed-Related Differential microRNA Expression and Analysis of Colostrum and Mature Milk Exosomes in Bamei and Landrace Pigs. Int. J. Mol. Sci. 2024, 25, 667. [Google Scholar] [CrossRef]
- Song-Zhao, G.X.; Srinivasan, N.; Pott, J.; Baban, D.; Frankel, G.; Maloy, K.J. Nlrp3 activation in the intestinal epithelium protects against a mucosal pathogen. Mucosal Immunol. 2014, 7, 763–774. [Google Scholar] [CrossRef]
- Kim, K.-U.; Kim, W.-H.; Jeong, C.H.; Yi, D.Y.; Min, H. More than Nutrition: Therapeutic Potential of Breast Milk-Derived Exosomes in Cancer. Int. J. Mol. Sci. 2020, 21, 7327. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.A.-O.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J. Pediatr. Surg. 2017, 52, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y.; et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Starkhammar, M.; Georén, S.K.; Swedin, L.; Dahlén, S.-E.; Adner, M.; Cardell, L.O. Intranasal administration of poly(I:C) and LPS in BALB/c mice induces airway hyperresponsiveness and inflammation via different pathways. PLoS ONE 2012, 7, e32110. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.; Vu, T.H.; Lee, S.; Lillehoj, H.S.; Hong, Y.H. Immunomodulatory effects of poly(I:C)-stimulated exosomes derived from chicken macrophages. Poult. Sci. 2021, 100, 101247. [Google Scholar] [CrossRef]
- Heinz, S.; Haehnel, V.; Karaghiosoff, M.; Schwarzfischer, L.; Müller, M.; Krause, S.W.; Rehli, M. Species-specific regulation of Toll-like receptor 3 genes in men and mice. J. Biol. Chem. 2003, 278, 21502–21509. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.S.; Park, H.G.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J.T. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 27–31. [Google Scholar] [CrossRef]
- Sales Conniff, A.; Encalada, G.; Patel, S.; Bhandary, M.; Al-Takrouri, F.; Heller, L.A.-O. Poly(I:C) transfection induces a pro-inflammatory cascade in murine mammary carcinoma and fibrosarcoma cells. RNA Biol. 2022, 19, 841–851. [Google Scholar] [CrossRef]
- Zhou, M.W.; Jiang, R.H.; Kim, K.D.; Lee, J.H.; Kim, C.D.; Yin, W.T.; Lee, J.H. Rosmarinic acid inhibits poly(I:C)-induced inflammatory reaction of epidermal keratinocytes. Life Sci. 2016, 155, 189–194. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, A.R.; Yun, C.H.; Han, S.H. Staphylococcus aureus lipoproteins augment inflammatory responses in poly I:C-primed macrophages. Cytokine 2018, 111, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.-E.; Kent, S.; Ashdown, H.; Poole, S.; Boksa, P.; Luheshi, G.N. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R759–R766. [Google Scholar] [CrossRef]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Müller, P.; et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nat. Biotechnol. 2000, 408, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.J.; Miska, E.A. The microRNAs of Caenorhabditis elegans. Semin. Cell Dev. Biol. 2010, 21, 728–737. [Google Scholar] [CrossRef]
- Jiang, R.A.-O.; Lönnerdal, B.A.-O. Milk-Derived miR-22-3p Promotes Proliferation of Human Intestinal Epithelial Cells (HIECs) by Regulating Gene Expression. Nutrients 2022, 14, 4901. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Kong, M.; Yang, J. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Biosci. Rep. 2020, 40, BSR20200527. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, X.A.-O.; Ge, L.; Gu, Y.; Lv, X.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. MiR-22-3p Inhibits Proliferation and Promotes Differentiation of Skeletal Muscle Cells by Targeting IGFBP3 in Hu Sheep. Animals 2022, 12, 114. [Google Scholar] [CrossRef]
- Deng, F.; Yan, J.; Lu, J.; Luo, M.; Xia, P.; Liu, S.; Wang, X.; Zhi, F.; Liu, D. M2 Macrophage-Derived Exosomal miR-590-3p Attenuates DSS-Induced Mucosal Damage and Promotes Epithelial Repair via the LATS1/YAP/β-Catenin Signalling Axis. J. Crohn’s Colitis 2021, 15, 665–677. [Google Scholar] [CrossRef]
- Chae, W.J.; Bothwell, A.L.M. Canonical and Non-Canonical Wnt Signaling in Immune Cells. Trends Immunol. 2018, 39, 830–847. [Google Scholar] [CrossRef]
- Avery, D.; Morandini, L.; Sheakley, L.S.; Shah, A.H.; Bui, L.; Abaricia, J.O.; Olivares-Navarrete, R. Canonical Wnt signaling enhances pro-inflammatory response to titanium by macrophages. Biomaterials 2022, 289, 121797. [Google Scholar] [CrossRef]
- Tewari, D.; Bawari, S.; Sharma, S.; DeLiberto, L.K.; Bishayee, A. Targeting the crosstalk between canonical Wnt/β-catenin and inflammatory signaling cascades: A novel strategy for cancer prevention and therapy. Pharmacol. Ther. 2021, 227, 107876. [Google Scholar] [CrossRef] [PubMed]
- Silva-García, O.; Valdez-Alarcón, J.J.; Baizabal-Aguirre, V.M. The Wnt/β-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediat. Inflamm. 2014, 2014, 310183. [Google Scholar] [CrossRef] [PubMed]
- Athanasios, A.; Charalampos, V.; Vasileios, T.; Ashraf, G. Protein-Protein Interaction (PPI) Network: Recent Advances in Drug Discovery. Curr. Drug Metab. 2017, 18, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Hassan, R.M.; Mersal, K.I.; Ammar, U.M.; Se-In, C.; He-Soo, H.; Kim, H.K.; Lee, A.; Lee, K.T.; et al. Design, synthesis and anti-inflammatory activity of imidazol-5-yl pyridine derivatives as p38α/MAPK14 inhibitor. Bioorganic Med. Chem. 2021, 31, 115969. [Google Scholar] [CrossRef]
- cNamee, E.N.; Collins, C.B.; Lebsack, M.D.; Rivera–Nieves, J. Cell-specific inhibition of p38alpha as a therapeutic strategy for inflammatory bowel disease. Gastroenterology 2010, 138, 1237–1239. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Wu, H.T.; Ni, Y.G.; Yin, Y.T.; Li, S.X.; Liao, D.F.; Qin, L. Crosstalk between Wnt5a and inflammatory signaling in inflammation. Sheng Li Xue Bao 2015, 67, 437–445. [Google Scholar]
- Pashirzad, M.; Shafiee, M.; Rahmani, F.; Behnam-Rassouli, R.; Hoseinkhani, F.; Ryzhikov, M.; Moradi Binabaj, M.; Parizadeh, M.R.; Avan, A.; Hassanian, S.M. Role of Wnt5a in the Pathogenesis of Inflammatory Diseases. J. Cell. Physiol. 2017, 232, 1611–1616. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.L.; Li, D.H.; Wang, J.S.; Zhao, F. Dual Role of Wnt5a in the Progression of Inflammatory Diseases. Chin. Med. Sci. J. 2022, 37, 265–274. [Google Scholar] [CrossRef]
- Rauner, M.; Stein, N.; Winzer, M.; Goettsch, C.; Zwerina, J.; Schett, G.; Distler, J.H.; Albers, J.; Schulze, J.; Schinke, T.; et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Miner. Res. 2012, 27, 575–585. [Google Scholar] [CrossRef]
- Mousavi, A. CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol. Lett. 2020, 217, 91–115. [Google Scholar] [CrossRef]
- Dotan, I.; Werner, L.; Vigodman, S.; Weiss, S.; Brazowski, E.; Maharshak, N.; Chen, O.; Tulchinsky, H.; Halpern, Z.; Guzner-Gur, H. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm. Bowel Dis. 2010, 16, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, J.; Jiang, X.; Bai, R.A.-O. CXCR4/CXCL12 axis: “old” pathway as “novel” target for anti-inflammatory drug discovery. Med. Res. Rev. 2024, 44, 1189–1220. [Google Scholar] [CrossRef]
- Kuhn, D.E.; Martin, M.M.; Feldman, D.S.; Terry, A.V.; Nuovo, G.J.; Elton, T.S. Experimental validation of miRNA targets. Methods 2008, 44, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Nam, J.-W.; Rissland, O.S.; Koppstein, D.; Abreu-Goodger, C.; Jan, C.H.; Agarwal, V.; Yildirim, M.A.; Rodriguez, A.; Bartel, D.P. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell 2014, 53, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Koppal, A.; Agius, P.; Sander, C.; Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010, 11, R90. [Google Scholar] [CrossRef]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Name | Sense (5′–3′) | Antisense (5′~3′) |
|---|---|---|
| mimic NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| miR-22-3p mimic | AAGCUGCCAGUUGAAGAACUGU | AGUUCUUCAACUGGCAGCUUUU |
| inhibitor NC | CAGUACUUUUGUGUAGUACAA | |
| miR-22-3p inhibitor | ACAGUUCUUCAACUGGCAGCUU |
| Gene Name | Nucleotide Sequence (5ʹ-3ʹ) | Product Length (bp) |
|---|---|---|
| FAS | F: CGTGAGGGTCAATTCTGCTGTC | 134 |
| R: GAATGATGGTTCTTGTCTGTGTAATCC | ||
| MAPK14 | F: TTATCTCATTAACAGGATGCCAAGCC | 127 |
| R: CTCCAGCAAGTCAACAGCCAAG | ||
| WNT5A | F: TCTTGGTGGTCCTTAGGTATGAATAAC | 135 |
| R: GTGGTCCTGATACAAGTGGCATAG | ||
| CXCL12 | F: ATGCCCTTGCCGATTCTTTGAG | 126 |
| R: CACACTTGTCTATTGTTGCTCTTCAG | ||
| CBL | F: GGACAAGAAGATGGTGGAGAAGTG | 143 |
| R: AGATAGTGCGGAGATGCTGGTAG | ||
| AKT3 | F: TTCTCTGGAGTAAACTGGCAAGATG | 135 |
| R: AGGTGGCGTTATTGTAATAGTCTGAG | ||
| ssc-miR-22-3p | F: CGCAAGCTGCCAGTTGAAGAACT | |
| R: miRNA qPCR 3ʹ primer (Universal primers) | ||
| U6 | F: GGAACGATACAGAGAAGATTAGC | 68 |
| R: TGGAACGCTTCACGAATTTGCG | ||
| GAPDH | F: AGTATGATTCCACCCACGGC | 139 |
| R: TACGTAGCACCAGCATCACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hu, H.; Fu, P.; Yang, Q.; Wang, P.; Gao, X.; Yang, J.; Gun, S.; Huang, X. Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14. Int. J. Mol. Sci. 2024, 25, 10715. https://doi.org/10.3390/ijms251910715
Li J, Hu H, Fu P, Yang Q, Wang P, Gao X, Yang J, Gun S, Huang X. Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14. International Journal of Molecular Sciences. 2024; 25(19):10715. https://doi.org/10.3390/ijms251910715
Chicago/Turabian StyleLi, Jie, Huihui Hu, Panpan Fu, Qiaoli Yang, Pengfei Wang, Xiaoli Gao, Jiaojiao Yang, Shuangbao Gun, and Xiaoyu Huang. 2024. "Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14" International Journal of Molecular Sciences 25, no. 19: 10715. https://doi.org/10.3390/ijms251910715
APA StyleLi, J., Hu, H., Fu, P., Yang, Q., Wang, P., Gao, X., Yang, J., Gun, S., & Huang, X. (2024). Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14. International Journal of Molecular Sciences, 25(19), 10715. https://doi.org/10.3390/ijms251910715





