Abstract
A 16-year-old patient, while an infant, incurred right-sided hemiparesis and had difficulty breast feeding. She was later diagnosed with a neonatal stroke and her genetic testing showed a missense mutation in her PROS1 (Protein S) gene. Both her grandfather and father, but not her mother, had hereditary Protein S (PS) deficiency. The patient was not prescribed any mediation due to her young age but was frequently checked by her physician. The patient’s plasma was first collected at the age of 13, and the isolated plasma from the patient and her father were analyzed by aPTT, thrombin generation, and enzyme-linked immunosorbent assays. These analyses showed low PS activity and clotting time associated with the missense mutation in the PROS1 gene. During the COVID-19 pandemic, the patient received her first Pfizer vaccination dose in 2021, followed by a booster dose in 2022. The plasma samples were collected 8 weeks post-immunization, after which her clotting parameters had improved for up to 6 months following vaccination. The patient’s plasma showed a significant reduction in thrombin generation and an improved aPTT clotting time. Mass spectrometry analysis revealed that her antithrombin-III level was significantly higher post-vaccination, and both thrombin and FXII levels were significantly lowered compared with her father. To our knowledge, this is the first report to document that COVID-19 vaccination can lower the risk of thrombosis in a patient with inherited thrombophilia. Although the effect was observed on a single mutation, it would be interesting to investigate the effect of COVID-19 vaccinations on other thrombophilia.
1. Introduction
A well-documented delicate balance exists between the procoagulant and anticoagulant proteins in the control of tissue hemostasis and homeostasis [1]. Disruption of this balance results in a spectrum of coagulation disorders [2]. For instance, Protein S (PS) is a key anticoagulant that has an essential function in the coagulation process by acting as a cofactor for Activated Protein C [3] and Tissue Factor Pathway Inhibitor [4]. Furthermore, we showed that PS directly binds FIXa, thereby inhibiting FIXa-mediated activation of FX and limiting subsequent thrombin formation [5].
Genetic and acquired PS deficiencies are extensively documented. Although the symptoms associated with acquired PS deficiency are temporary, genetic alterations in the PROS1 gene can result in pulmonary embolism, thrombophilia, venous thromboembolism and defects in development of the vascular system [6]. An association between hypercoagulability and altered PS activity has been reported in conditions such as liver and kidney disease, pregnancy, the use of oral (estrogen-based) contraceptives, hypoxia, chemotherapy [7], and COVID-19 [8]. Patients affected by COVID-19 have a high risk of venous thromboembolism, and PS deficiency increases the risk of thrombosis by 20% for those patients [9]. Also, lower PS activities were detected in serum of COVID-19 patients, and it was found that PS served as a marker for recurrent stroke [10] and poor prognosis [11].
Although vaccines for COVID-19 provide protection against viral-associated diseases, venous thromboembolism is nevertheless a common symptom post-vaccination [12]. However, in this case study, we present a novel finding that an mRNA-based COVID-19 vaccine improved anticoagulation and limited thrombin generation in a 16-year-old patient with a PS deficiency who had a history of neonatal stroke.
2. Case Presentation
Sanger DNA sequencing was used to determine the cause of the patient’s stroke. We determined that both she and her father had a methionine-643 to threonine (M643T) missense mutation (rs750531364) within the PROS1 gene on 3q11.1 (Table 1). Formstone et al. reported that this mutation increases the risk of thrombosis [13]. In addition, a variant in intron12 (rs1453447448) was also identified in the patient’s PROS1 gene. This mutation overlaps 11 transcripts and causes the substitution of threonine to cysteine or alanine. However, no prior reports of this mutation have been documented.

Table 1.
Genetic variants identified within the PROS1 gene in the patient and her father.
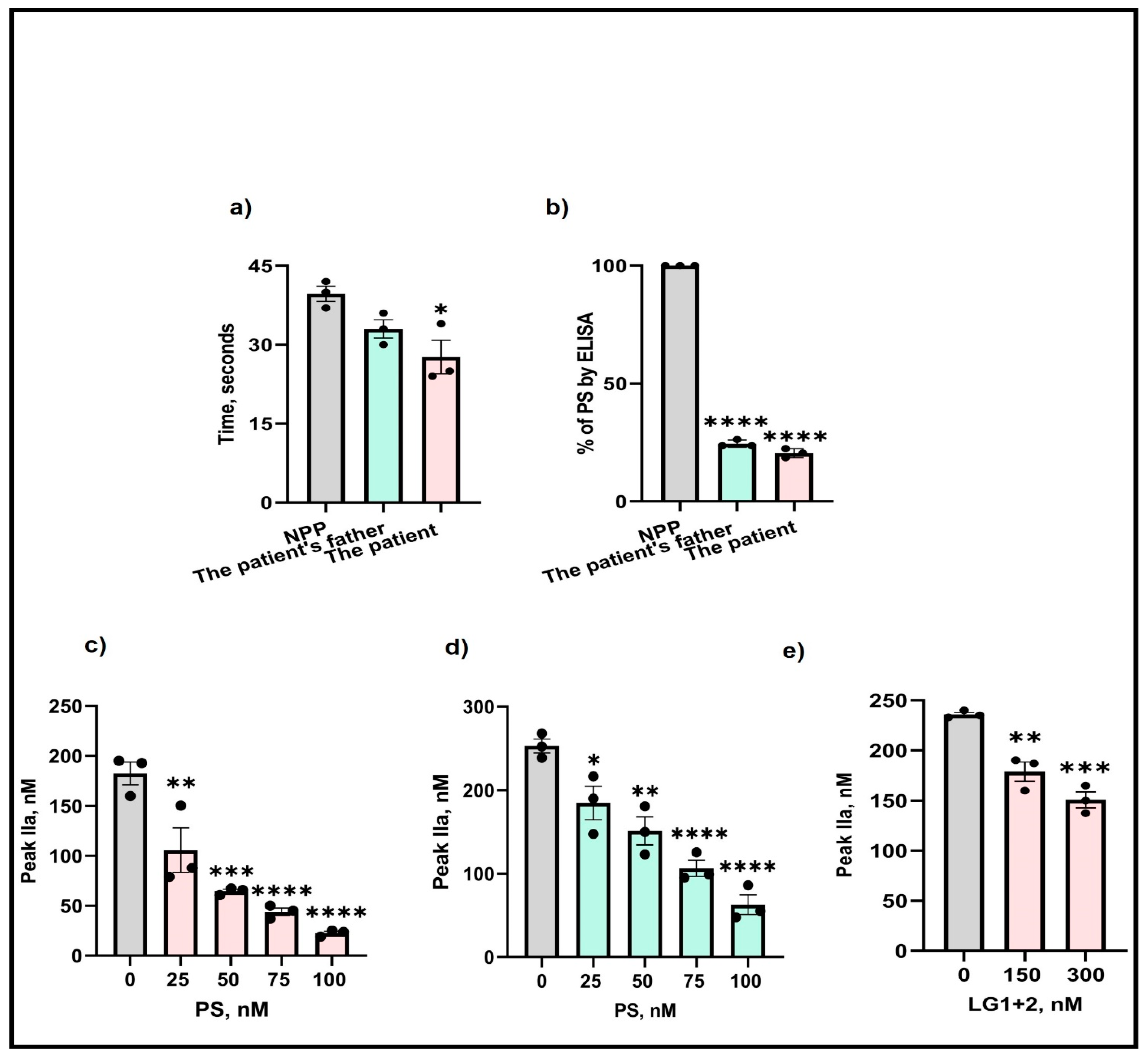
Next, to examine whether the M643T mutation affected the expression of PS, isolated plasma from the patient and her father were analyzed by aPTT and ELISA. Assessments of the clotting time in the patient’s plasma showed a 1.5-fold reduction in clotting time (Figure 1a) compared with the control (NPP—normal pooled plasma). Also, measurement of free PS by ELISA indicated an 80% and 74% reduction in the patient’s and her father’s plasma (Figure 1b), respectively.
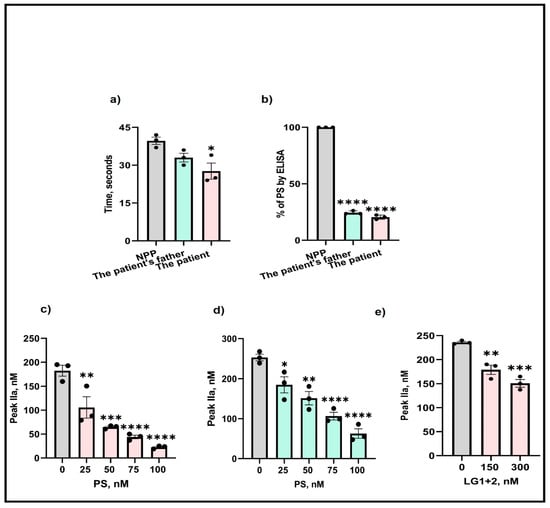
Figure 1.
Effects of M643T mutation on PS determined by (a) aPTT, (b) ELISA, and thrombin formation produced by (c) the patient, (d) the patient’s father, and (e) in presence of added PS LG1 + 2 domains. The values shown are the mean ± SD of three independent experiments in triplicate. Statistical analyses are performed by One-way ANOVA * p < 0.05, ** p, < 0.05, *** p <0.0001, **** p <0.0001.
To determine the effects of the M643T mutation on thrombin generation, isolated plasma from the patient and her father were supplemented with various concentrations (25–100 nM) of wild-type PS, and thrombin generation was measured. Before the addition of PS, the amount of thrombin produced in the PS-deficient patient’s plasma was 180 nM. However, supplementation with PS gradually decreased thrombin formation, reaching a significant 1.7-fold reduction (40 nM) after the addition of 100 nM PS (Figure 1c). Similar effects were observed for the father’s thrombin formation (Figure 1d).
The LG1 and LG2 domains of PS are important for binding to and inhibiting FIXa (our unpublished data). Therefore, we supplemented the plasmas with various LG1+2 concentrations (150–300 nM) and again assessed thrombin formation. The addition of LG1+2 to 300 nM resulted in a significant 1.2-fold reduction in the amount of thrombin produced, indicating the importance of these domains in the anticoagulant function of PS (Figure 1e).
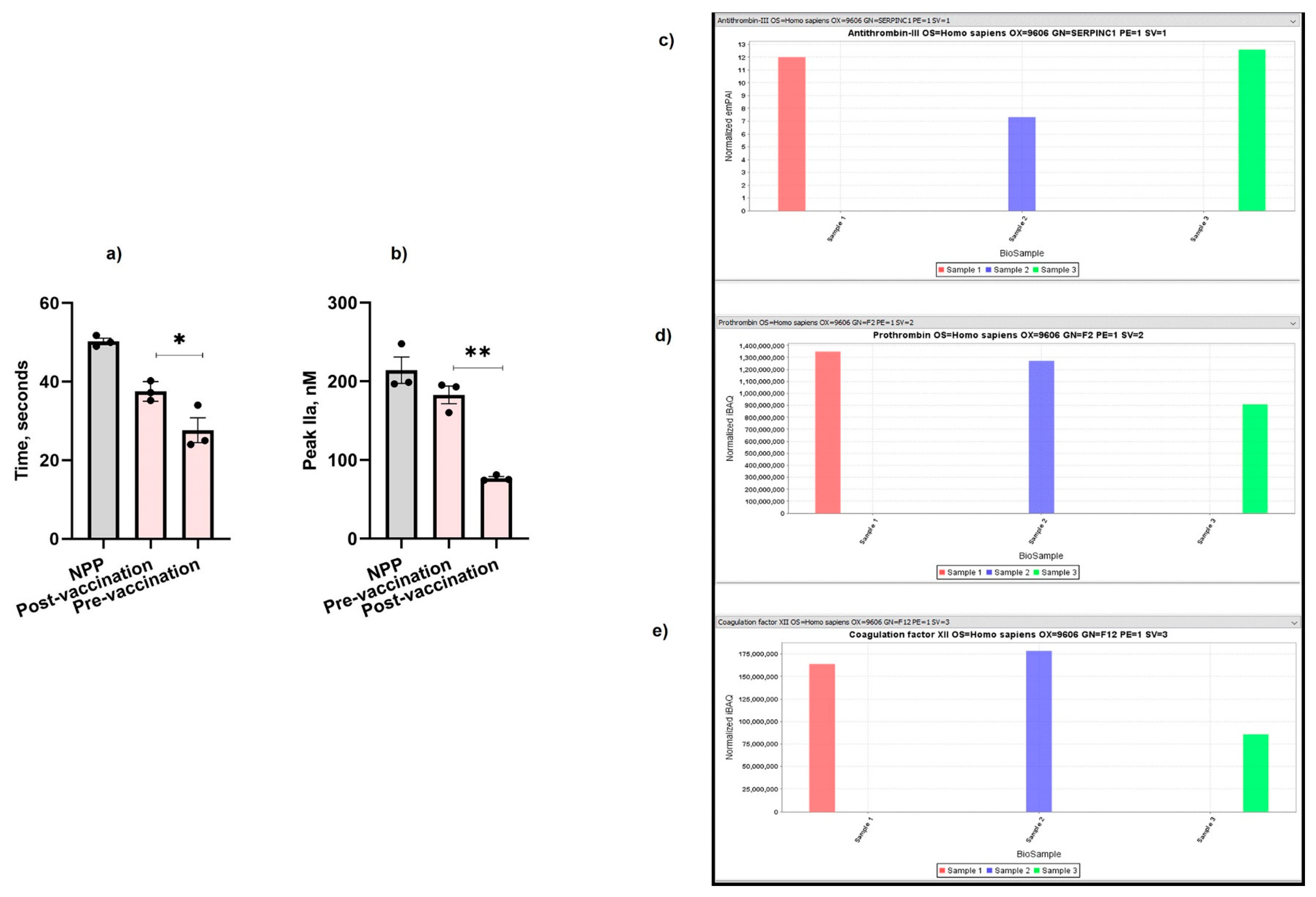
Following COVID-19 vaccination (Pfizer, New York City, NY, USA), the patient’s clotting time was increased by 12% (Figure 2a) and her thrombin formation was also significantly reduced by 2.4-fold post-vaccination (Figure 2b) compared to pre-vaccination (Figure 1a,b). The patient experienced an increase in the level of antithrombin-III (Figure 2c) (normalized exponentially modified protein abundance index (emPAI): 12.5; normalized intensity Based Absolute Quantitation (iBAQ): 2,500,000,000) compared with her father who was also vaccinated (normalized emPAI: 7.3; iBAQ: 1,900,000,000) and the control sample (normalized emPAI: 12; normalized iBAQ: 2,100,000,000). The level of thrombin was also significantly reduced in the patient (normalized iBAQ: 900,000,000) compared with the thrombin level of her father (normalized iBAQ: 1,290,000,000) and the control sample (normalized iBAQ: 1,330,000,000) (Figure 2d). Similarly, FXII was significantly lower in the patient’s plasma (normalized iBAQ: 87,000,000) compared to her father (normalized iBAQ: 180,000,000) and the control (normalized iBAQ: 163,000,000) (Figure 2e).
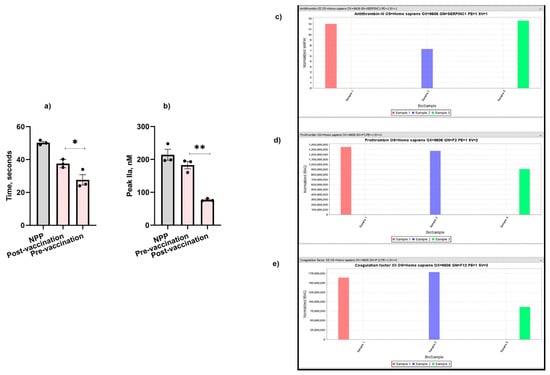
Figure 2.
Effect of COVID-19 vaccination on the patient’s (a) aPTT, (b) TGA, (c) antithrombin-III, (d) thrombin, and (e) FXII. Sample 1: Control. Sample 2: The patient’s father. Sample 3: The patient. The values shown are the mean ± SD of three independent experiments in triplicate. Statistical analyses are performed by One-way ANOVA * p < 0.05, ** p, < 0.05.
3. Discussion
Protein S deficiency is an autosomal-inherited thrombophilia which manifests as venous or arterial thrombosis. The symptoms associated with PS deficiency can vary from asymptomatic to severe, depending on the type of mutation and the age of onset [14]. In early infancy, compound heterozygous or homozygous PS deficiency may result in purpura fulminans [15,16], and standard anticoagulation therapy is usually recommended throughout adulthood [14]. There are over 453 variants recorded worldwide as of the year 2000 [17]. Since then, more variants have been identified in the PROS1 gene leading to various hereditary thrombophilia disorders [18]. In this case study, we identified several variants within the PROS1 gene in both the patient and her father. Most importantly, we identified a missense mutation (Table 1), rs750531364 c.1832T > C (p. Met611Thr), whereby methionine is substituted by threonine at residue 643 and causing PS deficiency. This variant resides in the LG2 domain of PS near the LG1+2 junction where residues E435 and E437 are responsible for binding FIXa (unpublished data), thereby limiting thrombin formation. Because of this mutation, her free PS levels are low (Figure 1b) and therefore PS cannot exert its effect on FIXa, resulting in a faster clotting time (Figure 1a) and higher thrombin formation (Figure 1c).
Since the pandemic, inherited PS deficiency has not been linked to prognosis of COVID-19, although Mele et al. (2022) reported that PS deficiency exacerbated the thrombotic burden leading to the death of a patient [19]. Acquired PS deficiency has been attributed to hypercoagulability and the increased risk of thrombosis in COVID-19 patients [20]. Several studies have linked mRNA vaccines and thrombosis [21], and one of the earliest pulmonary embolism cases induced by an mRNA-based COVID-19 vaccine was recorded in South Korea [12]. The exact mechanism behind these observations is unclear. It has been suggested that the virus spike protein expressed by the mRNA vaccines promotes the release of pro-inflammatory proteins and the subsequent activation of coagulation proteins [22] or that the spike protein activates TAM (TYRO3, AXL and MER) signaling receptors, leading to immuno-thrombosis [23]. Studies have also documented that COVID-19 vaccinations can alter the expression profile of non-coding RNA (ncRNA) such as microRNAs (miRNA) and long ncRNAs (lncRNAs) [24,25,26,27,28,29], resulting in various outcomes ranging from thrombosis [30] to reducing the severity of COVID-19 [31]. For instance, it has been reported that the expression of miR-195-5p and PS positively correlate [32] and both can serve as biomarkers of disease severity [33,34].
Initially, we selected various pro- and anti-coagulant proteins for the mass spectrometry analysis. However, there is a lack of plasma and published studies reporting the effect of COVID-19 vaccines on the increased the levels of FXII, FXIII, prothrombin, and antithrombin [35]. Therefore, we also selected FXII, antithrombin, and prothrombin for further analysis. Surprisingly, post-vaccination, the patient’s clotting time was improved (Figure 2a), which was also reflected on the lower thrombin formation (Figure 2b) compared to pre-vaccination values (Figure 1a,b). We also observed a reduction in the levels of FXII (Figure 2e) and prothrombin (Figure 2d) in the patient’s plasma, whereas her antithrombin-III (Figure 2c) level was significantly higher. Therefore, it is possible that the activation of FXI by FXII is hindered within the blood vessel walls, thereby affecting the activation of downstream coagulation factors. It would be interesting to test whether Pfizer’s COVID-19 vaccine has a similar effect on PS-deficient individuals having other PS mutations, and it would also be interesting to test the relevance of this observation in other inherited thrombophilia disorders.
Author Contributions
A.M., M.A.M. and D.P.-V. performed the experiments and collected experimental data. M.A.M. wrote the original manuscript. J.Z. performed the sequencing. L.T. introduced the patient’s mother to R.M.; R.M. conceived the research and reviewed and edited the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH R01 1 R01 HL151613 to R.M. J.Z. was funded by the Center for translational viral oncology (CTVO), Grant number: P20 GM121288-02.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB protocol number 912) from Lousiana State University Health Scince Center (LSUHSC). Consent has been obtained from the patient(s) to publish this paper.
Informed Consent Statement
We kindly thank the patient, her mother, and her father for providing the plasma samples and their consent for publication.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We acknowledge L.T. for her initial support for this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lane, D.A.; Philippou, H.; Huntington, J.A. Directing thrombin. Blood 2005, 106, 2605–2612. [Google Scholar] [CrossRef]
- Rodriguez, V.; Warad, D. Pediatric Coagulation Disorders. Pediatr. Rev. 2016, 37, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.J. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J. Biol. Chem. 1981, 256, 11128–11131. [Google Scholar] [CrossRef]
- Hackeng, T.M.; Sere, K.M.; Tans, G.; Rosing, J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc. Natl. Acad. Sci. USA 2006, 103, 3106–3111. [Google Scholar] [CrossRef]
- Chattopadhyay, R.; Sengupta, T.; Majumder, R. Inhibition of intrinsic Xase by protein S: A novel regulatory role of protein S independent of activated protein C. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2387–2393. [Google Scholar] [CrossRef]
- Burstyn-Cohen, T.; Heeb, M.J.; Lemke, G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J. Clin. Investig. 2009, 119, 2942–2953. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Nguyen, T. Protein S: Function, regulation, and clinical perspectives. Curr. Opin. Hematol. 2021, 28, 339–344. [Google Scholar] [CrossRef]
- Sim, M.M.S.; Wood, J.P. Dysregulation of Protein S in COVID-19. Best. Pract. Res. Clin. Haematol. 2022, 35, 101376. [Google Scholar] [CrossRef]
- Ferrari, E.; Sartre, B.; Squara, F.; Contenti, J.; Occelli, C.; Lemoel, F.; Levraut, J.; Doyen, D.; Dellamonica, J.; Mondain, V.; et al. High Prevalence of Acquired Thrombophilia without Prognosis Value in Patients with Coronavirus Disease 2019. J. Am. Heart Assoc. 2020, 9, e017773. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Gomez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Munoz Martin, A.J. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Yoo, S.J. Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report. Vaccines 2023, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Formstone, C.J.; Wacey, A.I.; Berg, L.P.; Rahman, S.; Bevan, D.; Rowley, M.; Voke, J.; Bernardi, F.; Legnani, C.; Simioni, P.; et al. Detection and characterization of seven novel protein S (PROS) gene lesions: Evaluation of reverse transcript-polymerase chain reaction as a mutation screening strategy. Blood 1995, 86, 2632–2641. [Google Scholar] [CrossRef]
- Gupta, A.; Tun, A.M.; Gupta, K.; Tuma, F. Protein S Deficiency. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pegelow, C.H.; Ledford, M.; Young, J.N.; Zilleruelo, G. Severe protein S deficiency in a newborn. Pediatrics 1992, 89, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Y.; Aygun, D.; Yilmaz, Y.; Kanra, G.; Secmeer, G.; Besbas, N.; Gurgey, A. Severe protein S deficiency associated with heterozygous factor V Leiden mutation in a child with purpura fulminans. Pediatr. Hematol. Oncol. 2003, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gandrille, S.; Borgel, D.; Sala, N.; Espinosa-Parrilla, Y.; Simmonds, R.; Rezende, S.; Lind, B.; Mannhalter, C.; Pabinger, I.; Reitsma, P.H.; et al. Protein S deficiency: A database of mutations--summary of the first update. Thromb. Haemost. 2000, 84, 918. [Google Scholar]
- Juhl, D.; Kuta, P.; Shneyder, M.; Wunsche, F.; Nowak-Gottl, U. Two Novel Variants in the Protein S Gene PROS1 Are Associated with Protein S Deficiency and Thrombophilia. Acta Haematol. 2021, 144, 222–226. [Google Scholar] [CrossRef]
- Mele, F.; Tafuri, S.; Stefanizzi, P.; Amati, D.A.; Calvano, M.; Leonardelli, M.; Macorano, E.; Duma, S.; De Gabriele, G.; Introna, F.; et al. Cerebral venous sinus thrombosis after COVID-19 vaccination and congenital deficiency of coagulation factors: Is there a correlation? Hum. Vaccin. Immunother. 2022, 18, 2095166. [Google Scholar] [CrossRef]
- Chirumamilla, Y.; Almerstani, Y.; Marcus, H.; Bachuwa, G. Protein S Deficiency and COVID-19: A Brutal Combination Leading to Acute Submassive Bilateral Pulmonary Embolism. Cureus 2023, 15, e41560. [Google Scholar] [CrossRef]
- Bekal, S.; Husari, G.; Okura, M.; Huang, C.A.; Bukari, M.S. Thrombosis Development After mRNA COVID-19 Vaccine Administration: A Case Series. Cureus 2023, 15, e41371. [Google Scholar] [CrossRef]
- Miri, C.; Bouchlarhem, A.; Boulouiz, S.; El Ouafi, N.; Bazid, Z. Pulmonary embolism with junctional tachycardia: A serious complication after COVID-19 vaccination. Ann. Med. Surg. 2022, 80, 103983. [Google Scholar] [CrossRef]
- Lemke, G.; Silverman, G.J. Blood clots and TAM receptor signalling in COVID-19 pathogenesis. Nat. Rev. Immunol. 2020, 20, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Gjorgjieva, T.; Chaloemtoem, A.; Shahin, T.; Bayaraa, O.; Dieng, M.M.; Alshaikh, M.; Abdalbaqi, M.; Del Monte, J.; Begum, G.; Leonor, C.; et al. Systems genetics identifies miRNA-mediated regulation of host response in COVID-19. Hum. Genom. 2023, 17, 49. [Google Scholar] [CrossRef]
- Chen, W.C.; Hu, S.Y.; Shen, C.F.; Cheng, M.H.; Hong, J.J.; Shen, C.J.; Cheng, C.M. COVID-19 Vaccination in Pregnancy: Pilot Study for Maternal and Neonatal MicroRNA Profiles. Vaccines 2023, 11, 1814. [Google Scholar] [CrossRef]
- Talotta, R. COVID-19 mRNA vaccines as hypothetical epigenetic players: Results from an in silico analysis, considerations and perspectives. Vaccine 2023, 41, 5182–5194. [Google Scholar] [CrossRef]
- Saeed, R.H.; Abdulrahman, Z.F.A.; Mohammad, D.K. The impact of COVID-19 on microRNA and CD marker expression in AML patients. Sci. Rep. 2024, 14, 14251. [Google Scholar] [CrossRef] [PubMed]
- Gilyazova, I.; Timasheva, Y.; Karunas, A.; Kazantseva, A.; Sufianov, A.; Mashkin, A.; Korytina, G.; Wang, Y.; Gareev, I.; Khusnutdinova, E. COVID-19: Mechanisms, risk factors, genetics, non-coding RNAs and neurologic impairments. Noncoding RNA Res. 2023, 8, 240–254. [Google Scholar] [CrossRef]
- Roustai Geraylow, K.; Hemmati, R.; Kadkhoda, S.; Ghafouri-Fard, S. miRNA expression in COVID-19. Gene Rep. 2022, 28, 101641. [Google Scholar] [CrossRef]
- Tay, J.W.; Romeo, G.; Hughes, Q.W.; Baker, R.I. Micro-ribonucleic Acid 494 regulation of protein S expression. J. Thromb. Haemost. 2013, 11, 1547–1555. [Google Scholar] [CrossRef]
- Rasizadeh, R.; Aghbash, P.S.; Nahand, J.S.; Entezari-Maleki, T.; Baghi, H.B. SARS-CoV-2-associated organs failure and inflammation: A focus on the role of cellular and viral microRNAs. Virol. J. 2023, 20, 179. [Google Scholar] [CrossRef]
- Rodriguez-Rius, A.; Lopez, S.; Martinez-Perez, A.; Souto, J.C.; Soria, J.M. Identification of a Plasma MicroRNA Profile Associated With Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Stoichitoiu, L.E.; Pinte, L.; Balea, M.I.; Nedelcu, V.; Badea, C.; Baicus, C. Anticoagulant protein S in COVID-19: Low activity, and associated with outcome. Rom. J. Intern. Med. 2020, 58, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Moatar, A.I.; Chis, A.R.; Romanescu, M.; Ciordas, P.D.; Nitusca, D.; Marian, C.; Oancea, C.; Sirbu, I.O. Plasma miR-195-5p predicts the severity of Covid-19 in hospitalized patients. Sci. Rep. 2023, 13, 13806. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Arefanian, H.; Channanath, A.M.; AlKhairi, I.; Cherian, P.; Devarajan, S.; Thanaraj, T.A.; Abu-Farha, M.; Abubaker, J.; Al-Mulla, F. Association of COVID-19 Vaccines ChAdOx1-S and BNT162b2 with Circulating Levels of Coagulation Factors and Antithrombin. Vaccines 2022, 10, 1226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).