Methyl Jasmonate (MeJA) Promotes the Self-Pollen Tube Growth of Camellia oleifera by Regulating Lignin Biosynthesis
Abstract
1. Introductions
2. Results
2.1. Appropriate Concentrations of MeJA Promoted Pollen Germination and Pollen Tube Elongation
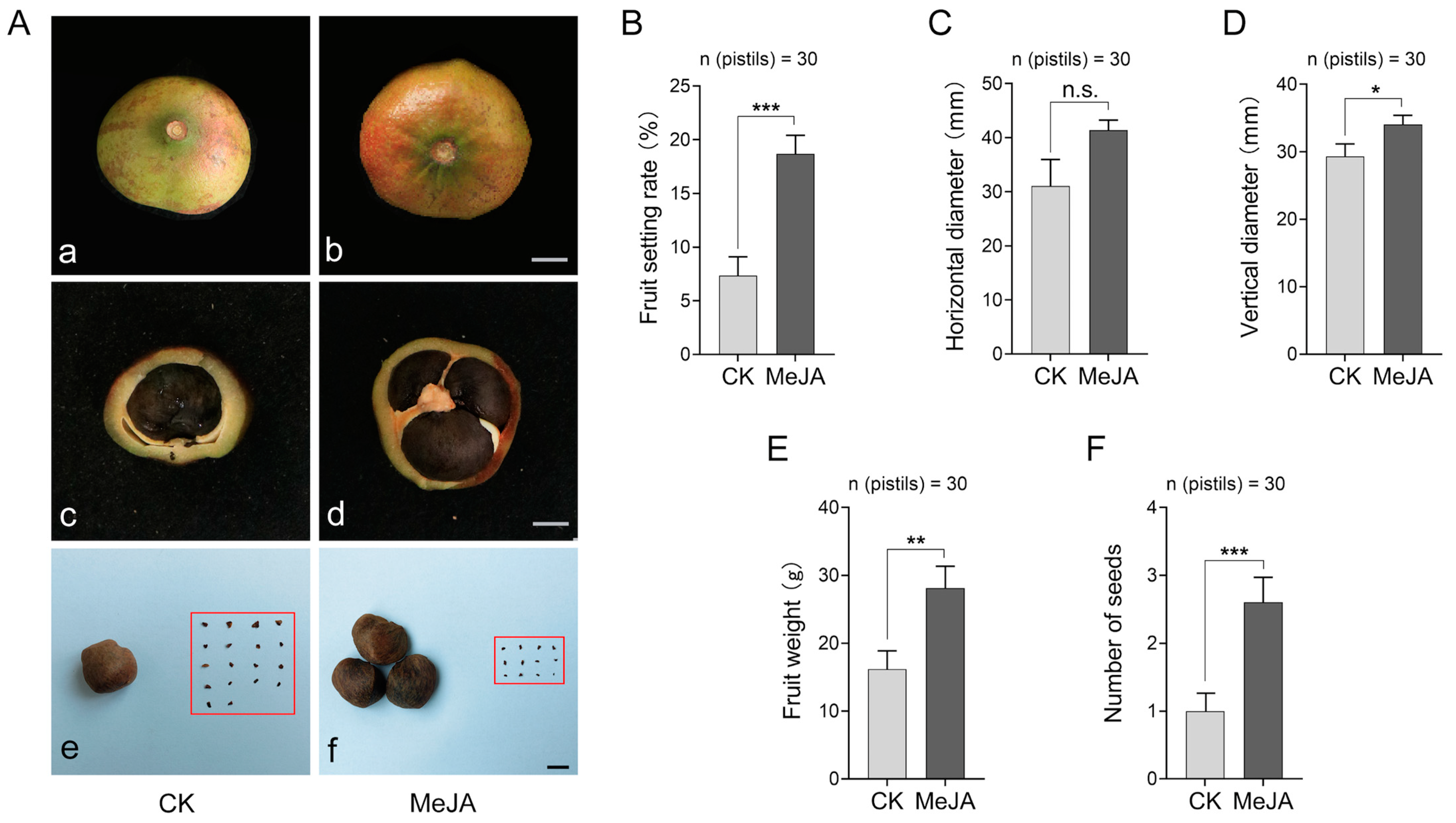
2.2. MeJA Improved the Fruit Setting Percentage in Self-Pollinated C. oleifera
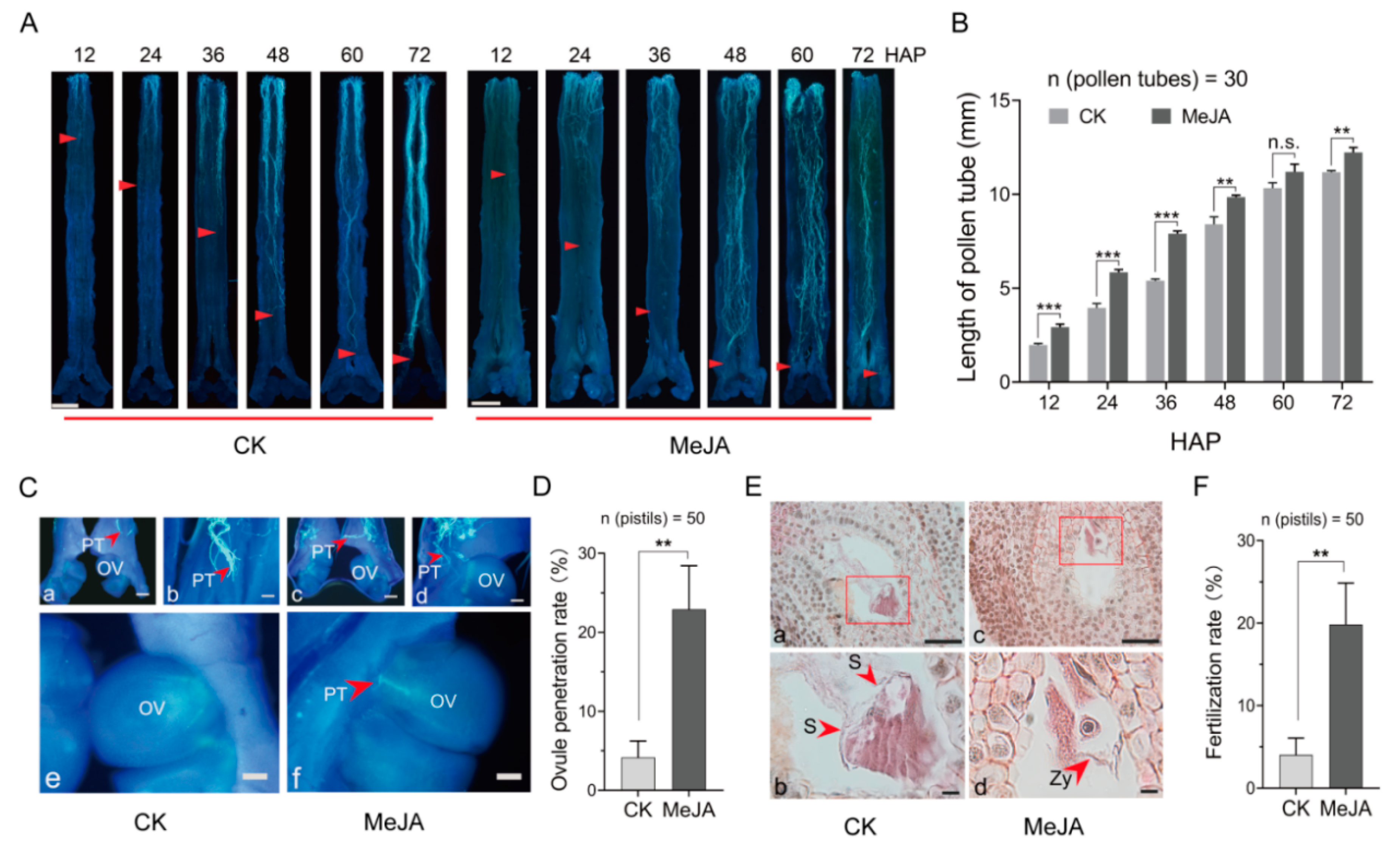
2.3. MeJA Increased the Rate of Ovule Penetration and Fertilization
2.4. Differential Expression of Pistil Genes in C. oleifera Treated with MeJA
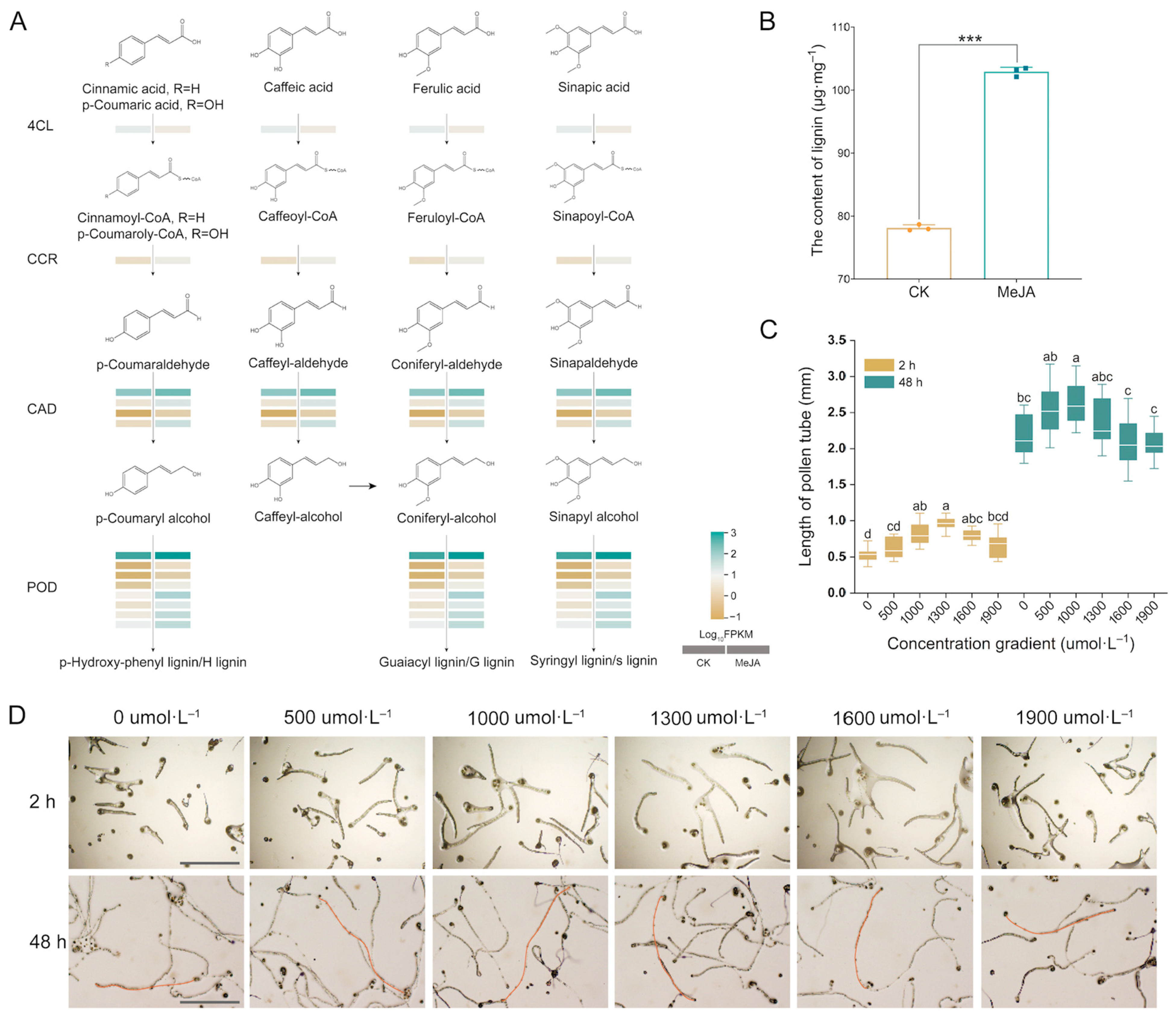
2.5. DEGs in Phenylpropanoid Biosynthesis
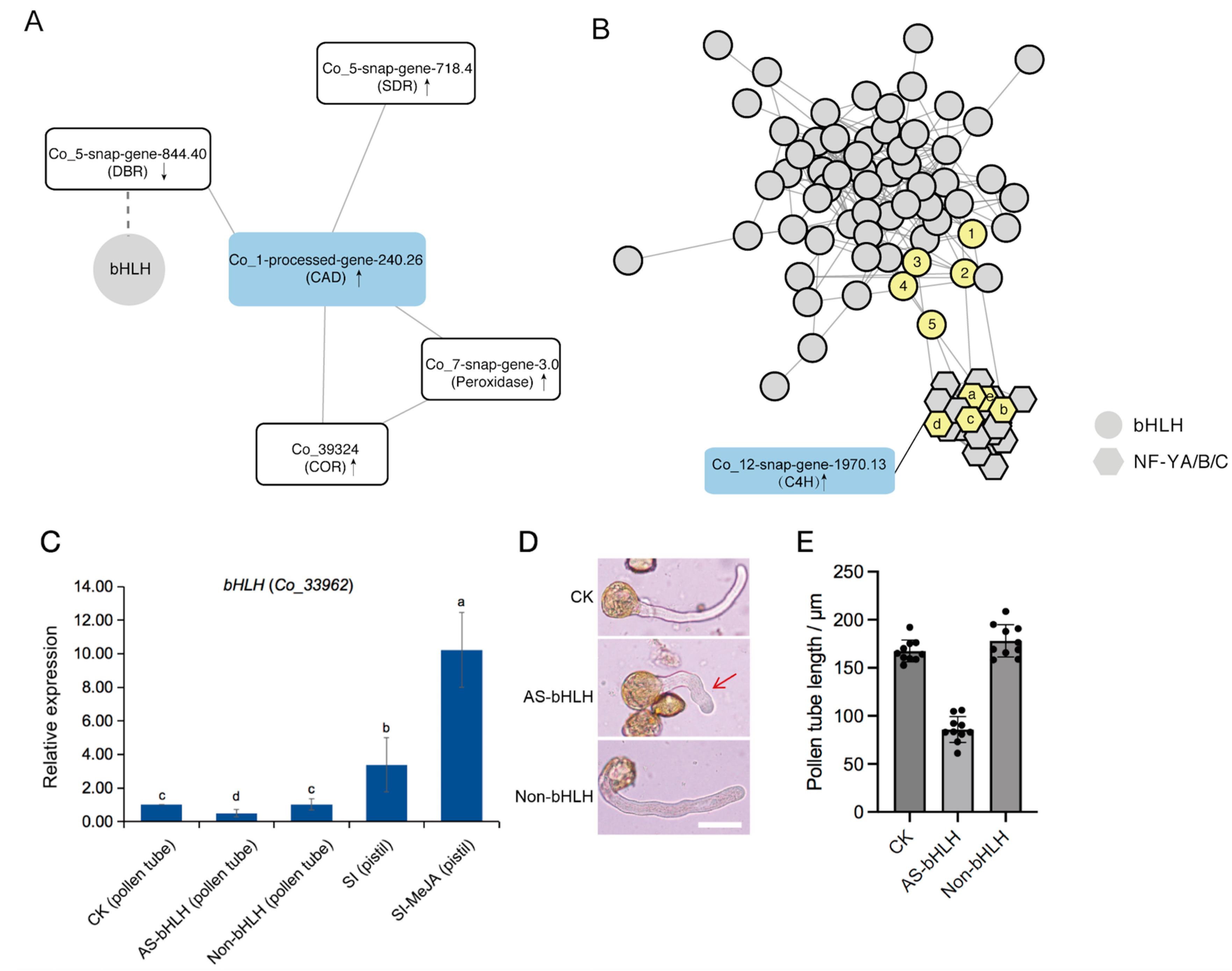
2.6. Regulatory Network of Key Genes with Transcription Factors
3. Discussion
3.1. Effect of MeJA on the Development of Pollen Tubes
3.2. Effect of MeJA on Lignin Biosynthesis
3.3. The Regulatory Mechanism of Lignin Biosynthesis
4. Materials and Methods
4.1. Plant Material and MeJA Treatment (Pollen Tube Growth In Vivo)
4.2. Pollen Tube Growth In Vitro
4.3. Aniline Blue Staining of Pollen Tubes
4.4. Fertilization Observation
4.5. Determination of the Lignin Content
4.6. Transcriptome Analysis
4.6.1. RNA Preparation and Transcriptome Sequencing
4.6.2. Screening and Analysis of the Differentially Expressed Genes
4.7. Gene Function Verification with the Antisense Oligodeoxynucleotide (AS-ODN) Method
4.8. qRT-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhuang, R. Oil-Tea Camellia in China, 2nd ed.; China Forestry Publishing House: Beijing, China, 2008. [Google Scholar]
- Lin, C.-Y.; Chen, S.-Y.; Lee, W.-T.; Yen, G.-C. Immunomodulatory effect of camellia oil (Camellia oleifera Abel.) on CD19 + B cells enrichment and IL-10 production in BALB/c mice. J. Funct. Foods 2022, 88, 104863. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.L.; Long, W.J.; Hu, Y.; Li, C.; Chen, A.Q.; Yu, R.Q. Rapid identification and quantification of cheaper vegetable oil adulteration in camellia oil by using excitation-emission matrix fluorescence spectroscopy combined with chemometrics. Food Chem. 2019, 293, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, L.; Yin, J.; Liu, G.; Ma, J.; Xia, S.; Gong, S.; Han, Q.; Li, T.; Chen, Y.; et al. Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway. Food Funct. 2022, 13, 4977–4992. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huo, R.; Cui, C.; Gao, Q.; Zong, J.; Wang, Y.; Sun, Y.; Hou, R. Anticancer activity and mechanism of total saponins from the residual seed cake of Camellia oleifera Abel. in hepatoma-22 tumor-bearing mice. Food Funct. 2019, 10, 2480–2490. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Quan, W.; Wang, A.; Gao, C.; Li, C. Applications of Chinese Camellia oleifera and its By-Products: A Review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, X.; He, J. Study on the antifungal activity and mechanism of tea saponin from Camellia oleifera cake. Eur. Food Res. Technol. 2022, 248, 783–795. [Google Scholar] [CrossRef]
- Ma, B.; Huang, Y.; Nie, Z.; Qiu, X.; Su, D.; Wang, G.; Yuan, J.; Xie, X.; Wu, Z. Facile synthesis of Camellia oleifera shell-derived hard carbon as an anode material for lithium-ion batteries. RSC Adv. 2019, 9, 20424–20431. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, D.Y.; Zou, F.; Gao, C.; Yang, Y.; Zhang, L.; Tan, X.F. Self-sterility in Camellia oleifera may be due to the prezygotic late-acting self-incompatibility. PLoS ONE 2014, 9, e99639. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, D.; Yang, Y.; Wang, B.; Liu, D.; Zou, F. Pollen Tube Growth and Double Fertilization in Camellia oleifera. J. Am. Soc. Hortic. Sci. 2015, 140, 12–18. [Google Scholar] [CrossRef]
- Nettancourt, D. Incompatibility and Incongruity in Wild and Cultivated Plants; Springer: Berlin/Heidelberg, Germany, 2001; pp. 217–251. [Google Scholar]
- Zhang, D.; Li, Y.Y.; Zhao, X.; Zhang, C.; Liu, D.K.; Lan, S.; Yin, W.; Liu, Z.J. Molecular insights into self-incompatibility systems: From evolution to breeding. Plant Commun. 2023, 5, 100719. [Google Scholar] [CrossRef] [PubMed]
- Broz, A.K.; Bedinger, P.A. Pollen-Pistil Interactions as Reproductive Barriers. Annu. Rev. Plant Biol. 2021, 72, 615–639. [Google Scholar] [CrossRef]
- Chang, Y.; Gong, W.; Xu, J.; Gong, H.; Song, Q.; Xiao, S.; Yuan, D. Integration of semi-in vivo assays and multi-omics data reveals the effect of galloylated catechins on self-pollen tube inhibition in Camellia oleifera. Hortic. Res. 2023, 10, uhac248. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef]
- Shiba, H.; Kimura, N.; Takayama, S.; Hinata, K.; Suzuki, A.; Isogai, A. Alteration of the self-incompatibility phenotype in Brassica by transformation of the antisense SLG gene. Biosci. Biotechnol. Biochem. 2000, 64, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- O‘Brien, M.; Kapfer, C.; Major, G.; Laurin, M.; Bertrand, C.; Kondo, K.; Kowyama, Y.; Matton, D.P. Molecular analysis of the stylar-expressed Solanum chacoense small asparagine-rich protein family related to the HT modifier of gametophytic self-incompatibility in Nicotiana. Plant J. 2002, 32, 985–996. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Zhang, B.; Tang, F.; Li, F.; Liao, Q.; Tang, D.; Peng, Z.; Jia, Y.; Gao, M.; et al. A nonS-locus F-box gene breaks self-incompatibility in diploid potatoes. Nat. Commun. 2021, 12, 4142. [Google Scholar] [CrossRef]
- Lynn, A.M.; Sullivan, L.L.; Galen, C. The cost of self-promotion: Ecological and demographic implications of the mentor effect in natural plant populations. New Phytol. 2023, 237, 1418–1431. [Google Scholar] [CrossRef]
- Montalt, R.; Prosper, L.; Carmen Vives, M.; Navarro, L.; Ollitrault, P.; Aleza, P. Breakdown of Self-Incompatibility in Citrus by Temperature Stress, Bud Pollination and Polyploidization. Agriculture 2022, 12, 273. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, F.; Chen, D.; Gu, Z. The effects of plant growth regulating substances on pollen germination and tube growth in Fengshui pear (Pyrus serotina). Acta Bot. Boreali-Occident. Sin. 2003, 4, 586–591. [Google Scholar]
- Zhang, X.; Liang, J.; Jing, S.; Chen, F. Influences of Plant Growth Regulators on Pollen Tube Growth and Germination in “Qinguan” Apple. Chin. Agric. Sci. Bull. 2014, 30, 213–217. [Google Scholar]
- Chang, Y.; Xu, J.; Guo, X.; Yang, G.; Deng, S.; Chen, Q.; Gong, H.; Song, Q.; Gong, W.; Yuan, D. Tannase increases fruit set by interfering with self-incompatibility of Camellia oleifera. Ind. Crops Prod. 2024, 210, 118189. [Google Scholar] [CrossRef]
- Yildiz, K.; Yilmaz, H. Effect of jasmonic acid, ACC and ethephon on pollen germination in strawberry. Plant Growth Regul. 2002, 38, 145–148. [Google Scholar] [CrossRef]
- Wu, J.; Qin, Y.; Zhao, J. Pollen tube growth is affected by exogenous hormones and correlated with hormone changes in styles in Torenia fournieri L. Plant Growth Regul. 2008, 55, 137–148. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Lin, Y.; Zhang, X.-L.; Pang, D.-W.; Zhao, J. IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. J. Exp. Bot. 2008, 59, 2529–2543. [Google Scholar] [CrossRef]
- Xu, J.; Chang, Y.; Gong, H.; Gong, W.; Yuan, D. Effects of different exogenous substances on pollen germination and pollen tube growthof Camellia oleifera. Acta Agric. Zhejiangensis 2023, 35, 789–798. [Google Scholar]
- Cheong, J.J.; Choi, Y.D. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wu, S.; Tian, L. Methyl jasmonate elicits distinctive hydrolyzable tannin, flavonoid, and phyto-oxylipin responses in pomegranate (Punica granatum L.) leaves. Planta 2021, 254, 89. [Google Scholar] [CrossRef]
- Shi, J.; Ma, C.; Qi, D.; Lv, H.; Yang, T.; Peng, Q.; Chen, Z.; Lin, Z. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biol. 2015, 15, 233. [Google Scholar] [CrossRef]
- Song, Q.; Gong, W.; Yu, X.; Ji, K.; Jiang, Y.; Chang, Y.; Yuan, D. Transcriptome and Anatomical Comparisons Reveal the Effects of Methyl Jasmonate on the Seed Development of Camellia oleifera. J. Agric. Food Chem. 2023, 71, 6747–6762. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Ranjan, A.; Singh, A.K.; Sirhindi, G. Methyl jasmonate reduces cadmium toxicity by enhancing phenol and flavonoid metabolism and activating the antioxidant defense system in pigeon pea (Cajanus cajan). Chemosphere 2023, 346, 140681. [Google Scholar] [CrossRef] [PubMed]
- Muradoğlu, F.; Yıldız, K.; Balta, F. Methyl Jasmonate Influences of Pollen Germination and Pollen Tube Growth of Apricot (Prunus armeniaca L.). Yznc Yil Niversitesi Tarim Bilim. Derg. 2010, 20, 183–188. [Google Scholar]
- Çetinbaş-Genç, A.; Vardar, F. Effect of methyl jasmonate on in-vitro pollen germination and tube elongation of Pinus nigra. Protoplasma 2020, 257, 1655–1665. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Lu, M.; Yang, J.; Tan, X. The Core Jasmonic Acid-Signalling Module CoCOI1/CoJAZ1/CoMYC2 Are Involved in Jas Mediated Growth of the Pollen Tube in Camellia oleifera. Curr. Issues Mol. Biol. 2022, 44, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Y.; Yang, D.; Liu, H.; Zhang, Y.; Wang, X.; Bai, F.; Cheng, S. Foliar methyl jasmonate (MeJA) application increased 2-acetyl-1-Pyrroline (2-AP) content and modulated antioxidant attributes and yield formation in fragrant rice. J. Plant Physiol. 2023, 282, 153946. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Yan, Y.J.; Sun, N.; Yang, Z.Y.; Zhao, S.P.; Wu, P.; Li, L.J. Exogenous methyl jasmonate treatment induced the transcriptional responses and accumulation of volatile terpenoids in Oenanthe javanica (Blume) DC. Int. J. Biol. Macromol. 2024, 265 Pt 2, 131017. [Google Scholar] [CrossRef]
- Song, Q.; Ji, K.; Yu, X.; Chen, L.; Wang, L.; Gong, W.; Yuan, D. Dynamic metabolic and transcriptomic profiling reveal synthetic characters and regulators of flavonoid biosynthesis in Camellia oleifera seeds. Ind. Crops Prod. 2022, 186, 115295. [Google Scholar] [CrossRef]
- Wei, X.; Guan, W.; Yang, Y.; Shao, Y.; Mao, L. Methyl jasmonate promotes wound healing by activation of phenylpropanoid metabolism in harvested kiwifruit. Postharvest Biol. Technol. 2021, 175, 111472. [Google Scholar] [CrossRef]
- Liu, C.F.; Yang, N.; Teng, R.M.; Li, J.W.; Chen, Y.; Hu, Z.H.; Li, T.; Zhuang, J. Exogenous methyl jasmonate and cytokinin antagonistically regulate lignin biosynthesis by mediating CsHCT expression in Camellia sinensis. Protoplasma 2023, 260, 869–884. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Li, Y.; Li, M.; Jin, P.; Zheng, Y. Involvement of PpWRKY70 in the methyl jasmonate primed disease resistance against Rhizopus stolonifer of peaches via activating phenylpropanoid pathway. Postharvest Biol. Technol. 2021, 174, 111466. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Barakate, A.; Stephens, J.; Goldie, A.; Hunter, W.N.; Marshall, D.; Hancock, R.D.; Lapierre, C.; Morreel, K.; Boerjan, W.; Halpin, C. Syringyl lignin is unaltered by severe sinapyl alcohol dehydrogenase suppression in tobacco. Plant Cell 2011, 23, 4492–4506. [Google Scholar] [CrossRef] [PubMed]
- Kitin, P.; Voelker, S.L.; Meinzer, F.C.; Beeckman, H.; Strauss, S.H.; Lachenbruch, B. Tyloses and phenolic deposits in xylem vessels impede water transport in low-lignin transgenic poplars: A study by cryo-fluorescence microscopy. Plant Physiol. 2010, 154, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Eudes, A.; Pollet, B.; Sibout, R.; Do, C.T.; Séguin, A.; Lapierre, C.; Jouanin, L. Evidence for a role of AtCAD 1 in lignification of elongating stems of Arabidopsis thaliana. Planta 2006, 225, 23–39. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Zhong, T.X.; Tang, R.; Song, J.L.; Fu, C.C.; Liu, Y.; Zhou, C.C.; Zhang, X.Q.; Chen, S.; Xie, X.M. Vascular preferential activity of the Pennisetum purpureum cinnamyl alcohol dehydrogenase promoter in transgenic tobacco plants. Plant Physiol. Biochem. 2018, 129, 357–367. [Google Scholar] [CrossRef]
- Kim, J.I.; Hidalgo-Shrestha, C.; Bonawitz, N.D.; Franke, R.B.; Chapple, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073. [Google Scholar] [CrossRef]
- El Houari, I.; Van Beirs, C.; Arents, H.E.; Han, H.; Chanoca, A.; Opdenacker, D.; Pollier, J.; Storme, V.; Steenackers, W.; Quareshy, M.; et al. Seedling developmental defects upon blocking CINNAMATE-4-HYDROXYLASE are caused by perturbations in auxin transport. New Phytol. 2021, 230, 2275–2291. [Google Scholar] [CrossRef]
- Yuan, M.; Shu, G.; Zhou, J.; He, P.; Xiang, L.; Yang, C.; Chen, M.; Liao, Z.; Zhang, F. AabHLH113 integrates jasmonic acid and abscisic acid signaling to positively regulate artemisinin biosynthesis in Artemisia annua. New Phytol. 2023, 237, 885–899. [Google Scholar] [CrossRef]
- Bernardini, A.; Lorenzo, M.; Chaves-Sanjuan, A.; Swuec, P.; Pigni, M.; Saad, D.; Konarev, P.V.; Graewert, M.A.; Valentini, E.; Svergun, D.I.; et al. The USR domain of USF1 mediates NF-Y interactions and cooperative DNA binding. Int. J. Biol. Macromol. 2021, 193, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xi, W.; Wang, X.; Li, H.; Liu, H.; Li, T.; Hou, J.; Liu, X.; Hao, C.; Zhang, X. TabHLH95-TaNF-YB1 module promotes grain starch synthesis in bread wheat. J. Genet. Genom. 2023, 50, 883–894. [Google Scholar] [CrossRef]
- Hu, G.; Gao, C.; Fan, X.; Gong, W.; Yuan, D. Pollination Compatibility and Xenia in Camellia oleifera. HortScience 2020, 55, 898–905. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, D.; Yang, Y.; Wang, B.; Liu, D.; Zou, F.; Tan, X. Anatomical Characteristics of Self-Incompatibility in Camellia oleifera. Sci. Silvae Sin. 2015, 51, 60–68. [Google Scholar]
- Gao, C.; Yuan, D.Y.; Wang, B.F.; Yang, Y.; Liu, D.M.; Han, Z.Q. A cytological study of anther and pollen development in Camellia oleifera. Genet. Mol. Res. 2015, 14, 8755–8765. [Google Scholar] [CrossRef]
- Foster, C.E.; Martin, T.M.; Pauly, M. Comprehensive compositional analysis of plant cell walls (Lignocellulosic biomass) part I: Lignin. J. Vis. Exp. JoVE 2010, 37, e1745. [Google Scholar]
- Lin, P.; Wang, K.; Wang, Y.; Hu, Z.; Yan, C.; Huang, H.; Ma, X.; Cao, Y.; Long, W.; Liu, W.; et al. The genome of oil-Camellia and population genomics analysis provide insights into seed oil domestication. Genome Biol. 2022, 23, 14. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Annika, G.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Liao, F.; Wang, L.; Yang, L.-B.; Zhang, L.; Peng, X.; Sun, M.-X. Antisense Oligodeoxynucleotide Inhibition as an Alternative and Convenient Method for Gene Function Analysis in Pollen Tubes. PLoS ONE 2013, 8, e59112. [Google Scholar] [CrossRef]
- Mizuta, Y.; Higashiyama, T. Antisense gene inhibition by phosphorothioate antisense oligonucleotide in Arabidopsis pollen tubes. Plant J. 2014, 78, 516–526. [Google Scholar] [CrossRef]
- Chang, Y.; Hu, S.; Xu, J.; Gong, H.; Guo, X.; Song, Q.; Gong, W.; Yuan, D. Identification of reference genes provides insights into the determinants of self-incompatibility in Camellia oleifera. Sci. Hortic. 2023, 321, 112301. [Google Scholar] [CrossRef]


| Concentration | Pollen Germination Rate/% | Length of Pollen Tube/μm | |
|---|---|---|---|
| 2 h | 48 h | ||
| 0 μmol·L−1 | 54.78 ± 1.46 b | 789.22 ± 37.73 c | 2664.71 ± 22.39 a |
| 0.5 μmol·L−1 | 59.17 ± 1.94 ab | 958.06 ± 48.87 b | 2698.69 ± 34.71 a |
| 5 μmol·L−1 | 62.66 ± 2.09 a | 1010.96 ± 26.54 ab | 2732.54 ± 39.42 a |
| 50 μmol·L−1 | 65.14 ± 2.57 a | 1126.67 ± 48.97 a | 1306.51 ± 66.72 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Guo, X.; Xu, H.; Wu, Q.; Xie, A.; Zhao, Z.; Tian, R.; Gong, W.; Yuan, D. Methyl Jasmonate (MeJA) Promotes the Self-Pollen Tube Growth of Camellia oleifera by Regulating Lignin Biosynthesis. Int. J. Mol. Sci. 2024, 25, 10720. https://doi.org/10.3390/ijms251910720
Chang Y, Guo X, Xu H, Wu Q, Xie A, Zhao Z, Tian R, Gong W, Yuan D. Methyl Jasmonate (MeJA) Promotes the Self-Pollen Tube Growth of Camellia oleifera by Regulating Lignin Biosynthesis. International Journal of Molecular Sciences. 2024; 25(19):10720. https://doi.org/10.3390/ijms251910720
Chicago/Turabian StyleChang, Yihong, Xinmiao Guo, Honggang Xu, Qixiao Wu, Anqi Xie, Zhixuan Zhao, Ruijie Tian, Wenfang Gong, and Deyi Yuan. 2024. "Methyl Jasmonate (MeJA) Promotes the Self-Pollen Tube Growth of Camellia oleifera by Regulating Lignin Biosynthesis" International Journal of Molecular Sciences 25, no. 19: 10720. https://doi.org/10.3390/ijms251910720
APA StyleChang, Y., Guo, X., Xu, H., Wu, Q., Xie, A., Zhao, Z., Tian, R., Gong, W., & Yuan, D. (2024). Methyl Jasmonate (MeJA) Promotes the Self-Pollen Tube Growth of Camellia oleifera by Regulating Lignin Biosynthesis. International Journal of Molecular Sciences, 25(19), 10720. https://doi.org/10.3390/ijms251910720






