Apelin Counteracts the Effects of Fusobacterium nucleatum on the Migration of Periodontal Ligament Cells In Vitro
Abstract
1. Introduction
2. Results
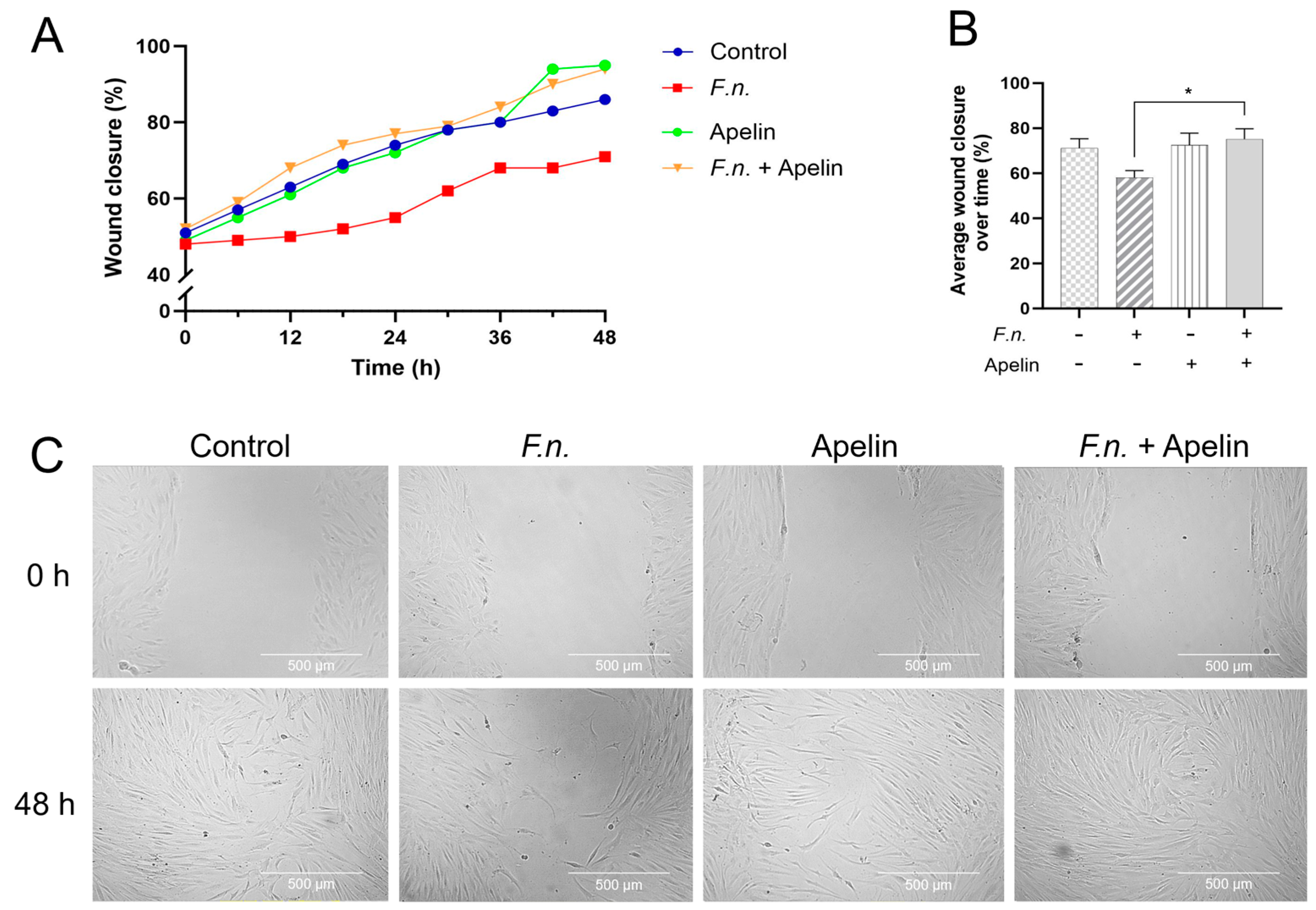
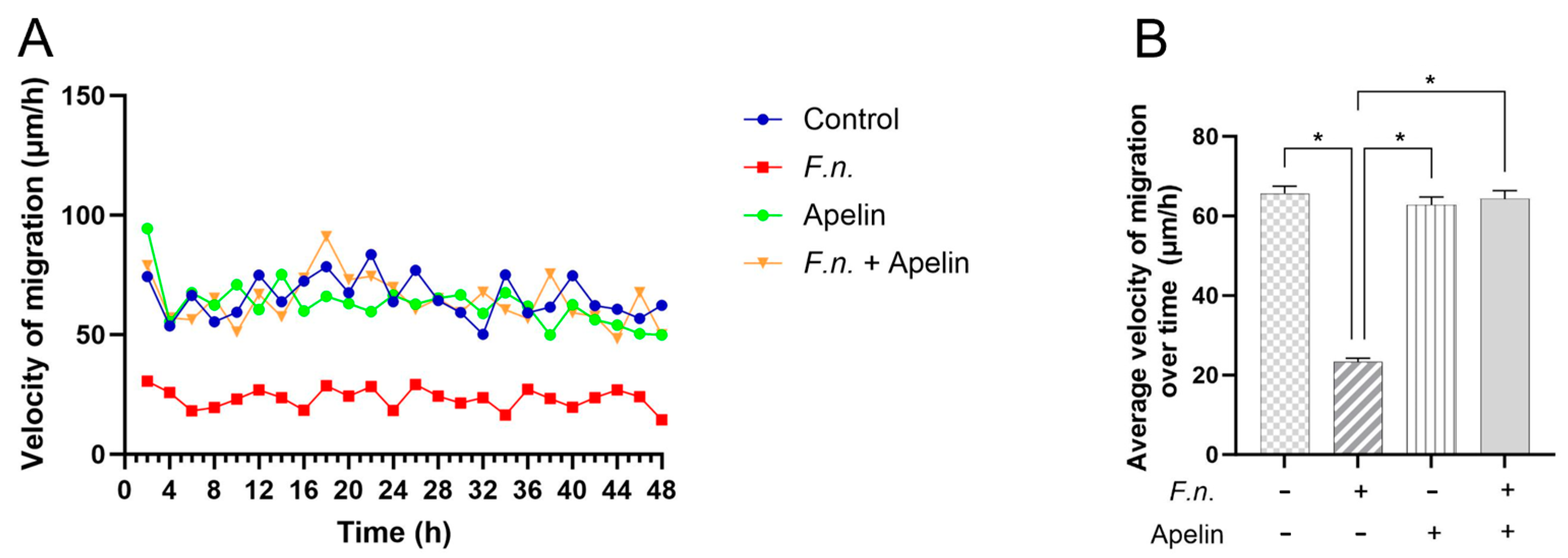
2.1. Effects of F. nucleatum and/or Apelin on In Vitro Wound Closure and Velocity of Cell Migration
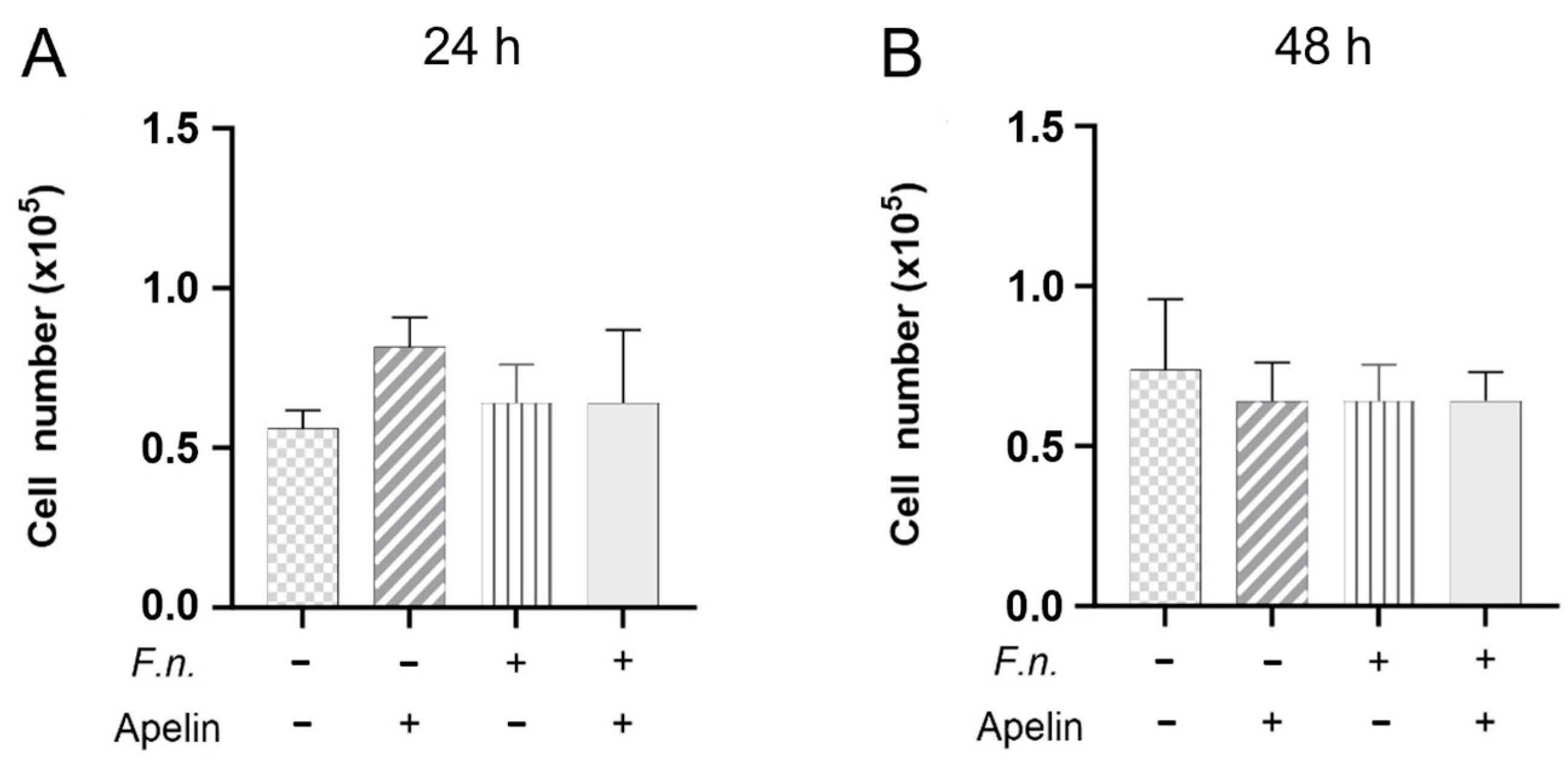
2.2. Effects of F. nucleatum and/or Apelin on Cell Proliferation
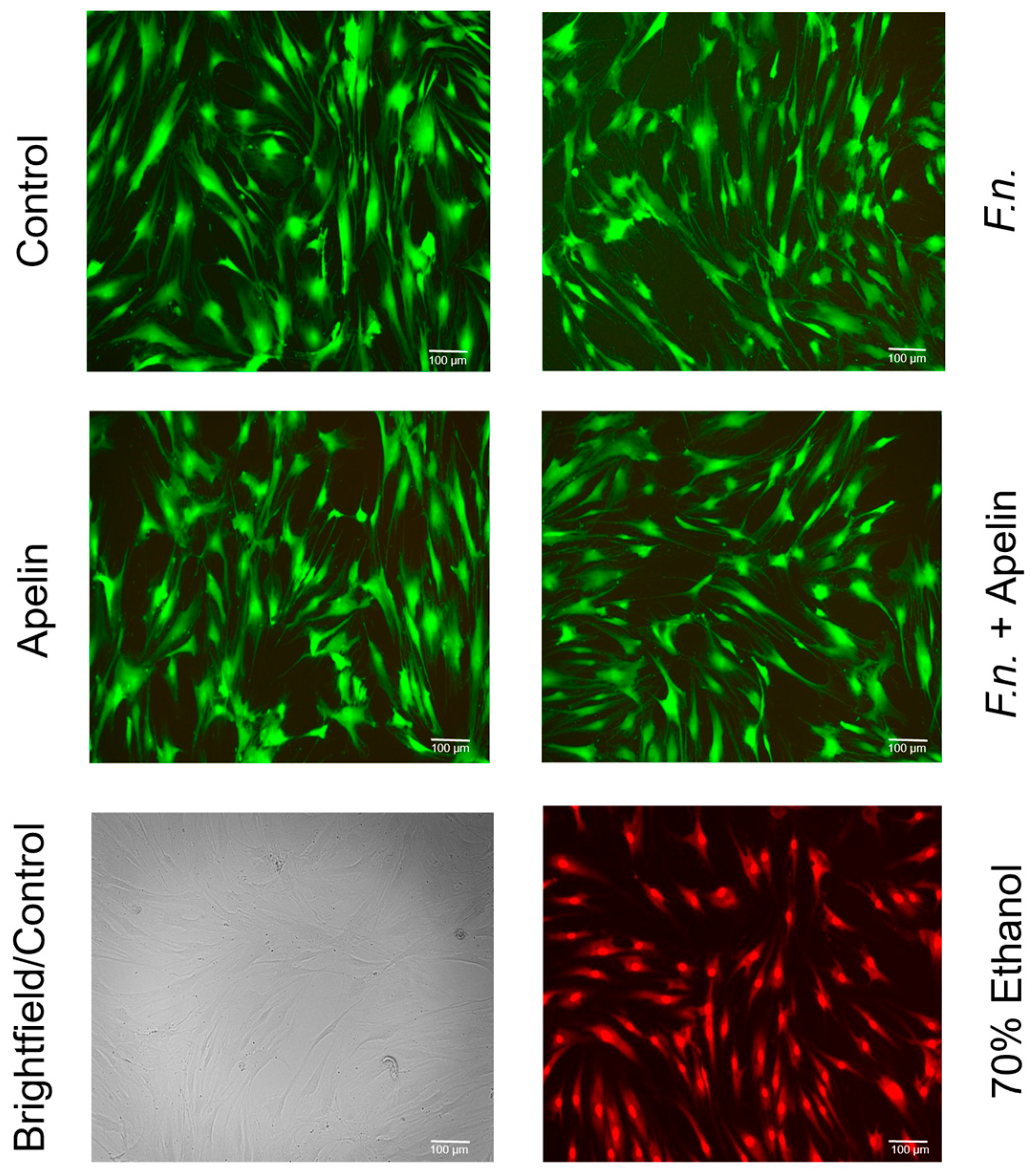
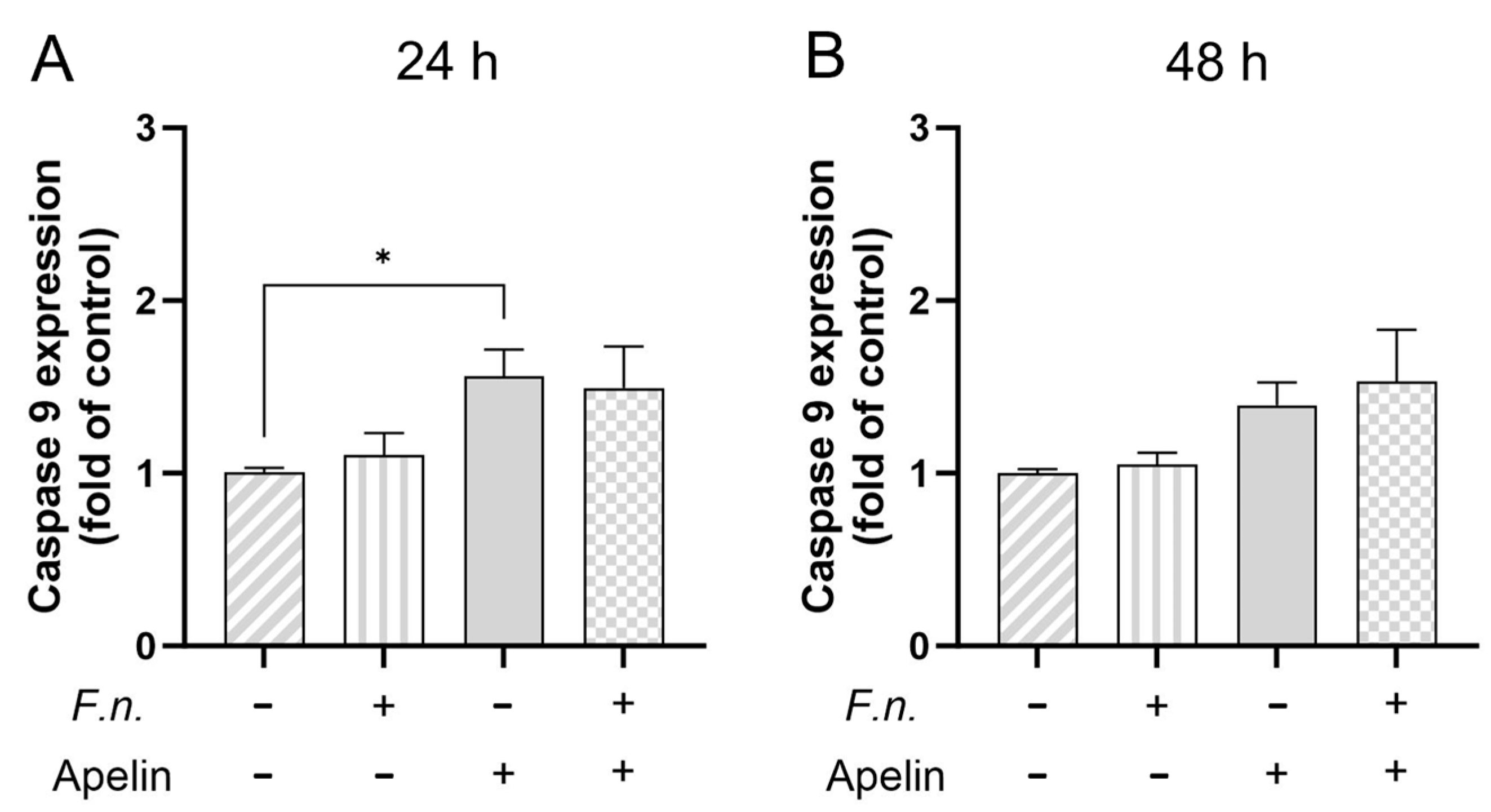
2.3. Effects of F. nucleatum and/or Apelin on Cell Viability and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. In Vitro Wound Healing Assay and Cell Migration
4.3. Analysis of Cell Number
4.4. Analysis of Cell Viability
4.5. Real-Time PCR
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2013, 35, 3–11. [Google Scholar] [CrossRef]
- Slots, J. Periodontal herpesviruses: Prevalence, pathogenicity, systemic risk. Periodontology 2000 2015, 69, 28–45. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, B.; Cheng, L.; Deng, S.; Chen, Q.; Hu, Y.; Ren, B.; Cheng, L.; Deng, S.; Chen, Q. Candida species in periodontitis: A new villain or a new target? J. Dent. 2024, 148, 105138. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.F.A.; Kamel, A.M.; Nayeem, M.; John, T.; Thacheril, A.; Tartaglia, G.; Tadakamadla, S. Smokeless tobacco and periodontitis: A systematic review with meta-analysis. J. Periodontal Res. 2024. [Google Scholar] [CrossRef]
- Leite, F.R.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Chisini, L.A.; Cademartori, M.G.; Francia, A.; Mederos, M.; Grazioli, G.; Conde, M.C.M.; Correa, M.B. Is the use of Cannabis associated with periodontitis? A systematic review and meta-analysis. J. Periodontal Res. 2019, 54, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Waziry, R.; Jawad, M.; Ballout, R.A.; Al Akel, M.; A Akl, E. The effects of waterpipe tobacco smoking on health outcomes: An updated systematic review and meta-analysis: Table 1. Leuk. Res. 2016, 46, dyw021–43. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Wang, W.; Jiang, X. Alcohol consumption and risk of periodontitis: A meta-analysis. J. Clin. Periodontol. 2016, 43, 572–583. [Google Scholar] [CrossRef]
- Aggarwal, K.; Gupta, J.; Kaur, R.K.; Bansal, D.; Jain, A. Effect of anxiety and psychologic stress on periodontal health: A systematic review and meta-analysis. Quintessence Int. 2022, 53, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Decker, A.M.; Kapila, Y.L.; Wang, H. The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontology 2000 2021, 87, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Harrel, S.K.; Nunn, M.E. The Effect of Occlusal Discrepancies on Periodontitis. II. Relationship of Occlusal Treatment to the Progression of Periodontal Disease. J. Periodontol. 2001, 72, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.; Jain, A.; Kapoor, A.; Malhotra, R.; Chahal, G.S. The Gender Bender effect in Periodontal Immune Response. Endocrine, Metab. Immune Disord.-Drug Targets 2016, 16, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.S.d.O.; de Freitas, M.R.; O Costa, F.; Cortelli, S.C.; Rovai, E.S.; Cortelli, J.R. The Effects of Patient Compliance in Supportive Periodontal Therapy on Tooth Loss: A systematic Review and Meta-analysis. J. Int. Acad. Periodontol. 2021, 23, 17–30. [Google Scholar]
- Schuch, H.S.; Peres, K.G.; Singh, A.; Peres, M.A.; Do, L.G. Socioeconomic position during life and periodontitis in adulthood: A systematic review. Community Dent. Oral Epidemiol. 2016, 45, 201–208. [Google Scholar] [CrossRef]
- Laine, M.L.; Crielaard, W.; Loos, B.G. Genetic susceptibility to periodontitis. Periodontology 2000 2011, 58, 37–68. [Google Scholar] [CrossRef]
- Loos, B.G.; John, R.P.; Laine, M.L. Identification of genetic risk factors for periodontitis and possible mechanisms of action. J. Clin. Periodontol. 2005, 32, 159–179. [Google Scholar] [CrossRef]
- Larsson, L.; Kavanagh, N.M.; Nguyen, T.V.N.; Castilho, R.M.; Berglundh, T.; Giannobile, W.V. Influence of epigenetics on periodontitis and peri-implantitis pathogenesis. Periodontology 2000 2022, 90, 125–137. [Google Scholar] [CrossRef]
- Barros, S.P.; Offenbacher, S. Modifiable risk factors in periodontal disease. Periodontology 2000 2013, 64, 95–110. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J. Clinical research activity in periodontal medicine: A systematic mapping of trial registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.; Ford, P.; Cullinan, M.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13, 3–10. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Kobayashi, T.; Bartold, P.M. Periodontitis and periodontopathic bacteria as risk factors for rheumatoid arthritis: A review of the last 10 years. Jpn. Dent. Sci. Rev. 2023, 59, 263–272. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Campos, J.R.; Martins, C.C.; Faria, S.F.S.; Carvalho, A.P.; Pereira, A.G.; Costa, F.O.; Cota, L.O.M. Association between components of metabolic syndrome and periodontitis: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 5557–5574. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Deschner, J.; Eick, S.; Damanaki, A.; Nokhbehsaim, M. The role of adipokines in periodontal infection and healing. Mol. Oral Microbiol. 2014, 29, 258–269. [Google Scholar] [CrossRef]
- Fève, B.; Bastard, C.; Fellahi, S.; Bastard, J.-P.; Capeau, J. New adipokines. Ann. d’Endocrinologie 2016, 77, 49–56. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Krysiak, R.; Handzlik-Orlik, G.; Okopien, B. The role of adipokines in connective tissue diseases. Eur. J. Nutr. 2012, 51, 513–528. [Google Scholar] [CrossRef]
- Kraus, D.; Winter, J.; Jepsen, S.; Jäger, A.; Meyer, R.; Deschner, J. Interactions of Adiponectin and Lipopolysaccharide from Porphyromonas gingivalis on Human Oral Epithelial Cells. PLoS ONE 2012, 7, e30716. [Google Scholar] [CrossRef] [PubMed]
- Nokhbehsaim, M.; Keser, S.; Nogueira, A.V.B.; Cirelli, J.A.; Jepsen, S.; Jäger, A.; Eick, S.; Deschner, J. Beneficial Effects of Adiponectin on Periodontal Ligament Cells under Normal and Regenerative Conditions. J. Diabetes Res. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.V.B.; Nokhbehsaim, M.; Tekin, S.; de Molon, R.S.; Spolidorio, L.C.; Memmert, S.; Damanaki, A.; Jäger, A.; Eick, S.; Deschner, J.; et al. Resistin Is Increased in Periodontal Cells and Tissues: In Vitro and In Vivo Studies. Mediat. Inflamm. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Nogueira, A.V.B.; Nokhbehsaim, M.; Eick, S.; Bourauel, C.; Jäger, A.; Jepsen, S.; Cirelli, J.A.; Deschner, J. Regulation of visfatin by microbial and biomechanical signals in PDL cells. Clin. Oral Investig. 2013, 18, 171–178. [Google Scholar] [CrossRef]
- Nogueira, A.V.B.; Nokhbehsaim, M.; Damanaki, A.; Eick, S.; Kirschneck, C.; Schröder, A.; Jantsch, J.; Deschner, J. Filifactor alocis and Tumor Necrosis Factor-Alpha Stimulate Synthesis of Visfatin by Human Macrophages. Int. J. Mol. Sci. 2021, 22, 1235. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Keser, S.; Nogueira, A.V.B.; Jäger, A.; Jepsen, S.; Cirelli, J.A.; Bourauel, C.; Eick, S.; Deschner, J. Leptin Effects on the Regenerative Capacity of Human Periodontal Cells. Int. J. Endocrinol. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Eick, S.; Nogueira, A.V.B.; Hoffmann, P.; Herms, S.; Fröhlich, H.; Jepsen, S.; Jäger, A.; Cirelli, J.A.; Deschner, J. Stimulation of MMP-1 and CCL2 by NAMPT in PDL Cells. Mediat. Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Kumar, S.; Hirani, T.; Patel, V.; Hirani, S.; Mohammed, I.; Shishoo, D. Expression of apelin among the individuals of chronic periodontitis, with and without type ii diabetes mellitus: A study using enzyme-linked immunosorbent assay. Adv. Hum. Biol. 2020, 10, 182–187. [Google Scholar] [CrossRef]
- Sarhat, E.R.; Rmaid, Z.J.; Jabir, T.H. Changes of Salivary Interleukine-17, Apelin, Omentin and Vaspin Levels in Normal Subjects and Diabetic Patients with Chronic periodontitis. Ann. Trop. Med. Pub. Health 2020, 23, 135–141. [Google Scholar] [CrossRef]
- Yoldaş, O.; Nogueira, A.V.B.; Kantar, P.M.; Ziskoven, P.C.; Deschner, J.; Buduneli, N. Gingival crevicular fluid levels of apelin correlates with clinical periodontal diagnosis. Clin. Oral Investig. 2023, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ziskoven, P.C.; Nogueira, A.V.B.; Gutierrez, L.S.; Weusmann, J.; Eick, S.; Buduneli, N.; Deschner, J. Apelin Enhances the Effects of Fusobacterium nucleatum on Periodontal Ligament Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 4733. [Google Scholar] [CrossRef]
- Ziskoven, P.C.; Nogueira, A.V.B.; Yoldaş, O.; Buduneli, N.; Wild, P.S.; Koeck, T.; Deschner, J. Apelin—A New Kid on the Block in Periodontology. Oral Health Prev. Dent. 2024, 22, 417–424. [Google Scholar] [CrossRef]
- Aukhil, I. Biology of wound healing. Periodontology 2000 2000, 22, 44–50. [Google Scholar] [CrossRef]
- Häkkinen, L.; Uitto, V.; Larjava, H. Cell biology of gingival wound healing. Periodontology 2000 2000, 24, 127–152. [Google Scholar] [CrossRef]
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and Surgical Wound Healing: A Current Review. ISRN Obes. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Alma, A.; Marconi, G.D.; Rossi, E.; Magnoni, C.; Paganelli, A. Obesity and Wound Healing: Focus on Mesenchymal Stem Cells. Life 2023, 13, 717. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Speéc. 2012, 4, 84–91. [Google Scholar] [CrossRef]
- Jepsen, S.; Suvan, J.; Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontology 2000 2020, 83, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Nokhbehsaim, M.; Deschner, B.; Winter, J.; Bourauel, C.; Jäger, A.; Jepsen, S.; Deschner, J. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1β in vitro. Clin. Oral Investig. 2011, 16, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. 2009, 2009, CD003875. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Somerman, M.J. Growth and Amelogenin-Like Factors in Periodontal Wound Healing. A Systematic Review. Ann. Periodontol. 2003, 8, 193–204. [Google Scholar] [CrossRef]
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J. Periodontal regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar] [CrossRef]
- Kasaj, A.; Willershausen, B.; Junker, R.; Stratul, S.-I.; Schmidt, M. Human periodontal ligament fibroblasts stimulated by nanocrystalline hydroxyapatite paste or enamel matrix derivative. An in vitro assessment of PDL attachment, migration, and proliferation. Clin. Oral Investig. 2011, 16, 745–754. [Google Scholar] [CrossRef]
- Giannopoulou, C.; Geinoz, A.; Cimasoni, G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J. Clin. Periodontol. 1999, 26, 49–55. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Russo, G.; Moccia, M.D.; Attin, T.; Schmidlin, P.R. Periodontal bacterial supernatants modify differentiation, migration and inflammatory cytokine expression in human periodontal ligament stem cells. PLoS ONE 2019, 14, e0219181. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef]
- Loberg, R.D.; Day, L.L.; Harwood, J.; Ying, C.; John, L.N.S.; Giles, R.; Neeley, C.K.; Pienta, K.J. CCL2 is a Potent Regulator of Prostate Cancer Cell Migration and Proliferation. Neoplasia 2006, 8, 578–586. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, X.; Chen, X.; Xia, J.; Cheng, B.; Tao, X. CCL2 promotes cell migration by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kim, S.; Oh, Y.; Moniruzzaman; Lee, G.; Cho, J. Effect of CCL2 on BV2 microglial cell migration: Involvement of probable signaling pathways. Cytokine 2016, 81, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, M.; Nemcovsky, C.E. In vitro models for evaluation of periodontal wound healing/regeneration. Periodontology 2000 2015, 68, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Unnisa, A.; Greig, N.H.; Kamal, M.A. Inhibition of Caspase 3 and Caspase 9 Mediated Apoptosis: A Multimodal Therapeutic Target in Traumatic Brain Injury. Curr. Neuropharmacol. 2023, 21, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Della-Fera, M.A.; Krieg, P.A.; Cox, C.M.; Robins, A.; Baile, C.A. A putative role for apelin in the etiology of obesity. Biochem. Biophys. Res. Commun. 2008, 368, 815–819. [Google Scholar] [CrossRef]
- Chen, L.; Tao, Y.; Feng, J.; Jiang, Y.R. Apelin Protects Primary Rat Retinal Pericytes from Chemical Hypoxia-Induced Apoptosis. J. Ophthalmol. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, T.; Li, Y.; Huang, L.; Yin, D. Fusobacterium nucleatum: The Opportunistic Pathogen of Periodontal and Peri-Implant Diseases. Front. Microbiol. 2022, 13, 860149. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Doğan, A. Apelin receptor (Aplnr) signaling promotes fibroblast migration. Tissue Cell 2019, 56, 98–106. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.-J.; Jiang, Y.-R. Apelin induces the proliferation, migration and expression of cytoskeleton and tight junction proteins in human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways. Int. J. Clin. Exp. Pathol. 2017, 10, 10711–10729. [Google Scholar]
- Shen, S.; Sun, T.; Ding, X.; Gu, X.; Wang, Y.; Ma, X.; Li, Z.; Gao, H.; Ge, S.; Feng, Q. The exoprotein Gbp of Fusobacterium nucleatum promotes THP-1 cell lipid deposition by binding to CypA and activating PI3K-AKT/MAPK/NF-κB pathways. J. Adv. Res. 2024, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Sun, T.; Shen, S.; Li, Z.; Ma, X.; Gu, X.; Zhang, X.; Peng, A.; Xu, X.; et al. Study of the inflammatory activating process in the early stage of Fusobacterium nucleatum infected PDLSCs. Int. J. Oral Sci. 2023, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; de Molon, R.S.; Nokhbehsaim, M.; Eick, S.; Kirschneck, C.; Cirelli, J.A.; Deschner, J.; Jäger, A.; et al. CXCL5, CXCL8, and CXCL10 regulation by bacteria and mechanical forces in periodontium. Ann. Anat.—Anat. Anz. 2020, 234, 151648. [Google Scholar] [CrossRef]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatumFacilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-κB Signaling Pathways in Human Gingival Fibroblasts. Oxidative Med. Cell. Longev. 2019, 2019, 1–22. [Google Scholar] [CrossRef]
- Rath-Deschner, B.; Nogueira, A.V.B.; Memmert, S.; Nokhbehsaim, M.; Cirelli, J.A.; Eick, S.; Miosge, N.; Kirschneck, C.; Kesting, M.; Deschner, J.; et al. Regulation of Anti-Apoptotic SOD2 and BIRC3 in Periodontal Cells and Tissues. Int. J. Mol. Sci. 2021, 22, 591. [Google Scholar] [CrossRef]
- Schminke, B.; Orde, F.V.; Gruber, R.; Schliephake, H.; Bürgers, R.; Miosge, N. The Pathology of Bone Tissue during Peri-Implantitis. J. Dent. Res. 2014, 94, 354–361. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cores Ziskoven, P.; Nogueira, A.V.B.; Eick, S.; Deschner, J. Apelin Counteracts the Effects of Fusobacterium nucleatum on the Migration of Periodontal Ligament Cells In Vitro. Int. J. Mol. Sci. 2024, 25, 10729. https://doi.org/10.3390/ijms251910729
Cores Ziskoven P, Nogueira AVB, Eick S, Deschner J. Apelin Counteracts the Effects of Fusobacterium nucleatum on the Migration of Periodontal Ligament Cells In Vitro. International Journal of Molecular Sciences. 2024; 25(19):10729. https://doi.org/10.3390/ijms251910729
Chicago/Turabian StyleCores Ziskoven, Pablo, Andressa V. B. Nogueira, Sigrun Eick, and James Deschner. 2024. "Apelin Counteracts the Effects of Fusobacterium nucleatum on the Migration of Periodontal Ligament Cells In Vitro" International Journal of Molecular Sciences 25, no. 19: 10729. https://doi.org/10.3390/ijms251910729
APA StyleCores Ziskoven, P., Nogueira, A. V. B., Eick, S., & Deschner, J. (2024). Apelin Counteracts the Effects of Fusobacterium nucleatum on the Migration of Periodontal Ligament Cells In Vitro. International Journal of Molecular Sciences, 25(19), 10729. https://doi.org/10.3390/ijms251910729





