Enzymatic Kinetic Resolution of Racemic 1-(Isopropylamine)-3-phenoxy-2-propanol: A Building Block for β-Blockers
Abstract
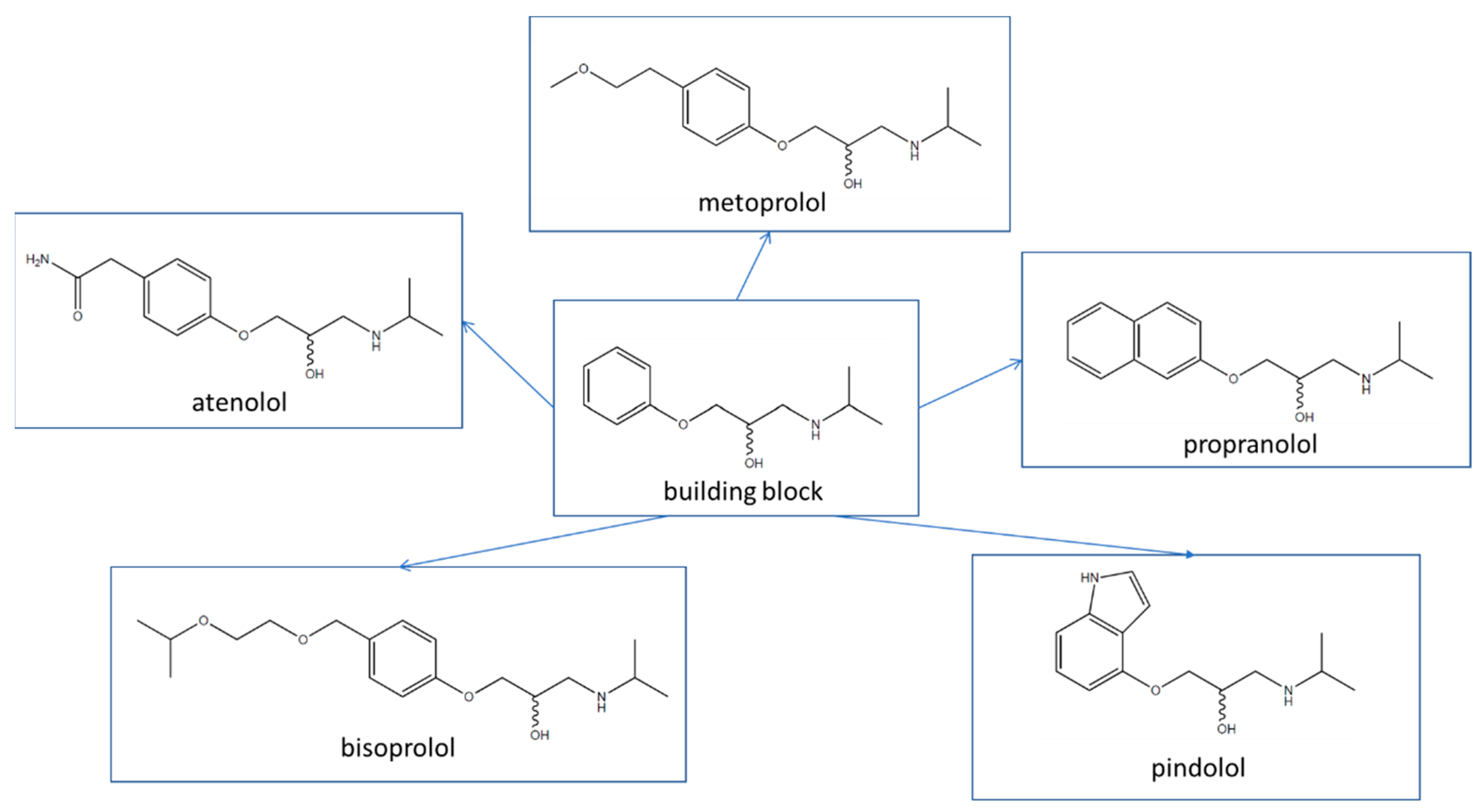
:1. Introduction
2. Results and Discussion
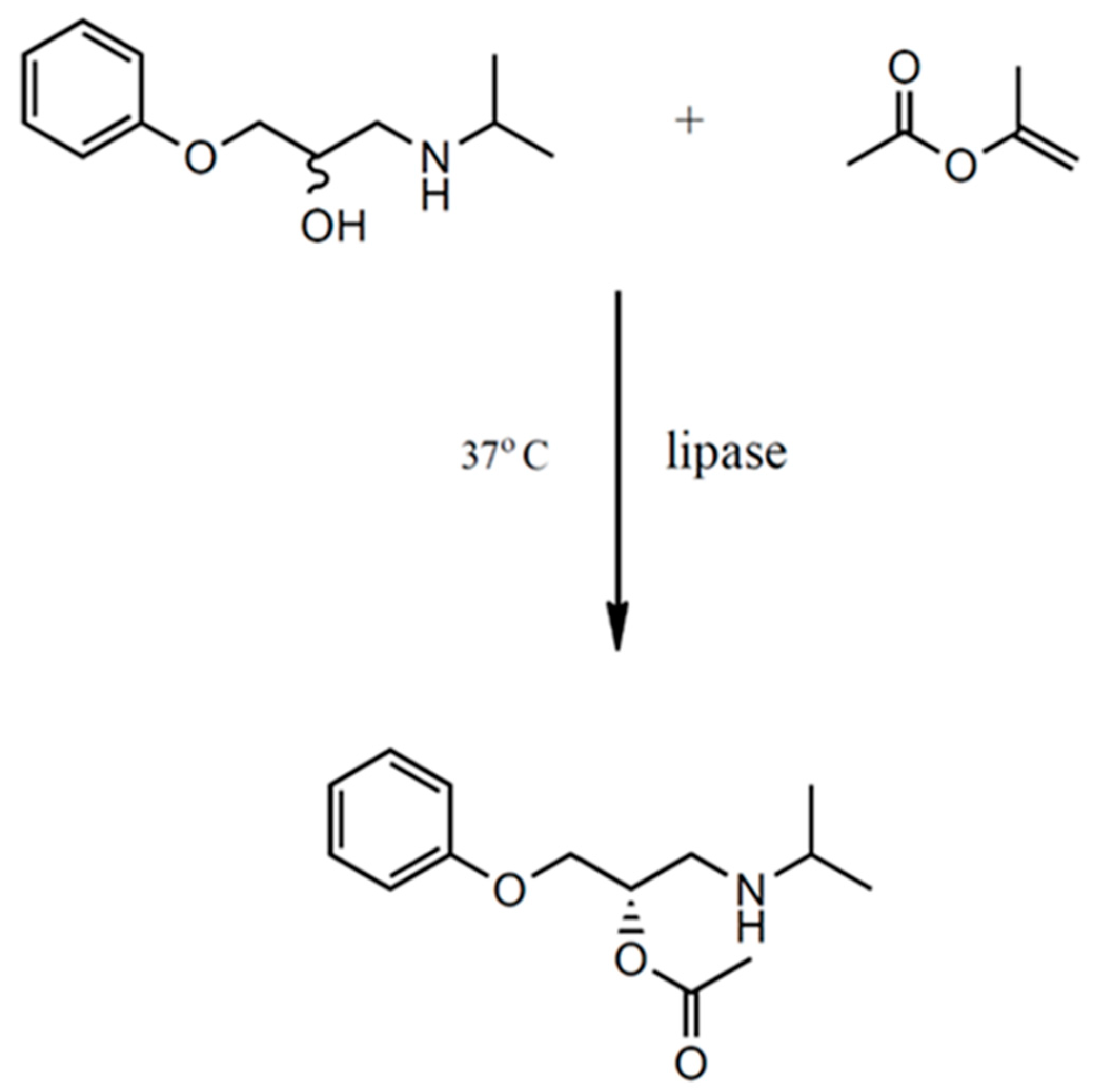
2.1. Enantioselective Biotransformation of Racemic β-Blocker Building Block
2.2. Effect of Reaction Time
2.3. Effect of the Biocatalysts
2.4. Effect of Reaction Medium
2.5. Effect of Ionic Liquids
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
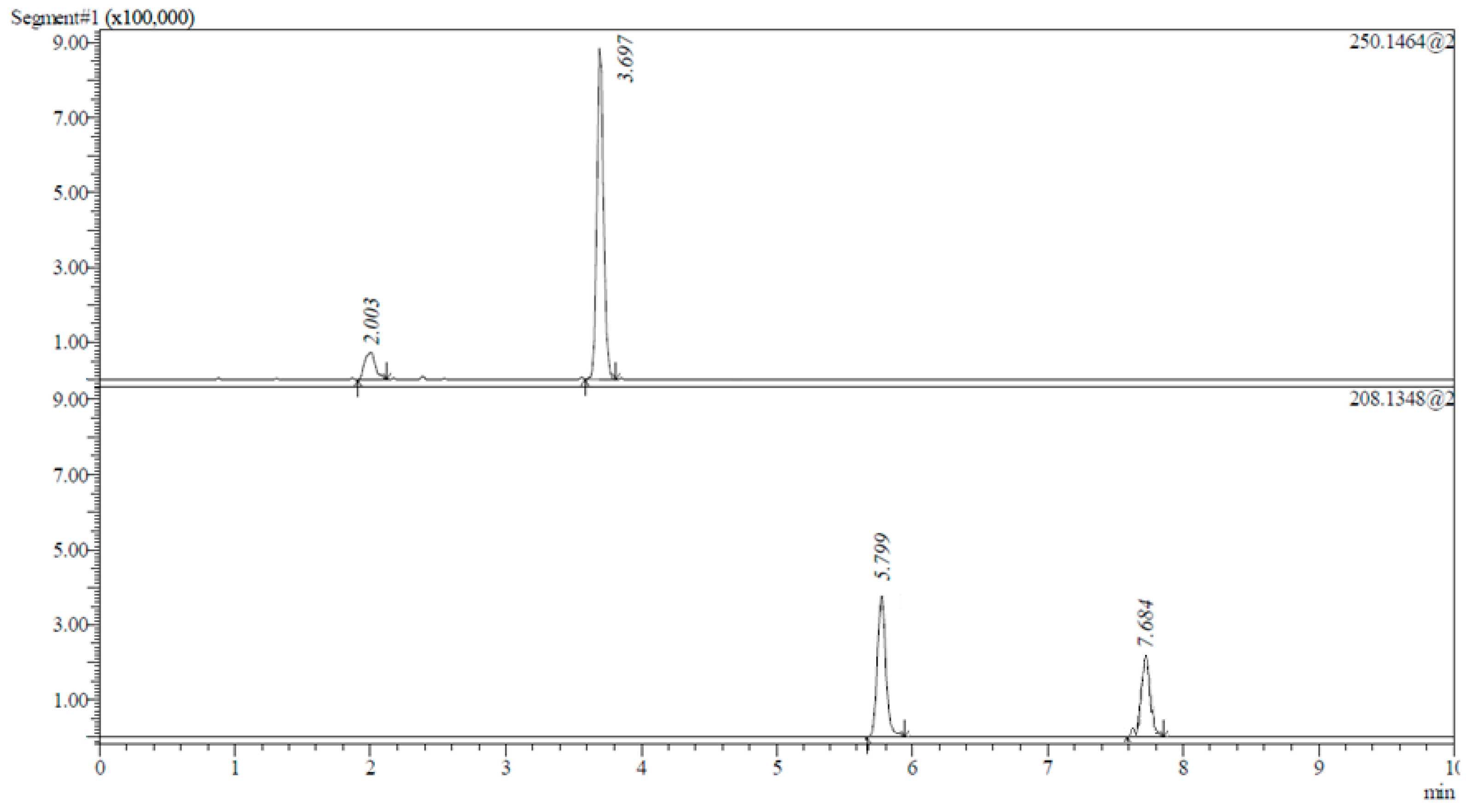
3.3. Chromatographic Conditions
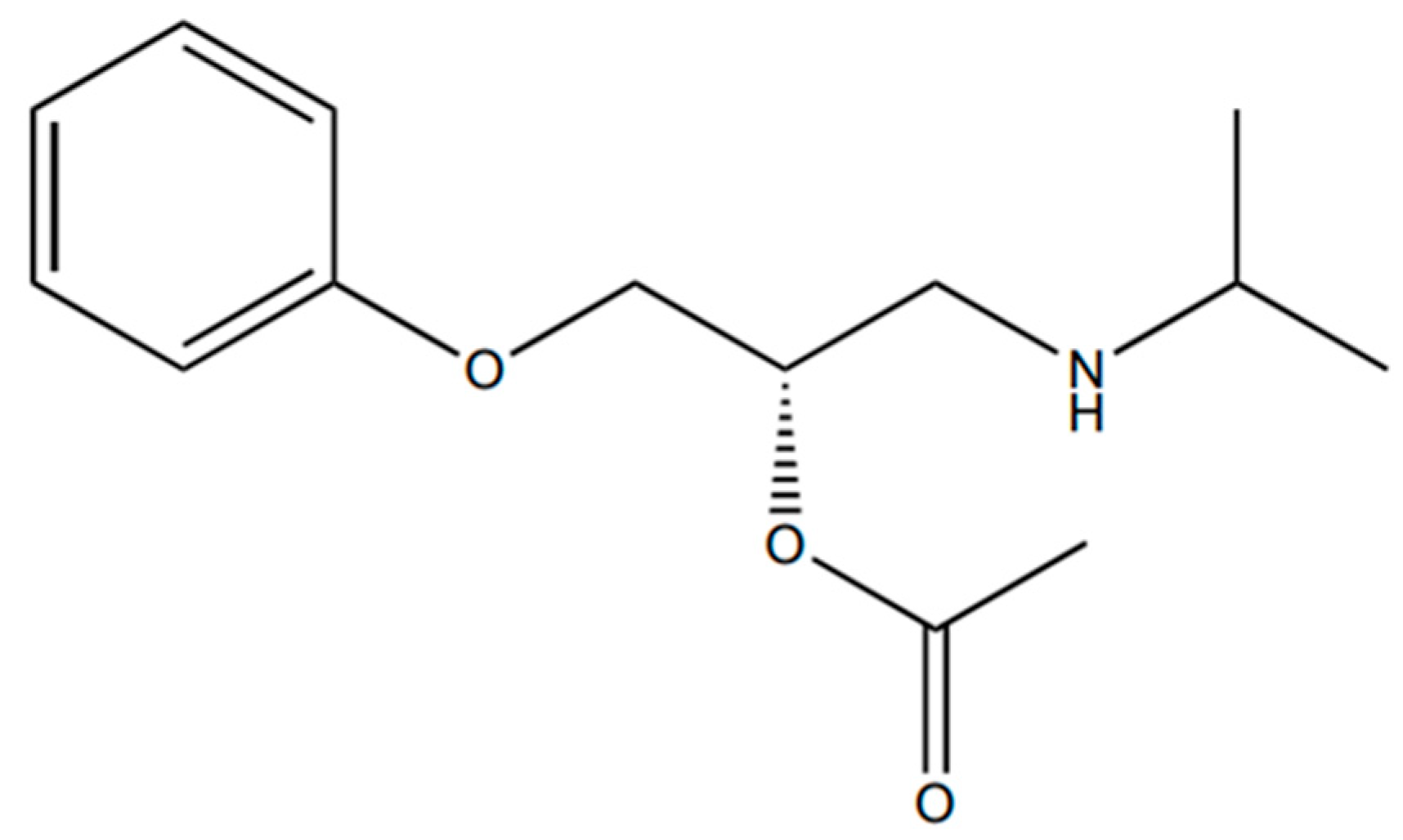
3.4. Chemical Acetylation of 1-(Isopropylamine)-3-Phenoxy-2-Propanol
3.5. Kinetic Resolution of β-Blockers Building Block
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Gallagher, K.; Teo, J.T.; Shah, A.M.; Gaughran, F. Interaction Between Race, Ethnicity, Severe Mental Illness, and Cardiovascular Disease. J. Am. Heart Assoc. 2022, 11, 12. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Z.; Greenlund, K. Association of depressive symptoms and hypertension prevalence, awareness, treatment and control among USA adults. J. Hypertens. 2022, 40, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M.; Kjeldsen, S.E.; Grassi, G.; Esler, M.; Mancia, G. The global burden of hypertension exceeds 1.4 billion people: Should a systolic blood pressure target below 130 become the universal standard? J. Hypertens. 2019, 37, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.J.; Delagrange, H.; van Valkengoed, I.G.M.; de Groot, C.J.M.; van den Born, B.J.H.; Gordijn, S.J.; Ganzevoort, W. Hypertensive Disorders of Pregnancy and Cardiovascular Disease Risk Across Races and Ethnicities: A Review. Front. Cardiovasc. Med. 2022, 9, 933822. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Hardy, S.T.; Fine, L.J.; Jaeger, B.C.; Wozniak, G.; Levitan, E.B.; Colantonio, L.D. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA J. Am. Med. Assoc. 2020, 324, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Stoschitzky, K.; Egginger, G.; Zernig, G.; Klein, W.; Lindner, W. Stereoselective features of (R)- and (S)-atenolol: Clinical pharmacological, pharmacokinetic, and radioligand binding studies. Chirality 1993, 5, 15–19. [Google Scholar] [CrossRef]
- Seebauer, C.T.; Graus, M.S.; Huang, L.; McCann, A.; Wylie-Sears, J.; Fontaine, F.; Karnezis, T.; Zurakowski, D.; Staffa, S.J.; Meunier, F.; et al. Non-beta blocker enantiomers of propranolol and atenolol inhibit vasculogenesis in infantile hemangioma. J. Clin. Investig. 2022, 132, 3. [Google Scholar] [CrossRef]
- Ali, I.; Alam, S.D.; Raja, R.; Shirsath, V.; Jain, A.K.; Yusuf, K.; Aljuwayid, A.M.; Sillanpaa, M. Chiral separation of beta-blockers by supercritical fluid chromatography using Chiralpak-IG and Chiralpak IBN-5 columns. Chirality 2022, 34, 848–855. [Google Scholar] [CrossRef]
- Britt, J.; Moffett, B.; Bronicki, R.; Checchia, P. Incidence of Adverse Events Requiring Intervention After Initiation of Oral Beta-Blocker in Pediatric Cardiac Intensive Care Patients. Pediatr. Cardiol. 2014, 35, 1062–1066. [Google Scholar] [CrossRef]
- Carlberg, B.; Samuelsson, O.; Lindholm, L.J. Atenolol in hypertension: Is it a wise choice? Lancet 2004, 364, 1684–1689. [Google Scholar] [CrossRef]
- Rosenthal, J.; Bahrmann, H.; Benkert, K.; Baumgart, P.; Bonner, G.; Klein, G.; Neiss, A.; Schnelle, K.; Frohlich, E. Analysis of adverse effects among patients with essential hypertension receiving an ACE inhibitor or a beta-blocker. Cardiology 1996, 87, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Solanki, J.D.; Mehta, H.B.; Panjwani, S.J.; Munshi, H.B.; Shah, C.J. Impact of Concomitant Use of Beta blocker, Statin, Aspirin, and Metformin on Central Hemodynamics and Arterial Stiffness in Hypertension. J. Pharmacol. Pharmacother. 2018, 9, 167–173. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Ali, A.Q.; Errayes, A.O. Green Chemistry: Principles, Applications, and Disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.A.; Muniruzzaman, M.; Salleh, H.M.; Alam, M.D.Z. Ionic Liquids as a Green Solvent for Lipase-Catalyzed Reactions. 2019; Materials Research Forum. In Industrial Applications of Green Solvents, Vol I; Inamuddin, Ahamed, M.I., Asiri, A.M., Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2019; Volume 50, pp. 21–60. [Google Scholar]
- Elgharbawy, A.A.M.; Moniruzzaman, M.; Goto, M. Recent advances of enzymatic reactions in ionic liquids: Part II. Biochem. Eng. J. 2020, 154, 107426. [Google Scholar] [CrossRef]
- Habulin, M.; Knez, Z. Optimization of (R,S)-1-phenylethanol kinetic resolution over Candida antarctica lipase B in ionic liquids. J. Mol. Catal. B-Enzym. 2009, 58, 24–28. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Imam, H.T.; Krasnan, V.; Rebros, M.; Marr, A.C. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. [Google Scholar] [CrossRef]
- Sheldon, R.A. Biocatalysis in ionic liquids: State-of-the-union. Green Chem. 2021, 23, 8406–8427. [Google Scholar] [CrossRef]
- Godoy, C.; Pardo-Tamayo, J.; Barbosa, O. Microbial Lipases and Their Potential in the Production of Pharmaceutical Building Blocks. Int. J. Mol. Sci. 2022, 23, 9933. [Google Scholar] [CrossRef]
- Bouzemi, N.; Grib, I.; Houiene, Z.; Aribi-Zouioueche, L. Enantiocomplementary Preparation of ( S)- and ( R)-Arylalkylcarbinols by Lipase-Catalysed Resolution and Mitsunobu Inversion: Impact of Lipase Amount. Catalysts 2014, 4, 215–225. [Google Scholar] [CrossRef]
- Serra, S.; De Simeis, D. Stereoselective Synthesis of Terpenoids through Lipase-Mediated Resolution Approaches. Catalysts 2020, 10, 504. [Google Scholar] [CrossRef]
- Soto, M.; Sanz-Machin, I.; Rodriguez-Solla, H.; Gotor-Fernandez, V. Chemoenzymatic Stereoselective Synthesis of trans-Flavan-4-ols via Lipase-Catalyzed Kinetic Resolutions. Catalysts 2021, 11, 1296. [Google Scholar] [CrossRef]
- Morandini, A.; Rossetti, A.; Sacchetti, A. Lipase-Catalyzed Kinetic Resolution of Alcohols as Intermediates for the Synthesis of Heart Rate Reducing Agent Ivabradine. Catalysts 2021, 11, 53. [Google Scholar] [CrossRef]
- Carvalho, A.; de Oliveira, B.; Lima, G.; Negreiro, J.; Oliveira, M.; de Lemos, T.; da Silva, M.; Fonseca, T.; Bezerra, R.; dos Santos, J.; et al. Resolution of Racemic Aryloxy-Propan-2-yl Acetates via Lipase-Catalyzed Hydrolysis: Preparation of Enantiomerically Pure/Enantioenriched Mexiletine Intermediates and Analogs. Catalysts 2022, 12, 1566. [Google Scholar] [CrossRef]
- Majewska, P. Lipase-Catalyzed Kinetic Resolution of Dimethyl and Dibutyl 1-Butyryloxy-1-carboxymethylphosphonates. Catalysts 2021, 11, 956. [Google Scholar] [CrossRef]
- Zappaterra, F.; Rodriguez, M.; Summa, D.; Semeraro, B.; Costa, S.; Tamburini, E. Biocatalytic Approach for Direct Esterification of Ibuprofen with Sorbitol in Biphasic Media. Int. J. Mol. Sci. 2021, 22, 3066. [Google Scholar] [CrossRef]
- Zappaterra, F.; Presini, F.; Venturi, V.; Lerin, L.; Giovannini, P.; Costa, S. Biocatalytic Insights for The Synthesis of New Potential Prodrugs: Design of two Ibuprofen Derivatives. Appl. Sci. 2023, 13, 9852. [Google Scholar] [CrossRef]
- Chalupka, J.; Sikora, A.; Marszall, M.P. The Utilization of Two-Phase Catalytic System in Enantioselective Biotransformation of Racemic Atenolol. Catalysts 2022, 12, 1068. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Ghosh, S.; Bhaumik, J.; Banoth, L.; Banerjee, U.C. Lipase-catalyzed green synthesis of enantiopure atenolol. Rsc Adv. 2015, 5, 15850–15860. [Google Scholar] [CrossRef]
- Wei, T.; Yang, K.P.; Bai, B.; Zang, J.; Yu, X.; Mao, D.B. Enzymatic Hydrolytic Resolution of Racemic Ibuprofen Ethyl Ester Using an Ionic Liquid as Cosolvent. Molecules 2016, 21, 905. [Google Scholar] [CrossRef] [PubMed]
- Chalupka, J.; Duleba, J.; Sikora, A.; Siodmiak, T.; Marszall, M.P. The Application of Two-Phase Catalytic System in Enantioselective Separation of Racemic (R,S)-1-Phenylethanol. Catalysts 2023, 13, 292. [Google Scholar] [CrossRef]
- Sih, C.J.; Wu, S.H. Resolution of Enantiomers via Biocatalysis. Top. Stereochem. 1989, 19, 63–125. [Google Scholar]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative-analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Siódmiak, T.; Tarczykowska, A.; Sroka, W.D.; Ziegler-Borowska, M.; Marszałł, M.P. Enantioselective acetylation of (R,S)-atenolol: The use of Candida rugosa lipases immobilized onto magnetic chitosan nanoparticles in enzyme-catalyzed biotransformation. J. Mol. Catal. B Enzym. 2016, 134, 43–50. [Google Scholar] [CrossRef]
- Agustian, J.; Kamaruddin, A.H.; Aboul-Enein, H.Y. Enantio-conversion and-selectivity of racemic atenolol kinetic resolution using free Pseudomonas fluorescens lipase (Amano) conducted via transesterification reaction. RSC Adv. 2016, 6, 26077–26085. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Effect of the immobilization protocol on the properties of lipase B from Candida antarctica in organic media: Enantiospecifc production of atenolol acetate. J. Mol. Catal. B Enzym. 2011, 71, 124–132. [Google Scholar] [CrossRef]
- Lund, I.T.; Bøckmann, P.L.; Jacobsen, E.E. Highly enantioselective CALB-catalyzed kinetic resolution of building blocks for β-blocker atenolol. Tetrahedron 2016, 72, 7288–7292. [Google Scholar] [CrossRef]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Ziegler-Borowska, M.; Marszałł, M.P. Enantioseparation of (RS)-atenolol with the use of lipases immobilized onto new-synthesized magnetic nanoparticles. Tetrahedron-Asymmetry 2017, 28, 374–380. [Google Scholar] [CrossRef]
- Sikora, A.; Sroka, W.D.; Siódmiak, T.; Marszall, M.P. Kinetic resolution of (R, S)-atenolol with the use of lipases in various organic solvents. Curr. Org. Synth. 2017, 14, 747–754. [Google Scholar] [CrossRef]
- Chiou, T.-W.; Chang, C.-C.; Lai, C.-T.; Tai, D.-F. Kinetic resolution of propranolol by a lipase-catalyzed N-acetylation. Bioorg. Med. Chem. Lett. 1997, 7, 433–436. [Google Scholar] [CrossRef]
- Barbosa, O.; Ariza, C.; Ortiz, C.; Torres, R. Kinetic resolution of (R/S)-propranolol (1-isopropylamino-3-(1-naphtoxy)-2-propanolol) catalyzed by immobilized preparations of Candida antarctica lipase B (CAL-B). New Biotechnol. 2010, 27, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.; Herar, A.; Kreye, L.; Wendel, V.; Capewell, A.; Meyer, H.; Scheper, T.; Kolisis, F. Factors affecting the lipase-catalyzed transesterification reactions of 3-hydroxy esters in organic-solvents. Tetrahedron-Asymmetry 1993, 4, 1007–1016. [Google Scholar] [CrossRef]
- Escorcia, A.M.; Molina, D.; Daza, M.C.; Doerr, M. Acetylation of (R, S)-propranolol catalyzed by Candida antarctica lipase B: An experimental and computational study. J. Mol. Catal. B Enzym. 2013, 98, 21–29. [Google Scholar] [CrossRef]
- Escorcia, A.M.; Daza, M.C.; Doerr, M. Computational study of the enantioselectivity of the O-acetylation of (R, S)-propranolol catalyzed by Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2014, 108, 21–31. [Google Scholar] [CrossRef]
- Gamboa-Velázquez, G.; Juaristi, E. Mechanoenzymology in the Kinetic Resolution of β-Blockers: Propranolol as a Case Study. ACS Org. Inorg. Au 2022, 2, 343–350. [Google Scholar] [CrossRef]
- Sikora, A.; Siodmiak, T.; Marszall, M.P. Kinetic Resolution of Profens by Enantioselective Esterification Catalyzed by Candida antarctica and Candida rugosa Lipases. Chirality 2014, 26, 663–669. [Google Scholar] [CrossRef]




| No | Acetylating Agent | Reaction Medium | Lipase | Ionic Liquid |
|---|---|---|---|---|
| 1 | vinyl acetate | toluene | OF | n/a |
| 2 | vinyl acetate | toluene | MY | n/a |
| 3 | isopropenyl acetate | toluene | OF | n/a |
| 4 | isopropenyl acetate | toluene | MY | n/a |
| 5 | vinyl acetate | dichloromethane | OF | n/a |
| 6 | vinyl acetate | dichloromethane | MY | n/a |
| 7 | isopropenyl acetate | dichloromethane | OF | n/a |
| 8 | isopropenyl acetate | dichloromethane | MY | n/a |
| 9 | vinyl acetate | toluene | OF | [EMIM][BF4] |
| 10 | vinyl acetate | toluene | MY | [EMIM][BF4] |
| 11 | isopropenyl acetate | toluene | OF | [EMIM][BF4] |
| 12 | isopropenyl acetate | toluene | MY | [EMIM][BF4] |
| 13 | vinyl acetate | dichloromethane | OF | [EMIM][BF4] |
| 14 | vinyl acetate | dichloromethane | MY | [EMIM][BF4] |
| 15 | isopropenyl acetate | dichloromethane | OF | [EMIM][BF4] |
| 16 | isopropenyl acetate | dichloromethane | MY | [EMIM][BF4] |
| 17 | vinyl acetate | toluene | OF | [EMIM][MSF3] |
| 18 | vinyl acetate | toluene | MY | [EMIM][MSF3] |
| 19 | isopropenyl acetate | toluene | OF | [EMIM][MSF3] |
| 20 | isopropenyl acetate | toluene | MY | [EMIM][MSF3] |
| 21 | vinyl acetate | dichloromethane | OF | [EMIM][MSF3] |
| 22 | vinyl acetate | dichloromethane | MY | [EMIM][MSF3] |
| 23 | isopropenyl acetate | dichloromethane | OF | [EMIM][MSF3] |
| 24 | isopropenyl acetate | dichloromethane | MY | [EMIM][MSF3] |
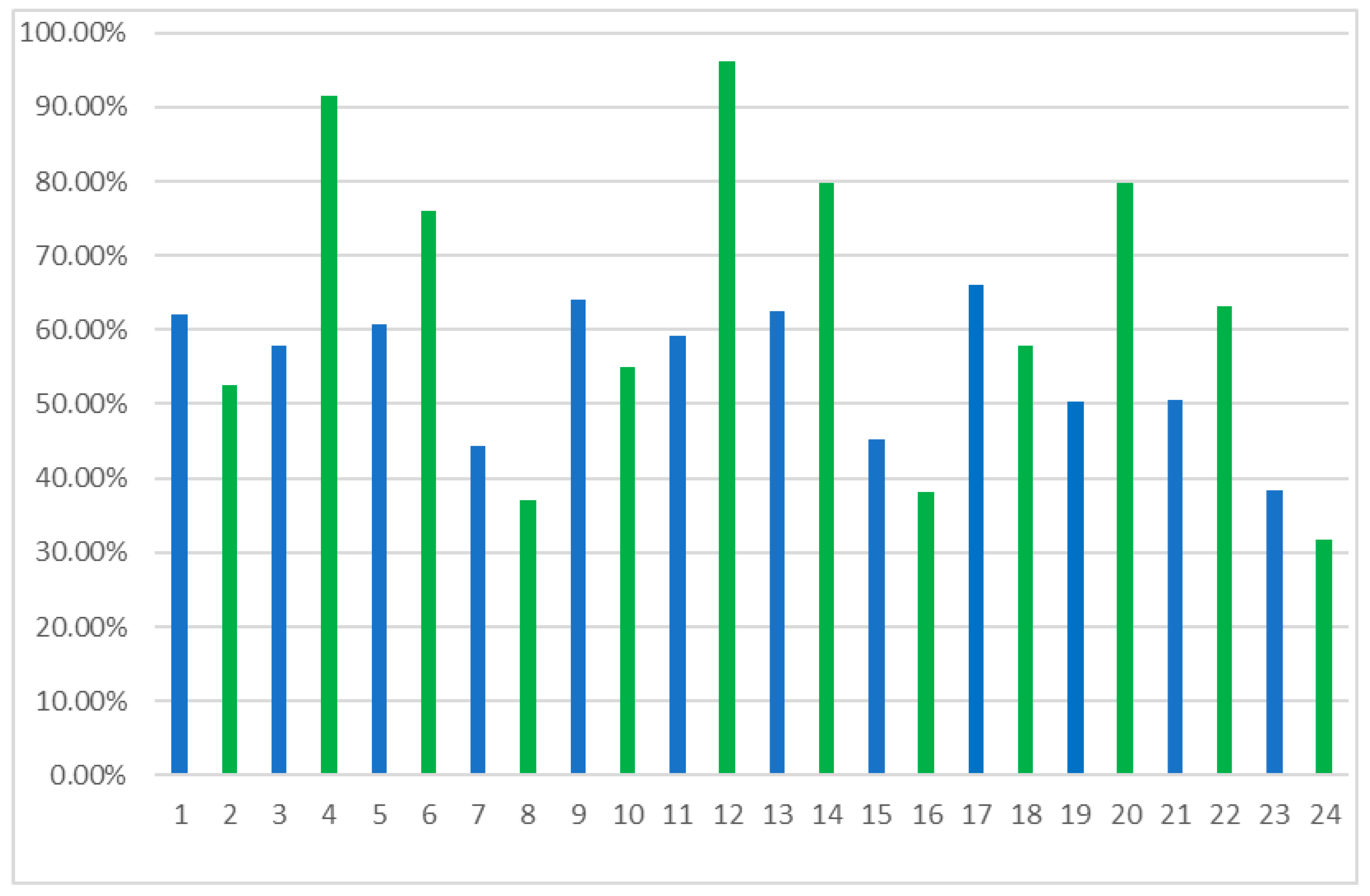
| No | ees | eep | C | E |
|---|---|---|---|---|
| 1 | 5.3% | 62.2% | 7.8% | 5.5 |
| 2 | 1.0% | 52.4% | 1.9% | 3.2 |
| 3 | 8.1% | 58.0% | 12.2% | 4.1 |
| 4 | 24.5% | 91.6% | 21.1% | 28.9 |
| 5 | 2.1% | 60.7% | 3.3% | 4.2 |
| 6 | 2.3% | 76.1% | 2.9% | 7.5 |
| 7 | 14.0% | 44.4% | 24.0% | 3.0 |
| 8 | 10.1% | 37.0% | 21.4% | 2.4 |
| 9 | 5.4% | 64.0% | 7.7% | 4.8 |
| 10 | 1.0% | 55.1% | 1.9% | 3.5 |
| 11 | 8.3% | 59.1% | 12.3% | 4.2 |
| 12 | 28.2% | 96.2% | 22.7% | 67.5 |
| 13 | 2.1% | 62.5% | 3.3% | 4.4 |
| 14 | 2.3% | 79.9% | 2.8% | 9.1 |
| 15 | 14.4% | 45.3% | 24.1% | 3.1 |
| 16 | 10.6% | 38.1% | 21.7% | 2.5 |
| 17 | 4.6% | 65.9% | 6.5% | 5.1 |
| 18 | 0.9% | 57.8% | 1.5% | 3.8 |
| 19 | 6.7% | 50.2% | 11.8% | 3.2 |
| 20 | 22.3% | 79.8% | 21.8% | 11.1 |
| 21 | 1.8% | 50.6% | 3.5% | 3.1 |
| 22 | 1.9% | 63.1% | 3.0% | 4.5 |
| 23 | 11.7% | 38.5% | 23.3% | 2.5 |
| 24 | 8.4% | 31.7% | 20.9% | 2.1 |
| Author | Racemic Compound | Literature | E-Ratio | Lipase | Acetylating Agent | Reaction Medium |
|---|---|---|---|---|---|---|
| Agustian et al. | (R, S)-atenolol | [37] | 17 | Pseudomonas fluorescence | Vinyl acetate | Tetrahydrofuran |
| Barbosa et al. | (R, S)-atenolol | [38] | 65 | Candida antarctica B | Vinyl acetate | Toluene, Hexane |
| Dvivedee et al. | (R,S)-atenolol | [31] | 210 | Candida antarctica A, Psudomonas cepacia (PCL), Mucor meihei (MML), Candida rugosa (CRL L8525), Candida rugosa (CRL L1754), Candida rugosa (CRL 62316), Candida cylindracea (CCL), Aspergillus niger (ANL), porcine pancreas lipase (PPL), lipase AY “Amano 30” (CRL). | Vinyl acetate | [EMIM][BF4] and toluene, acetonitrile |
| Lund et al. | (R, S)-atenolol | [39] | 278 | Candida antarctica B | Vinyl butanoate | Toluene |
| Sikora et al. | (R,S)-atenolol | [36,40,41] | 67 | Candida rugosa (OF), Candida rugosa (MY), Alcaigenes fecalis (QLM), Pseudomonas strutzeri (TL), Candida antarctica (CALBY), Aspergillus niger, Burkholderia cepacia, Burkoholderia cepacia (SL) Alcaigenes sp. (PL). | Isopropenyl acetate, vinyl acetate | Toluene, acetonitrile, tetrahydrofuran, chloroform |
| Chiou et al. | (R, S)-propranolol | [42] | 21 | Burkholderia cepacia (Amano PS), Candida rugosa MY, Candida rugosa OF, Amano L10, Amano AP15, Amano D20, Candida rugosa (Sigma) | Isopropenyl acetate, vinyl acetate | Toluene, chloroform, isopropyl ether, tetrahydrofuran, acetonitrile |
| Barbosa et al. | (R, S)-propranolol | [43] | 57 | Candida antarctica B | Vinyl acetate | Toluene |
| Bornscheuer et al. | (R, S)-propranolol | [44] | - | Candida antarctica B | Vinyl acetate, cyclohexyl acetate | Dodecane |
| Escorcia et al. | (R, S)-propranolol | [45,46] | 63 | Candida antarctica B | Vinyl acetate | The mixture of toluene and methanol |
| Gamboa-Velazquez et al. | (R, S)-propranolol | [47] | 259 | Candida antarctica B | Not applicable | The mixture of dioxane and water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chałupka, J.; Marszałł, M.P.; Sikora, A. Enzymatic Kinetic Resolution of Racemic 1-(Isopropylamine)-3-phenoxy-2-propanol: A Building Block for β-Blockers. Int. J. Mol. Sci. 2024, 25, 10730. https://doi.org/10.3390/ijms251910730
Chałupka J, Marszałł MP, Sikora A. Enzymatic Kinetic Resolution of Racemic 1-(Isopropylamine)-3-phenoxy-2-propanol: A Building Block for β-Blockers. International Journal of Molecular Sciences. 2024; 25(19):10730. https://doi.org/10.3390/ijms251910730
Chicago/Turabian StyleChałupka, Joanna, Michał Piotr Marszałł, and Adam Sikora. 2024. "Enzymatic Kinetic Resolution of Racemic 1-(Isopropylamine)-3-phenoxy-2-propanol: A Building Block for β-Blockers" International Journal of Molecular Sciences 25, no. 19: 10730. https://doi.org/10.3390/ijms251910730






