A Synbiotic Combining Chitin–Glucan and Lactobacillus acidophilus NCFM Induces a Colonic Molecular Signature Soothing Intestinal Pain and Inflammation in an Animal Model of IBS
Abstract
:1. Introduction
2. Results
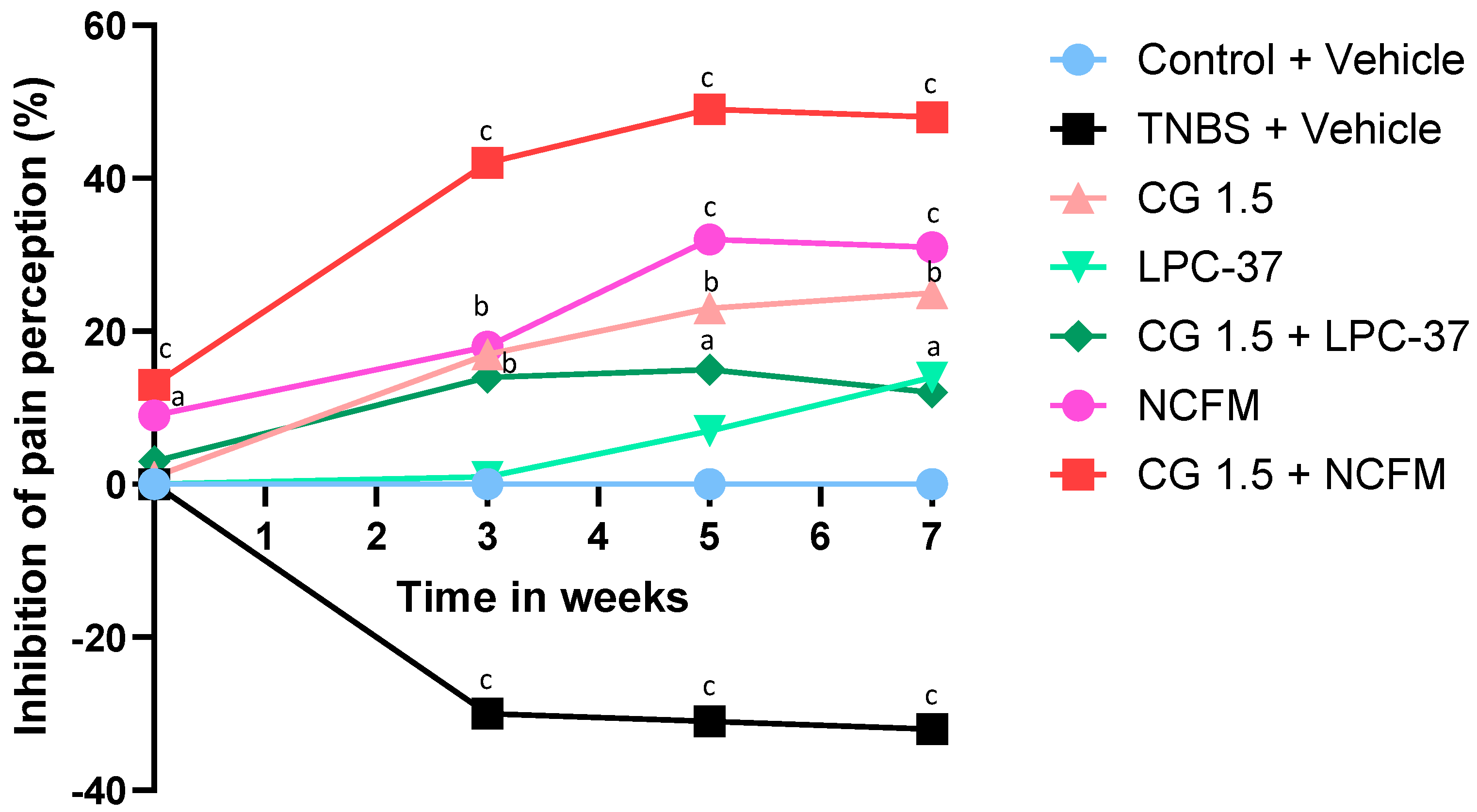
2.1. Antinociceptive Effects of the Compounds
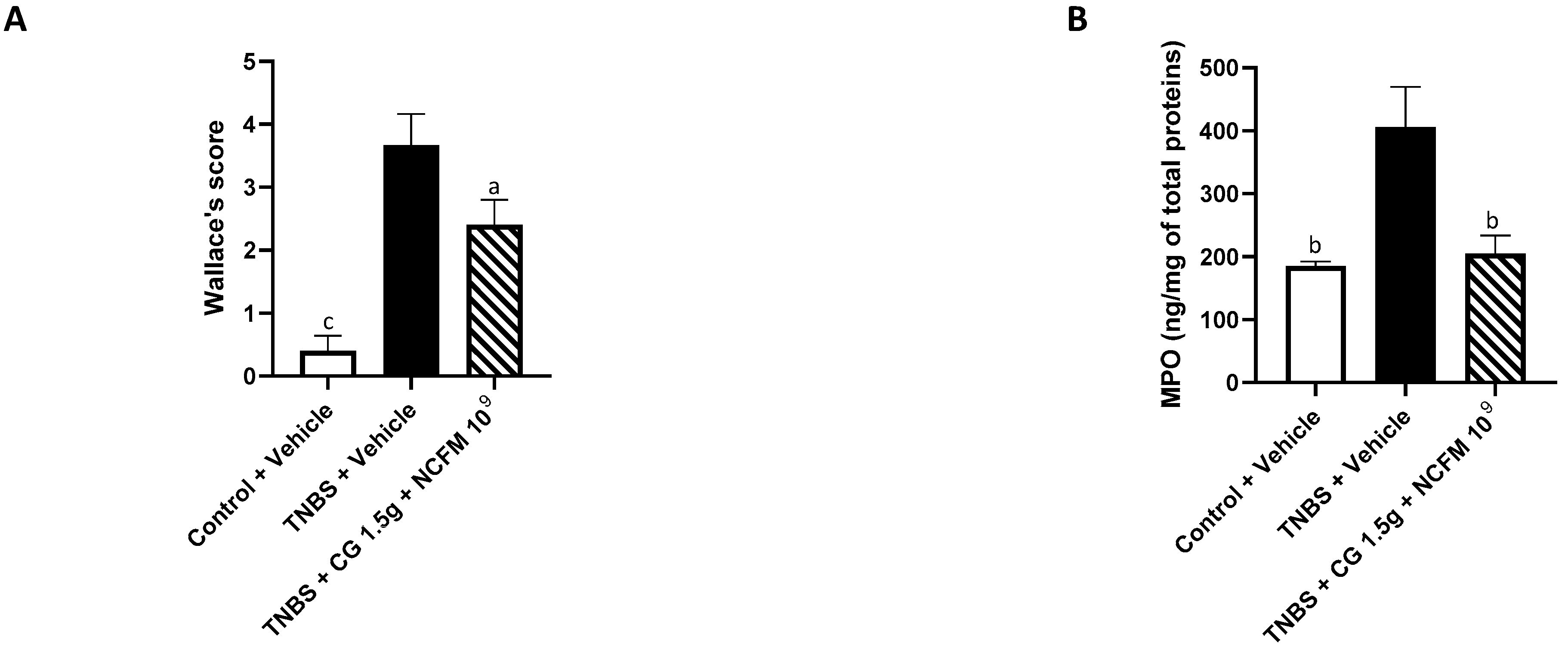
2.2. Anti-Inflammatory Effects of the Synbiotic
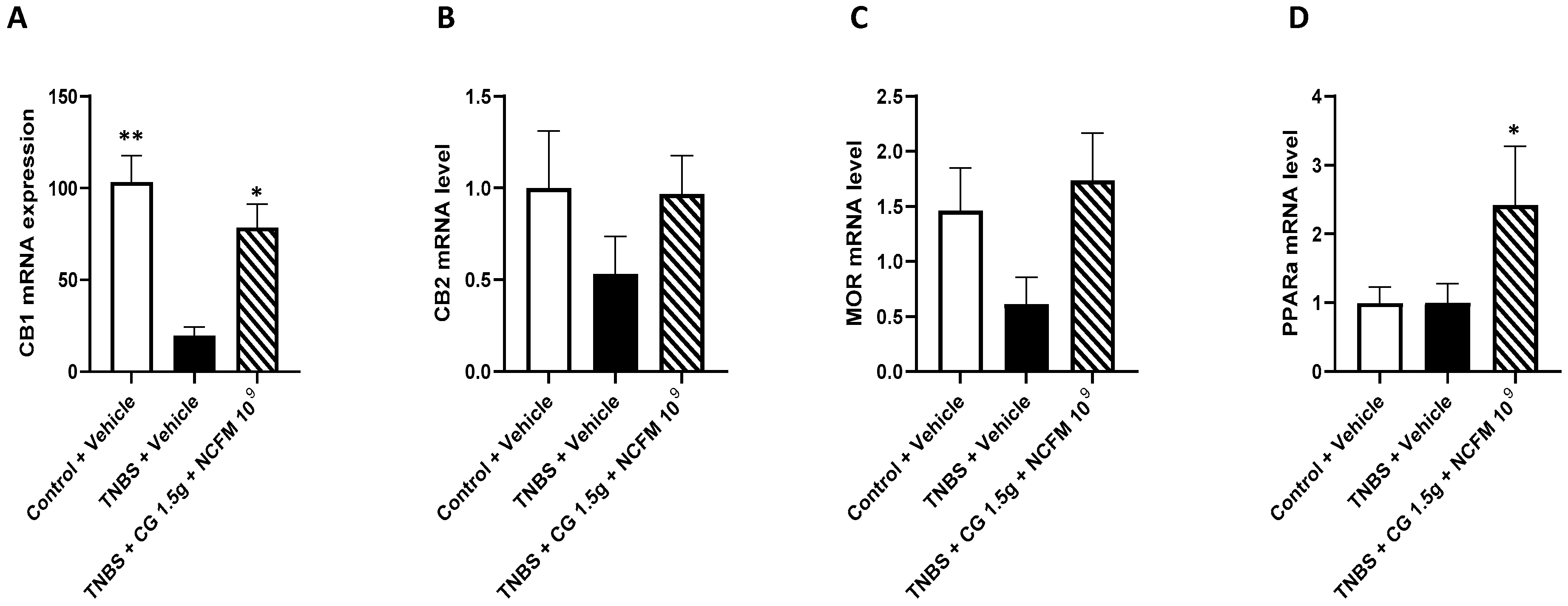
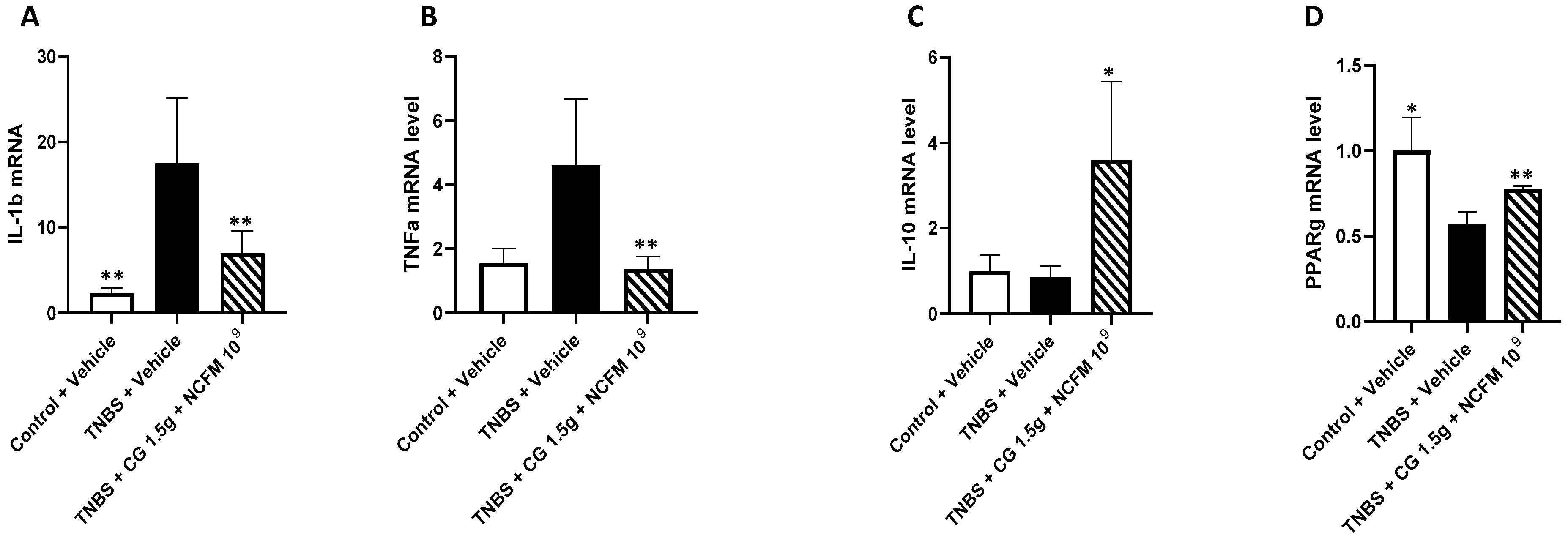
2.3. Genes mRNA Quantification in the Colon of Rats
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chitin–Glucan and Bacteria
5.2. Model of TNBS-Induced Long Lasting Visceral Hypersensitivity in Rats
5.2.1. Rats
5.2.2. TNBS-Induced Visceral Hypersensitivity
5.2.3. Evaluation of Visceral Pain by Colorectal Distension
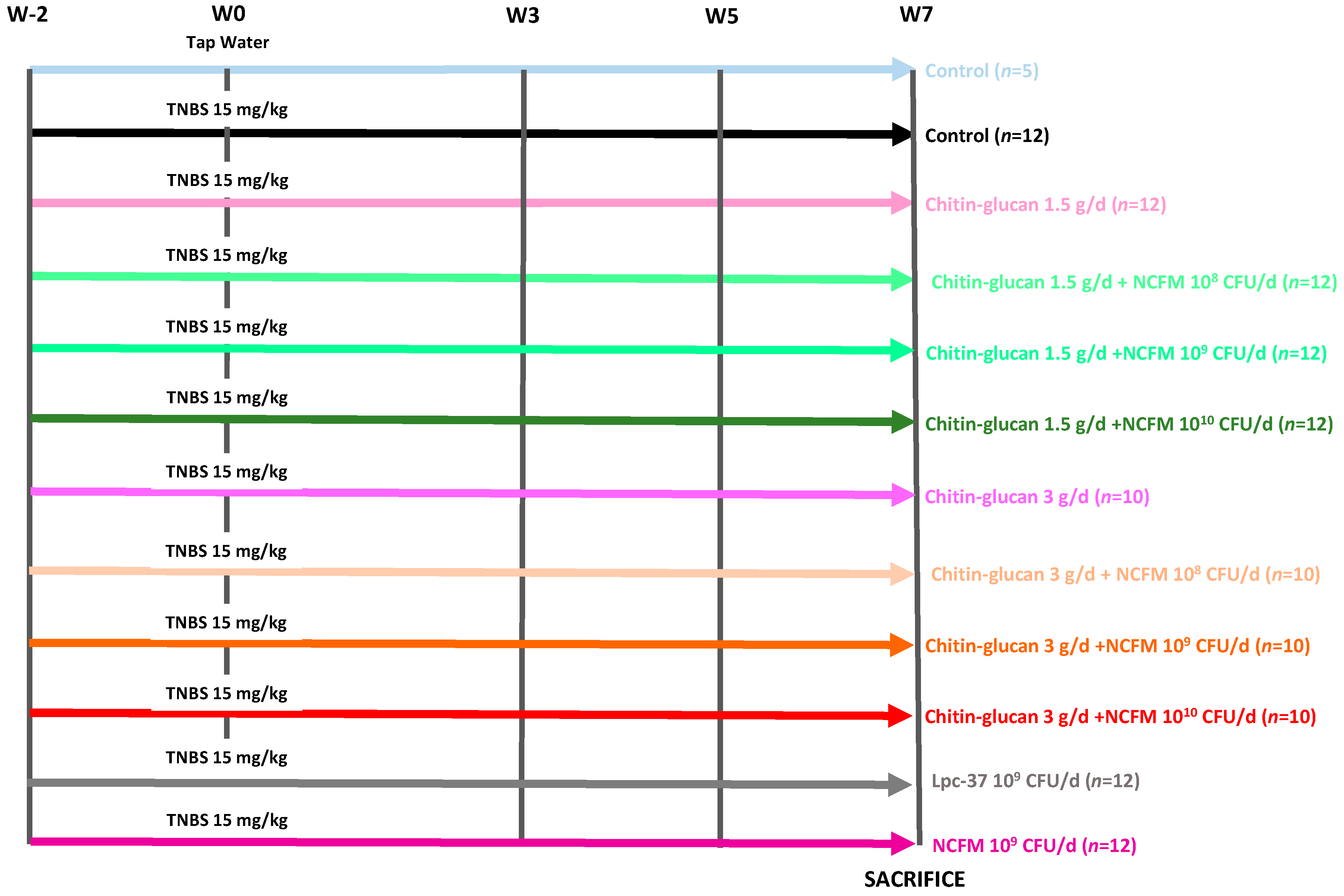
5.3. Experimental Design
5.4. Evaluation of Macroscopic Inflammatory Lesions (Wallace Score)
5.5. Quantification of Colonic Myeloperoxidase (MPO)
5.6. mRNA Quantification in Colon Samples
5.7. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camilleri, M.; Choi, M.G. Review article: Irritable bowel syndrome. Aliment. Pharmacol. Ther. 1997, 11, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Prior, M. Irritable bowel syndrome with constipation: A European-focused systematic literature review of disease burden. J. Med. Econ. 2013, 16, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Wadhwa, V.; LeClair, J.; Mikami, S.; Park, R.; Jones, M.; Sethi, N.; Brown, A.; Lembo, A. In-patient discharge rates for the irritable bowel syndrome—An analysis of national trends in the United States from 1997 to 2010. Aliment. Pharmacol. Ther. 2013, 38, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Andrews, C.N.; MacQueen, G.; Korownyk, C.; Marsiglio, M.; Graff, L.; Kvern, B.; Lazarescu, A.; Liu, L.; Paterson, W.G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc. Gastroenterol. 2019, 2, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical practice guideline on the pharmacological management of irritable bowel syndrome with diarrhea. Gastroenterology 2022, 163, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Sultan, S.; Lembo, A.; Verne, G.N.; Smalley, W.; Heidelbaugh, J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology 2022, 163, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson Ung, E.; Ringstrom, G.; Sjövall, H.; Simrén, M. How patients with long-term experience of living with irritable bowel syndrome manage illness in daily life: A qualitative study. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Kurland, M.; Lydick, E.; Locke, G.R., 3rd; Yawn, B.P. The patient’s perspective of irritable bowel syndrome. J. Fam. Pr. 2001, 50, 521–525. [Google Scholar] [PubMed]
- Di Rosa, C.; Altomare, A.; Terrigno, V.; Carbone, F.; Tack, J.; Cicala, M.; Guarino, P.L. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2023, 15, 1647. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA Panel on Dietetic Products NaAN. Scientific opinion on the safety of ‘Chitin-glucan’ as a novel food ingredient. EFSA J. 2010, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Berecochea-Lopez, A.; Decordé, K.; Ventura, E.; Godard, M.; Bornet, A.; Teissèdre, P.L.; Cristol, J.P.; Rouanet, J.M. Fungal chitin-glucan from Aspergillus niger efficiently reduces aortic fatty streak accumulation in the high-fat fed hamster, an animal model of nutritionally induced atherosclerosis. J. Agric. Food Chem. 2009, 57, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Verstrepen, L.; Ghyselinck, J.; Van den Abbeele, P.; Marzorati, M.; Modica, S.; Ranjanoro, T.; Maquet, V. Chitin Glucan Shifts Luminal and Mucosal Microbial Communities, Improve Epithelial Barrier and Modulates Cytokine Production In Vitro. Nutrients 2021, 13, 3249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Neyrinck, A.M.; Zhang, Z.; Seethaler, B.; Nazare, J.A.; Robles Sánchez, C.; Roumain, M.; Muccioli, G.G.; Bindels, L.B.; Cani, P.D.; et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes 2020, 12, 1810530. [Google Scholar] [CrossRef] [PubMed]
- Valibouze, C.; Dubuquoy, C.; Chavatte, P.; Genin, M.; Maquet, V.; Modica, S.; Desreumaux, P.; Rousseaux, C. Chitin-glucan improves important pathophysiological features of irritable bowel syndrome. World J. Gastroenterol. 2024, 30, 2258–2271. [Google Scholar] [CrossRef]
- McFarland, L.V.; Karakan, T.; Karatas, A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. eClinicalMedicine 2021, 41, 101154. [Google Scholar] [CrossRef]
- Goodoory, V.C.; Khasawneh, M.; Black, C.J.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of Probiotics in Irritable Bowel Syndrome: Systematic Review and Meta-analysis. Gastroenterology 2023, 165, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Klaenhammer, T. Invited Review: The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J. Dairy. Sci. 2001, 84, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Merour, E.; Geboes, K.; Chamaillard, M.; Ouwehand, A.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain—A randomised clinical study. Aliment. Pharmacol. Ther. 2014, 40, 200–207. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, B.; Slack, T.; Wong, S.W.; Lam, F.; Muhlmann, M.; Koestenbauer, J.; Dark, J.; Newstead, G. Randomized controlled trial of probiotics after colonoscopy. ANZ J. Surg. 2017, 87, E65–E69. [Google Scholar] [CrossRef] [PubMed]
- Foligne, B.; Nutten, S.; Grangette, C.; Dennin, V.; Goudercourt, D.; Poiret, S.; Dewulf, J.; Brassart, D.; Mercenier, A.; Pot, B. Correlation between in vitro and in vivo immune modulatory properties of lactic acid bacteria. World J. Gastroenterol. 2007, 13, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Engelbrektson, A.L.; Korzenik, J.R.; Sanders, M.E.; Clement, B.G.; Leyer, G.; Klaenhammer, T.R.; Kitts, C.L. Analysis of treatment effects on the microbial ecology of the human intestine. FEMS Microbiol. Ecol. 2006, 57, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Griffin, S.M.; Ibarra, A.; Ellsiepen, E.; Hellhammer, J. Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: A randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol. Stress. 2020, 13, 100277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, A.C.; Farmer, A.D.; Ness, T.J.; Greenwood-Van Meerveld, B. Critical evaluation of animal models of visceral pain for therapeutics development: A focus on irritable bowel syndrome. Neurogastroenterol. Motil. 2020, 32, e13776. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Bourdu, S.; Dapoigny, M.; Chapuy, E.; Artigue, F.; Vasson, M.P.; Dechelotte, P.; Bommelaer, G.; Eschalier, A.; Ardid, D. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 2005, 128, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C.; et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 2005, 201, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Esquerre, N.; Basso, L.; Dubuquoy, C.; Djouina, M.; Chappard, D.; Blanpied, C.; Desreumaux, P.; Vergnolle, N.; Vignal, C.; Body-Malapel, M. Aluminum Ingestion Promotes Colorectal Hypersensitivity in Rodents. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 185–196. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, A.M.; Schukking, I.; Brummer, R.J.M.; van de Burgwal, L.H.M.; Larsen, O.F.A. Comparing probiotic and drug interventions in irritable bowel syndrome: A meta-analysis of randomised controlled trials. Benef. Microbes 2022, 13, 183–194. [Google Scholar] [CrossRef]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef]
- Umeano, L.; Iftikhar, S.; Alhaddad, S.F.; Paulsingh, C.N.; Riaz, M.F.; Garg, G.; Mohammed, L. Effectiveness of Probiotic Use in Alleviating Symptoms of Irritable Bowel Syndrome: A Systematic Review. Cureus 2024, 16, e58306. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Talley, N.J. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: A systematic review. Gastroenterol. 2011, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Moossavi, S.; Cremon, C.; Barbaro, M.R.; Moraveji, S.; Talmon, G.; Rezaei, N.; Hughes, P.A.; Bian, Z.X.; Choi, C.H.; et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2017, 30, e13192. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Tack, J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Pfeiler, E.A.; Brown, J.B.; Zadeh, M.; Gramarossa, M.; Managlia, E.; Bere, P.; Sarraj, B.; Khan, M.W.; Pakanati, K.C.; et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4623–4630. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, L.; Dharancy, S.; Nutten, S.; Pettersson, S.; Auwerx, J.; Desreumaux, P. Role of peroxisome proliferator-activated receptor gamma and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet 2002, 360, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Speca, S.; Langlois, A.; Davila, A.M.; Dubuquoy, C.; Grauso, M.; Martin Mena, A.; Figeac, M.; Metzger, D.; Rousseaux, C.; et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates lactase expression and activity in the gut. EMBO Mol. Med. 2017, 9, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S. PPARalpha agonists—An emerging concept in the management of neuropathic and visceral pain. Psychopharmacology 2012, 221, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Margiotta, F.; Martelli, A.; Testai, L.; Calderone, V.; et al. The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR alpha Receptor Activation in Pain Relief. Nutrients 2022, 14, 3137. [Google Scholar] [CrossRef] [PubMed]
- Ranaivo, H.; Zhang, Z.; Alligier, M.; Van Den Berghe, L.; Sothier, M.; Lambert-Porcheron, S.; Feugier, N.; Cuerq, C.; Machon, C.; Neyrinck, A.M.; et al. Chitin-glucan supplementation improved postprandial metabolism and altered gut microbiota in subjects at cardiometabolic risk in a randomized trial. Sci. Rep. 2022, 12, 8830. [Google Scholar] [CrossRef]
- Adolph, T.E.; Meyer, M.; Jukic, A.; Tilg, H. Heavy arch: From inflammatory bowel diseases to metabolic disorders. Gut 2024, 73, 1376–1387. [Google Scholar] [CrossRef]
- Ghusn, W.; Loftus, E.V., Jr.; Johnson, A.M. Reviewing the impact of obesity on inflammatory bowel disease and considerations for optimizing management. Curr. Opin. Gastroenterol. 2024, 40, 268–275. [Google Scholar] [CrossRef]
- Je, Y.; Han, K.; Chun, J.; Kim, Y.; Kim, J.H.; Youn, Y.H.; Park, H.; Im, J.P.; Kim, J.S. Association of Waist Circumference with the Risk of Inflammatory Bowel Disease: A Nationwide Cohort Study of Million Individuals in Korea. J. Crohn’s Colitis 2023, 17, 681–692. [Google Scholar] [CrossRef]
- Drouet, M.; Dubuquoy, L.; Desreumaux, P.; Bertin, B. Visceral fat and gut inflammation. Nutrition 2012, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]

| Score | Criteria of Macroscopic Evaluation |
|---|---|
| 0 | No inflammation |
| 1 | Hyperemia without ulcerations |
| 2 | Hyperemia with thickening of the mucosa without ulcerations |
| 3 | 1 ulceration without thickening of the colonic wall |
| 4 | 2 or more ulcerative or inflammatory sites |
| 5 | 2 or more ulcerative or inflammatory sites with an extent > 1 cm |
| 6 | Ulcerative or inflammatory site > 2 cm |
| 7 | Ulcerative or inflammatory site > 3 cm |
| 8 | Ulcerative or inflammatory site > 4 cm |
| 9 | Ulcerative or inflammatory site > 5 cm |
| Rat Genes | Primer Sequences (5′ → 3′) |
|---|---|
| GAPDH | F:5′-CTG-TTC-TAG-AGA-CAG-CCG-CAT-CT-3′ R: 5′-ACA-CCG-ACC-TTC-ACC-ATC-TTG-3′ |
| IL-1b | S: 5′-TgAAAgCTCTCCACCTCAATggAC-3′ AS: 5′-TgCAgCCATCTTTAggAAgACACg-3′ |
| IL-10 | S: 5′-CAgTCAgCCAgACCCACAT-3′ AS: 5′-gCTCCACTgCCTTgCTTT-3′ |
| TNF-α | S: 5′-AgCACAgAAAgCATgATCCgAg-3′ AS: 5′-CCTggTATgAAgTggCAAATCg-3′ |
| PPARγ | F: 5′-CTgACCCAATggTTgCTgATTAC-3′ R: 5′-ggACgCAggCTCTACTTTgATC -3′ |
| PPARα | F: 5′-ACgATgCTgTCCTCCTTgATg-3′ R: 5′-gTgTgATAAAgCCATTgCCgT-3′ |
| MOR | F: 5′-TTCTgCATTgCTTTgggTTACACg-3′ R: 5′-CTgACAgCAACCTgATTCCACgTA-3′ |
| CB1 | F: 5′-ATgAAgTCgATCCTAgATggCCTTg-3′ R: 5′-gCTCCCCACACTggATg-3’ |
| CB2 | F: 5′-gATAgCTCggATgCggCTAgACgTg-3′ R: 5-CAgCATggggCTgTggATCgAgg-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capirchio, L.; Rousseaux, C.; Dubuquoy, C.; Ouwehand, A.C.; Maquet, V.; Modica, S.; Louis, E.; Desreumaux, P.; Tack, J. A Synbiotic Combining Chitin–Glucan and Lactobacillus acidophilus NCFM Induces a Colonic Molecular Signature Soothing Intestinal Pain and Inflammation in an Animal Model of IBS. Int. J. Mol. Sci. 2024, 25, 10732. https://doi.org/10.3390/ijms251910732
Capirchio L, Rousseaux C, Dubuquoy C, Ouwehand AC, Maquet V, Modica S, Louis E, Desreumaux P, Tack J. A Synbiotic Combining Chitin–Glucan and Lactobacillus acidophilus NCFM Induces a Colonic Molecular Signature Soothing Intestinal Pain and Inflammation in an Animal Model of IBS. International Journal of Molecular Sciences. 2024; 25(19):10732. https://doi.org/10.3390/ijms251910732
Chicago/Turabian StyleCapirchio, Lena, Christel Rousseaux, Caroline Dubuquoy, Arthur C. Ouwehand, Véronique Maquet, Salvatore Modica, Edouard Louis, Pierre Desreumaux, and Jan Tack. 2024. "A Synbiotic Combining Chitin–Glucan and Lactobacillus acidophilus NCFM Induces a Colonic Molecular Signature Soothing Intestinal Pain and Inflammation in an Animal Model of IBS" International Journal of Molecular Sciences 25, no. 19: 10732. https://doi.org/10.3390/ijms251910732
APA StyleCapirchio, L., Rousseaux, C., Dubuquoy, C., Ouwehand, A. C., Maquet, V., Modica, S., Louis, E., Desreumaux, P., & Tack, J. (2024). A Synbiotic Combining Chitin–Glucan and Lactobacillus acidophilus NCFM Induces a Colonic Molecular Signature Soothing Intestinal Pain and Inflammation in an Animal Model of IBS. International Journal of Molecular Sciences, 25(19), 10732. https://doi.org/10.3390/ijms251910732






