Genome-Wide Identification and Characterization of Diterpenoid Pathway CYPs in Andrographis paniculata and Analysis of Their Expression Patterns under Low Temperature Stress
Abstract
:1. Introduction
2. Results
2.1. Identification and Classification of Total CYPs in Andrographis Paniculata
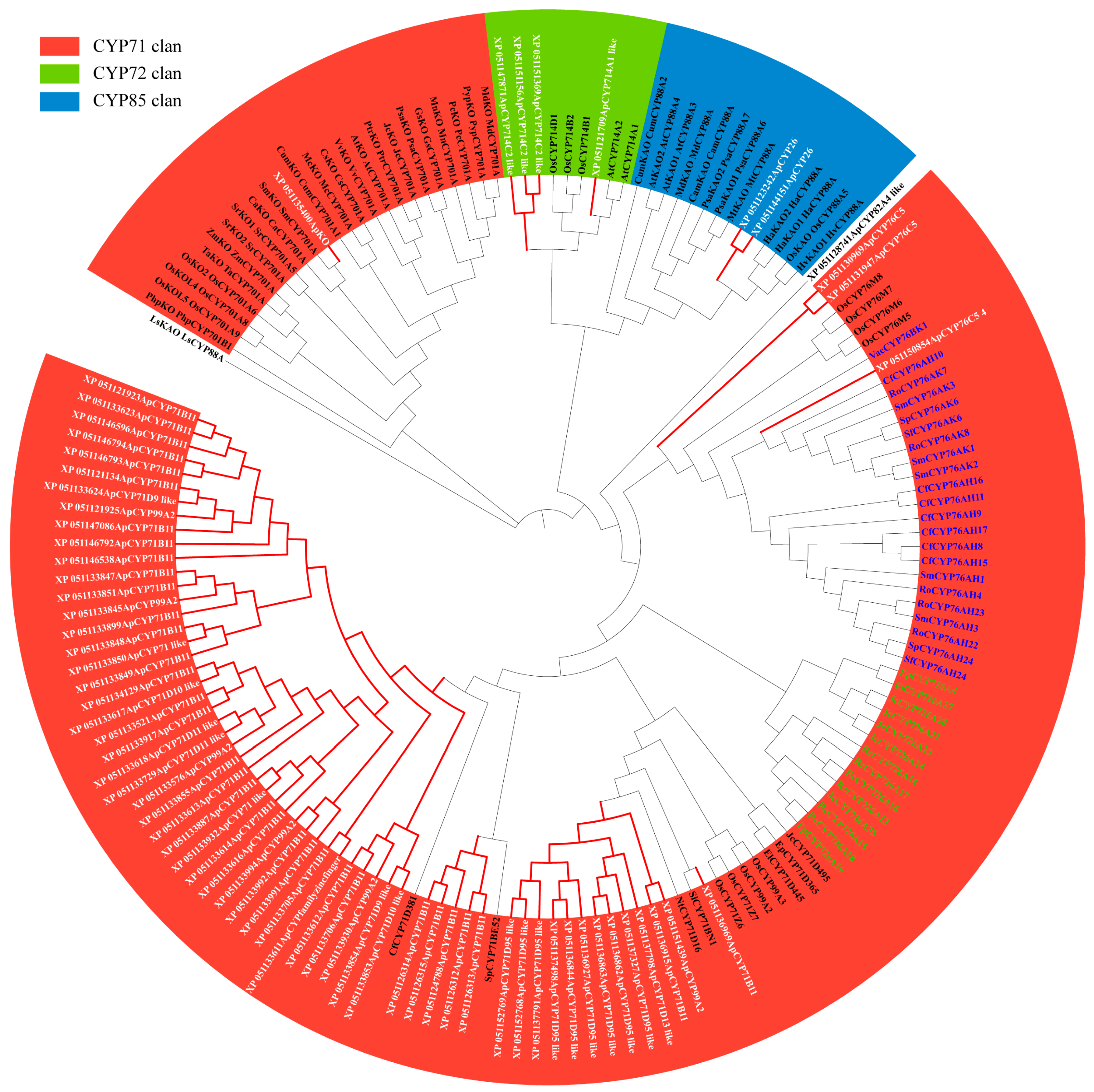
2.2. Identification of Diterpenoid Pathway CYPs in Andrographis Paniculata
2.3. Analysis of Basic Information for Diterpenoid Pathway CYPs
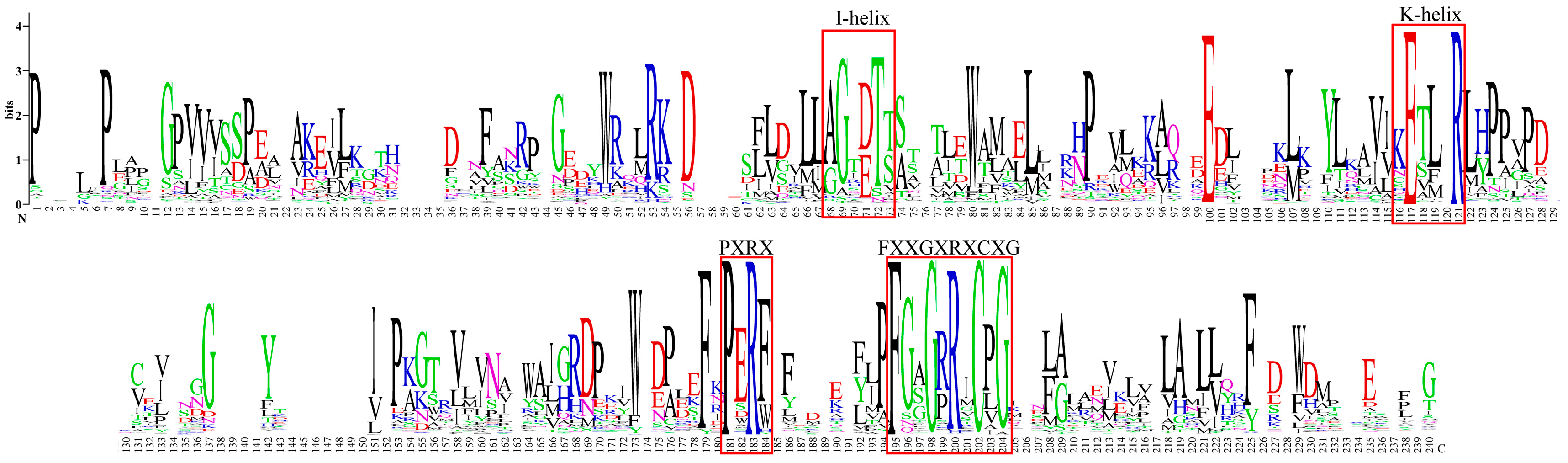
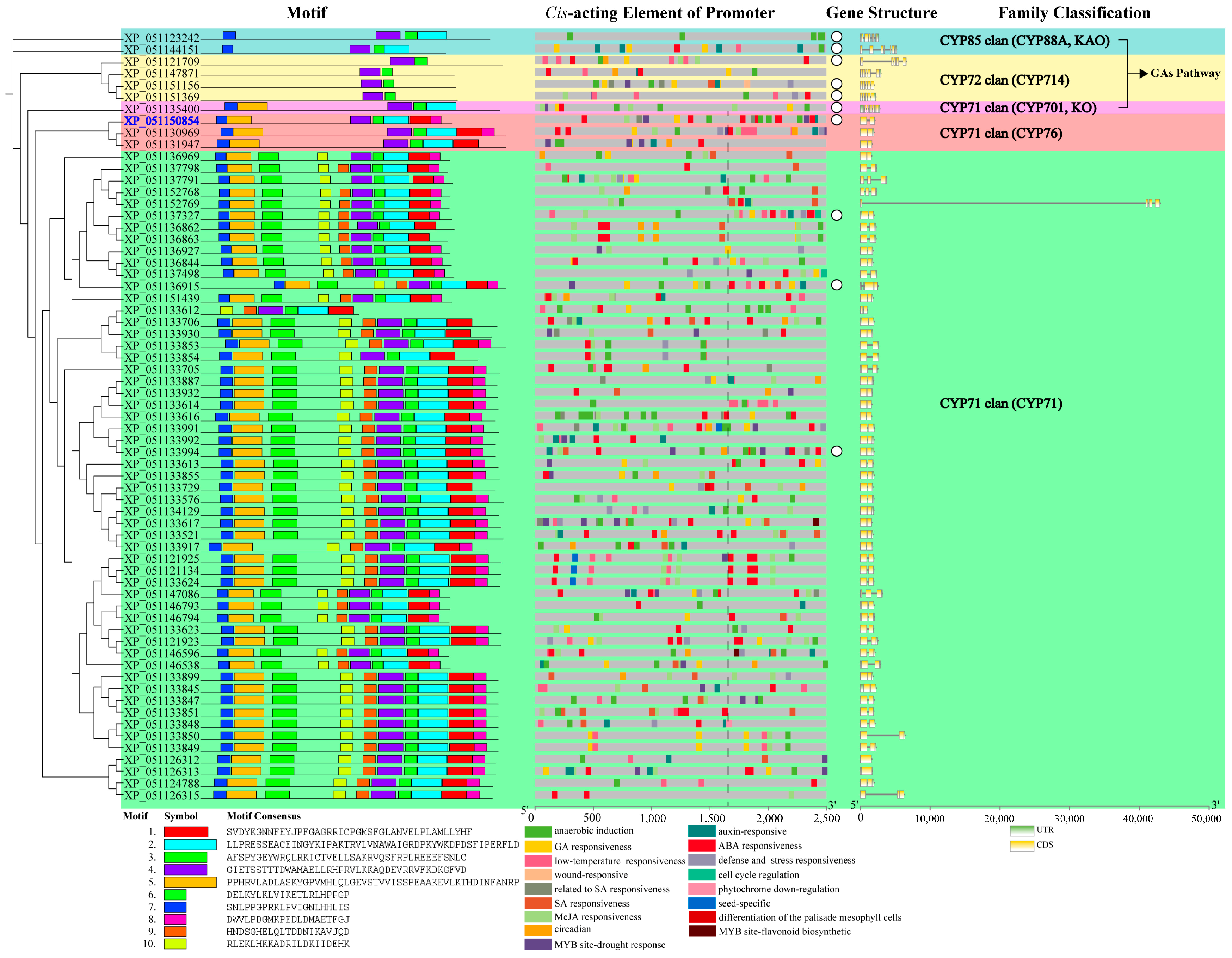
2.4. Analysis of Feature Information for Diterpenoid Pathway CYPs
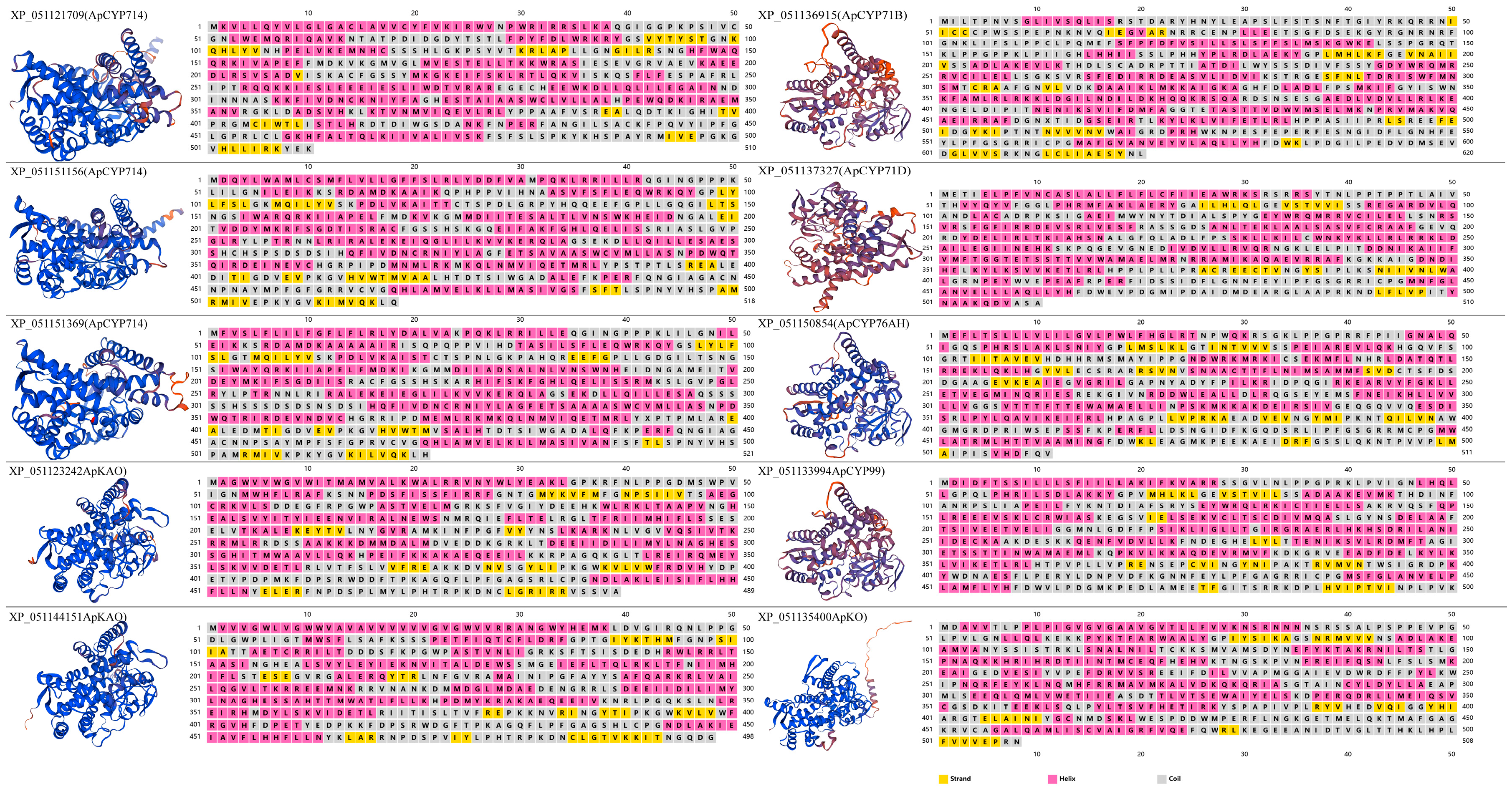
2.5. Structural Analysis of Representative Diterpenoid Pathway CYPs
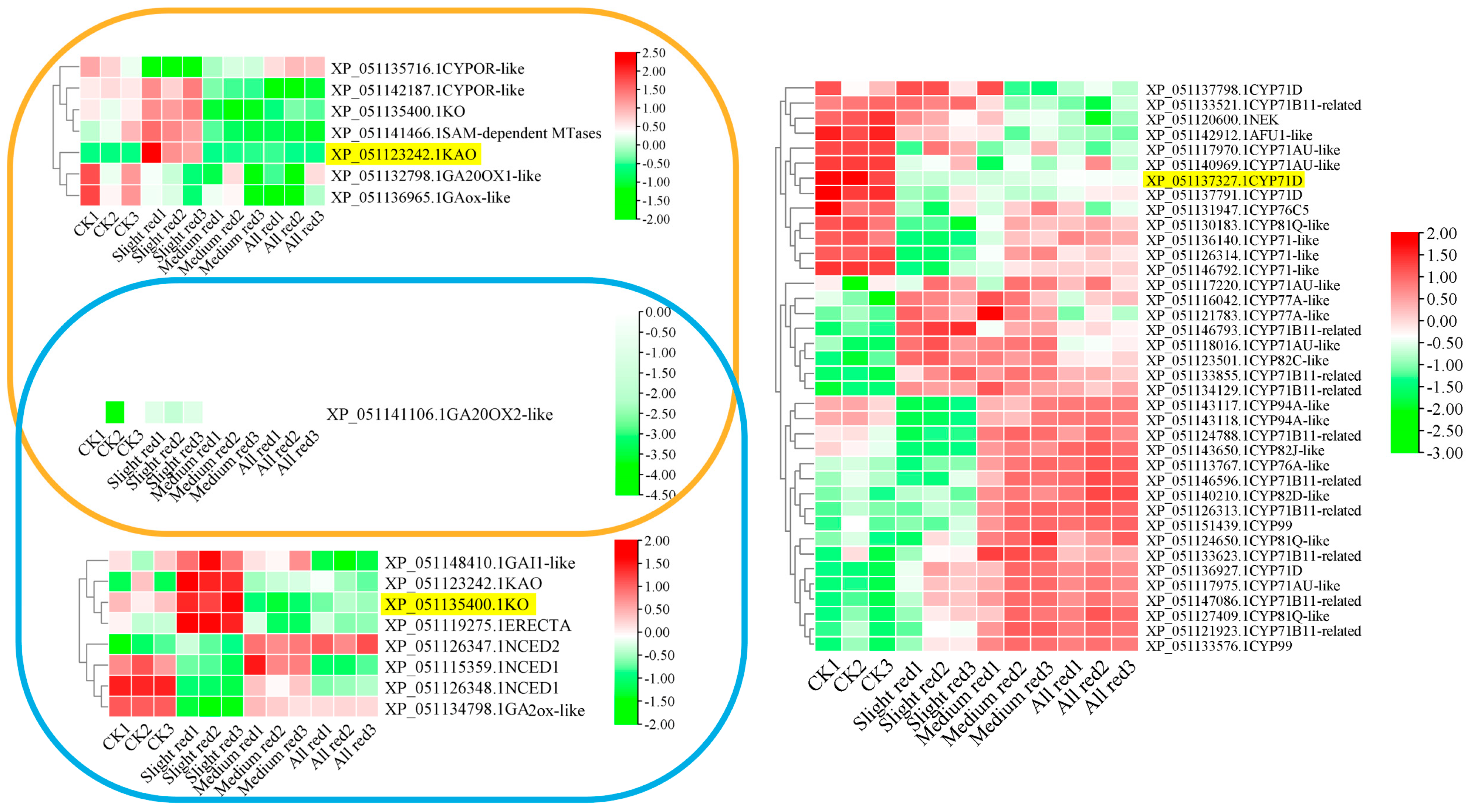
2.6. Protein Interactions of LT-Responsive Representative CYPs
2.7. LT Expression Profiling of KO, KAO, and CYP71D-Interacting Genes
3. Discussion
4. Materials and Methods
4.1. Identification of Total CYPs in Andrographis Paniculata
4.2. Construction of Phylogenetic Trees
4.3. Identification of Conserved Domains in Andrographis Paniculata CYPs
4.4. Analysis of Gene Structure
4.5. Analysis of Protein Characteristics
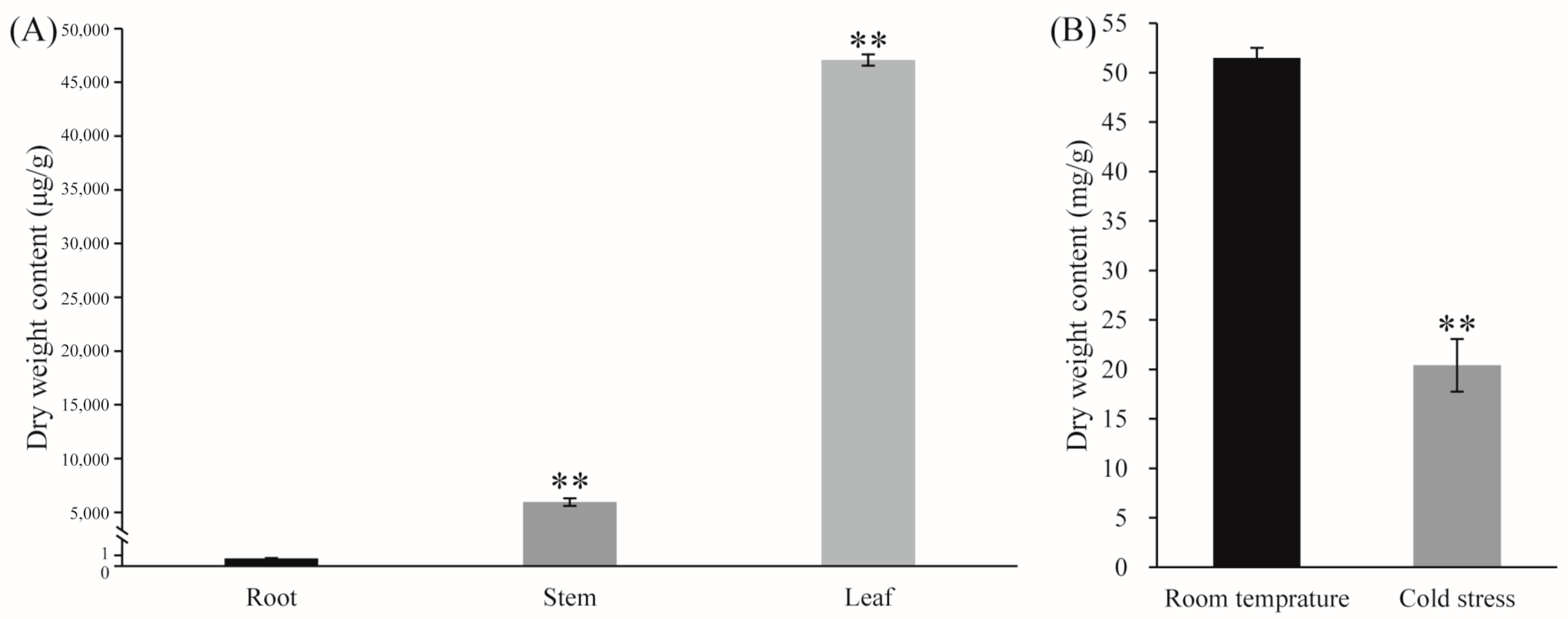
4.6. Quantification of Andrographolide Content
4.6.1. Growth Conditions
4.6.2. LT Treatment Conditions
4.6.3. Detection of Andrographolide Using Liquid Chromatography–Tandem Mass Spectrometry
4.6.4. Statistical Analysis
4.7. Gene Expression Analysis under LT Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, M.-Y.; Xu, S.-Q.; Zhang, W.-T.; Gu, Y.; Mei, Y.; Li, J.-Y.; Zhou, F.; Wang, J.-H. Advances in scientific research on Andrographis paniculata. Chin. Agric. Sci. Bull. 2022, 38, 155–164. [Google Scholar]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.W.; Sagineedu, S.R.; Wong, W.S.F. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Xu, S.-Q.; Li, J.-Y.; Gu, Y.; Mei, Y.; Zhang, W.-T.; Zhou, F.; Wang, J.-H. Transcriptome analysis and discovery of genes related to andrographolide synthesis of Andrographis paniculata under UV-C treatment. J. South. Agric. 2022, 53, 618–627. [Google Scholar]
- Tan, H.-T.; Gong, D.-Y.; Li, B. Advances in the biosynthetic pathway of dendrobine and terpenoid CYP450 enzymes. J. Chongqing Technol. Bus. Univ. (Nat. Sci. Ed.) 2022, 39, 1–13. [Google Scholar]
- Li, D. Cloning and Function Analysis of CYP82Ds Genes from Camellia sinensis. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Li, P.; Lu, X. Research advances in cytochrome P450-catalysed pharmaceutical terpenoid biosynthesis in plants. J. Exp. Bot. 2019, 70, 4619–4630. [Google Scholar] [CrossRef]
- Bathe, U.; Tissier, A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef]
- Mitu, S.; Ogbourne, S.; Klein, A.; Tran, T.; Cummins, S. The CYP450 multigene family of Fontainea and insights into diterpenoid synthesis. BMC Plant Biol. 2021, 21, 191. [Google Scholar] [CrossRef]
- Hu, J.-D.; Qiu, S.; Wang, F.-Y.; Li, Q.; Xiang, C.-L.; Di, P.; Wu, Z.-D.; Jiang, R.; Li, J.-X.; Zeng, Z.; et al. Functional divergence of CYP76AKs shapes the chemodiversity of abietane-type diterpenoids in genus Salvia. Nat. Commun. 2023, 14, 4696. [Google Scholar] [CrossRef]
- Mizobuchi, R.; Sato, H.; Fukuoka, S.; Tanabata, T.; Tsushima, S.; Imbe, T.; Yano, M. Mapping a quantitative trait locus for resistance to bacterial grain rot in rice. Rice 2013, 6, 13. [Google Scholar] [CrossRef]
- King, A.J.; Brown, G.D.; Gilday, A.D.; Larson, T.R.; Graham, I.A. Production of bioactive diterpenoids in the Euphorbiaceae depends on evolutionarily conserved gene clusters. Plant Cell 2014, 26, 3286–3298. [Google Scholar] [CrossRef]
- Kuang, X.-J.; Sun, S.-J.; Wei, J.-H.; Li, Y.; Sun, C. Iso-Seq analysis of the Taxus cuspidata transcriptome reveals the complexity of Taxol biosynthesis. BMC Plant Biol. 2019, 19, 210. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wu, Q.-Y.; Ge, Y.; Huang, Z.-Y.; Hong, R.; Li, A.-T.; Xu, J.-H.; Yu, H.-L. Hydroxylases involved in terpenoid biosynthesis: A review. Bioresour. Bioprocess. 2023, 10, 39. [Google Scholar] [CrossRef]
- Eilon, S.; Peter, H.; Tai-Ping, S. Highlights in gibberellin research: A tale of the dwarf and the slender. Plant Physiol. 2024, 195, 111. [Google Scholar]
- He, J.; Chen, Q.-W.; Xin, P.-Y.; Yuan, J.; Ma, Y.-H.; Wang, X.-M.; Xu, M.-M.; Chu, J.-F.; Peters, R.J.; Wang, G.-D. CYP72A enzymes catalyse 13-hydrolyzation of gibberellins. Nat. Plants. 2019, 5, 1057–1065. [Google Scholar] [CrossRef]
- Wang, J.-W.; Liu, Z.-Y.; She, H.-B.; Xu, Z.-S.; Zhang, H.-L.; Fang, Z.-W.; Qian, W. Genome-wide identification and characterization of U-Box gene family members and analysis of their expression patterns in Phaseolus vulgaris L. under cold stress. Int. J. Mol. Sci. 2024, 25, 7968. [Google Scholar] [CrossRef]
- Kumar, M.S.; Babu, P.R.; Rao, K.V.; Reddy, V.D. Organization and classification of cytochrome P450 genes in castor (Ricinus communis L.). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 131–143. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.-X.; Li, Q.-H.; Wang, Z.-W.; Zeng, W.-W.; Yin, H.; Qi, K.-J.; Zou, Y.; Hu, J.; Huang, B.-S.; et al. Genome-wide identification, comparative analysis and functional roles in flavonoid biosynthesis of cytochrome P450 superfamily in pear (Pyrus spp.). BMC Genomic Data 2023, 24, 58. [Google Scholar] [CrossRef]
- Liu, X.-L.; Cao, F.-L.; Cai, J.-F.; Wang, H.-L. The molecular cloning and expression analysis of a CYP71 gene in Ginkgo biloba L. Not. Bot. Horti Agrobo. Cluj-Napoca 2016, 44, 77–84. [Google Scholar] [CrossRef]
- Xia, T.-F.; Yang, Y.-P.; Zheng, H.-Y.; Han, X.-Y.; Jin, H.-B.; Xiong, Z.-J.; Qian, W.-Q.; Xia, L.-Q.; Ji, X.; Li, G.-W.; et al. Efficient expression and function of a receptor-like kinase in wheat powdery mildew defense require an intron located MYB binding site. Plant Biotechnol. J. 2021, 19, 897–909. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Nelson, D.R.; Zhang, J.; Wan, X.-Y.; Peters, R.J. Plant (di)terpenoid evolution: From pigments to hormones and beyond. Nat. Prod. Rep. 2023, 40, 452–469. [Google Scholar] [CrossRef]
- Mai, T.D.; Kim, H.M.; Park, S.Y.; Ma, S.H.; Do, J.H.; Choi, W.; Jang, H.M.; Hwang, H.B.; Song, E.G.; Shim, J.S.; et al. Metabolism of phenolic compounds catalyzed by Tomato CYP736A61. Enzyme Microb. Technol. 2024, 176, 110425. [Google Scholar] [CrossRef]
- Thyssen, G.N.; Naoumkina, M.; McCarty, J.C.; Jenkins, J.N.; Florane, C.; Li, P.; Fang, D.D. The P450 gene CYP749A16 is required for tolerance to the sulfonylurea herbicide trifloxysulfuron sodium in cotton (Gossypium hirsutum L.). BMC Plant Biol. 2018, 18, 186. [Google Scholar] [CrossRef]
- Nelson, D.R.; Ming, R.; Alam, M.; Schuler, M.A. Comparison of cytochrome P450 genes from six plant genomes. Trop. Plant Biol. 2008, 1, 216–235. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Sun, W.-T.; Zhang, X.-Z.; Wan, S.-T.; Wang, R.-W.; Li, C. Regulation on oxidation selectivity for β-amyrin by Class Ⅱ cytochrome P450 enzymes. Synth. Biol. J. 2021, 2, 804. [Google Scholar]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef]
- Wang, E.; Wang, R.; DeParasis, J.; Loughrin, J.H.; Gan, S.; Wagner, G.J. Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural-product-based aphid resistance. Nat. Biotechnol. 2001, 19, 371–374. [Google Scholar] [CrossRef]
- Karunanithi, P.S.; Dhanota, P.; Addison, J.B.; Tong, S.; Fiehn, O.; Zerbe, P. Functional characterization of the cytochrome P450 monooxygenase CYP71AU87 indicates a role in marrubiin biosynthesis in the medicinal plant Marrubium vulgare. BMC Plant Biol. 2019, 19, 114. [Google Scholar] [CrossRef]
- Ignea, C.; Athanasakoglou, A.; Ioannou, E.; Georgantea, P.; Trikka, F.A.; Loupassaki, S.; Roussis, V.; Makris, A.M.; Kampranis, S.C. Carnosic acid biosynthesis elucidated by a synthetic biology platform. Proc. Natl. Acad. Sci. USA 2016, 113, 3681–3686. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, G.; Chen, T.; Ma, X.; Wang, R.; Jin, B.; Yang, J.; Kang, L.; Tang, J.; Lai, C.-J.-S.; et al. Expansion within the CYP71D subfamily drives the heterocyclization of tanshinones synthesis in Salvia miltiorrhiza. Nat. Commun. 2021, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, I.; Andersen-Ranberg, J.; Jensen, N.B.; Wubshet, S.G.; Heskes, A.M.; Forman, V.; Hallström, B.; Hamberger, B.; Motawia, M.S.; Olsen, C.E.; et al. Total biosynthesis of the cyclic AMP booster forskolin from Coleus forskohlii. Elife 2017, 6, e23001. [Google Scholar] [CrossRef] [PubMed]
- Heskes, A.M.; Sundram, T.C.M.; Boughton, B.A.; Jensen, N.B.; Hansen, N.L.; Crocoll, C.; Cozzi, F.; Rasmussen, S.; Hamberger, B.; Hamberger, B.; et al. Biosynthesis of bioactive diterpenoids in the medicinal plant Vitex agnus-castus. Plant J. 2018, 93, 943–958. [Google Scholar] [CrossRef]
- Guo, J.; Ma, X.-H.; Cai, Y.; Ma, Y.; Zhan, Z.-L.; Zhou, Y.-J.J.; Liu, W.J.; Guan, M.-X.; Yang, J.; Cui, G.-H.; et al. Cytochrome P450 promiscuity leads to a bifurcating biosynthetic pathway for tanshinones. New Phytol. 2016, 210, 525–534. [Google Scholar] [CrossRef]
- Chen, Z.-X. Prokaryotic Expression, Purification and Crystallography Studies on CYP76AH3 from Salvia miltiorrhiza and CYP725A4 from Taxus wallichiana. Ph.D. Thesis, Peking University Health Science Center, Beijing, China, 2022. [Google Scholar]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. New Phytol. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Weckwerth, P.R.; Poretsky, E.; Murphy, K.M.; Sims, J.; Saldivar, E.; Christensen, S.A.; Char, S.N.; Yang, B.; Tong, A.D.; et al. Genetic elucidation of complex biochemical traits mediating maize innate immunity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Peters, R.J. Gibberellin Phytohormone Metabolism. Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Springer: Berlin/Heidelberg, Germany, 2013; pp. 233–249. [Google Scholar]
- Magome, H.; Nomura, T.; Hanada, A.; Takeda-Kamiya, N.; Ohnishi, T.; Shinma, Y.; Katsumata, T.; Kawaide, H.; Kamiya, Y.; Yamaguchi, S. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Triterpene structural diversification by plant cytochrome P450 enzymes. Front. Plant Sci. 2017, 8, 1886. [Google Scholar] [CrossRef]
- Huang, X.-F.; Qu, T.-T.; Ding, Y.; Lei, T.-T.; Zhang, J.; Tang, X.-M. Chilling resistance of gibberellin-deficiency tomato fruits. Food Ferment. Ind. 2020, 46, 8. [Google Scholar]
- Zhu, Z.; Ding, Y.; Zhao, J.-H.; Nie, Y.; Zhang, Y.; Sheng, J.-P.; Tang, X.-M. Effects of postharvest gibberellic acid treatment on chilling tolerance in cold-stored tomato (Solanum lycopersicum L.) Fruit. Food Bioprocess Technol. 2016, 9, 1202–1209. [Google Scholar] [CrossRef]
- Sun, W.; Leng, L.; Yin, Q.-G.; Xu, M.-M.; Huang, M.-K.; Xu, Z.-C.; Zhang, Y.-J.; Yao, H.; Wang, C.-X.; Xiong, C.; et al. The genome of the medicinal plant Andrographis paniculata provides insight into the biosynthesis of the bioactive diterpenoid neoandrographolide. Plant J. 2018, 97, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.-C.; Peters, R.J. Characterization of CYP76AH4 clarifies phenolic diterpenoid biosynthesis in the Lamiaceae. Org. Biomol. Chem. 2013, 11, 7650–7652. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Cardi, T.; Campanelli, G.; Sestili, S.; JoséDíez, M.; Soler, S.; Prohens, J.; Tripodi, P. ddRAD sequencing-based genotyping for population structure analysis in cultivated tomato provides new insights into the genomic diversity of Mediterranean’da serbo’type long shelf-life germplasm. Hortic. Res. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- CAS 5508-58-7. Available online: https://www.merckmillipore.com/INTL/en/product/Andrographolide-CAS-5508-58-7-Calbiochem,EMD_BIO-172060?ReferrerURL=https%3A%2F%2Fwww.google.com%2F (accessed on 1 October 2024).


| No. | CYP Subfamily | Number of Andrographis Paniculata CYPs | Number of Arabidopsis Thaliana CYPs | Number of Terrestrial Plants Diterpenoid Pathway CYPs |
|---|---|---|---|---|
| 1 | 51 | 1 | 1 | 0 |
| 2 | 71 | 80 | 48 | 11 |
| 3 | 72 | 20 | 8 | 0 |
| 4 | 73 | 2 | 1 | 0 |
| 5 | 74 | 3 | 2 | 0 |
| 6 | 75 | 7 | 1 | 0 |
| 7 | 76 | 23 | 9 | 26 |
| 8 | 77 | 3 | 4 | 0 |
| 9 | 78 | 8 | 6 | 0 |
| 10 | 79 | 8 | 6 | 0 |
| 11 | 81 | 8 | 16 | 0 |
| 12 | 82 | 19 | 5 | 0 |
| 13 | 83 | 15 | 3 | 0 |
| 14 | 84 | 4 | 1 | 0 |
| 15 | 85 | 3 | 1 | 0 |
| 16 | 86 | 17 | 10 | 0 |
| 17 | 87 | 5 | 1 | 0 |
| 18 | 88 | 2 | 2 | 12 |
| 19 | 89 | 0 | 6 | 0 |
| 20 | 90 | 9 | 4 | 0 |
| 21 | 93 | 4 | 1 | 0 |
| 22 | 94 | 12 | 6 | 0 |
| 23 | 96 | 0 | 12 | 0 |
| 24 | 97 | 4 | 3 | 0 |
| 25 | 98 | 2 | 3 | 0 |
| 26 | 701 | 1 | 1 | 23 |
| 27 | 702 | 0 | 6 | 0 |
| 28 | 703 | 1 | 1 | 0 |
| 29 | 704 | 5 | 3 | 0 |
| 30 | 705 | 0 | 25 | 0 |
| 31 | 706 | 2 | 7 | 0 |
| 32 | 707 | 10 | 4 | 0 |
| 33 | 708 | 0 | 3 | 0 |
| 34 | 709 | 2 | 3 | 0 |
| 35 | 710 | 1 | 4 | 0 |
| 36 | 711 | 1 | 1 | 0 |
| 37 | 712 | 0 | 2 | 0 |
| 38 | 714 | 4 | 2 | 5 |
| 39 | 715 | 5 | 1 | 0 |
| 40 | 716 | 13 | 2 | 0 |
| 41 | 718 | 1 | 1 | 0 |
| 42 | 720 | 1 | 1 | 0 |
| 43 | 721 | 0 | 1 | 0 |
| 44 | 722 | 2 | 1 | 0 |
| 45 | 724 | 2 | 1 | 0 |
| 46 | 726 | 0 | 0 | 14 |
| 47 | 734 | 4 | 1 | 0 |
| 48 | 735 | 1 | 2 | 0 |
| 49 | 736 | 8 | 0 | 0 |
| 50 | 749 | 5 | 0 | 0 |
| 51 | CYP family | 17 | 0 | 0 |
| Total | 345 | 233 | 91 |
| Subfamily | Gene Number | Number of Cis-Elements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABA | Anaerobic | Auxin | Stress | GAs | LT | MeJA | Drought | Circadian | Salicylic Acid | Cell Differentiation | Flavonoid Biosynthesis | ||
| CYP88A | 2 | 1 | 6 | 4 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| CYP701A | 1 | 1 | 3 | 0 | 0 | 2 | 0 | 3 | 1 | 0 | 0 | 0 | 0 |
| CYP714s | 4 | 5 | 6 | 2 | 3 | 5 | 8 | 4 | 2 | 2 | 4 | 0 | 0 |
| CYP76s | 3 | 17 | 7 | 3 | 2 | 3 | 6 | 5 | 7 | 3 | 2 | 1 | 0 |
| CYP71s | 55 | 120 | 78 | 30 | 25 | 40 | 53 | 97 | 29 | 25 | 55 | 4 | 2 |
| Total | 65 | 144 | 100 | 39 | 31 | 53 | 68 | 109 | 39 | 30 | 61 | 5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Li, J.; Xu, S.; Gu, Y.; Wang, J. Genome-Wide Identification and Characterization of Diterpenoid Pathway CYPs in Andrographis paniculata and Analysis of Their Expression Patterns under Low Temperature Stress. Int. J. Mol. Sci. 2024, 25, 10741. https://doi.org/10.3390/ijms251910741
Sun M, Li J, Xu S, Gu Y, Wang J. Genome-Wide Identification and Characterization of Diterpenoid Pathway CYPs in Andrographis paniculata and Analysis of Their Expression Patterns under Low Temperature Stress. International Journal of Molecular Sciences. 2024; 25(19):10741. https://doi.org/10.3390/ijms251910741
Chicago/Turabian StyleSun, Mingyang, Jingyu Li, Shiqiang Xu, Yan Gu, and Jihua Wang. 2024. "Genome-Wide Identification and Characterization of Diterpenoid Pathway CYPs in Andrographis paniculata and Analysis of Their Expression Patterns under Low Temperature Stress" International Journal of Molecular Sciences 25, no. 19: 10741. https://doi.org/10.3390/ijms251910741






