Biochemistry and Diseases Related to the Interconversion of Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine
Abstract
1. Introduction
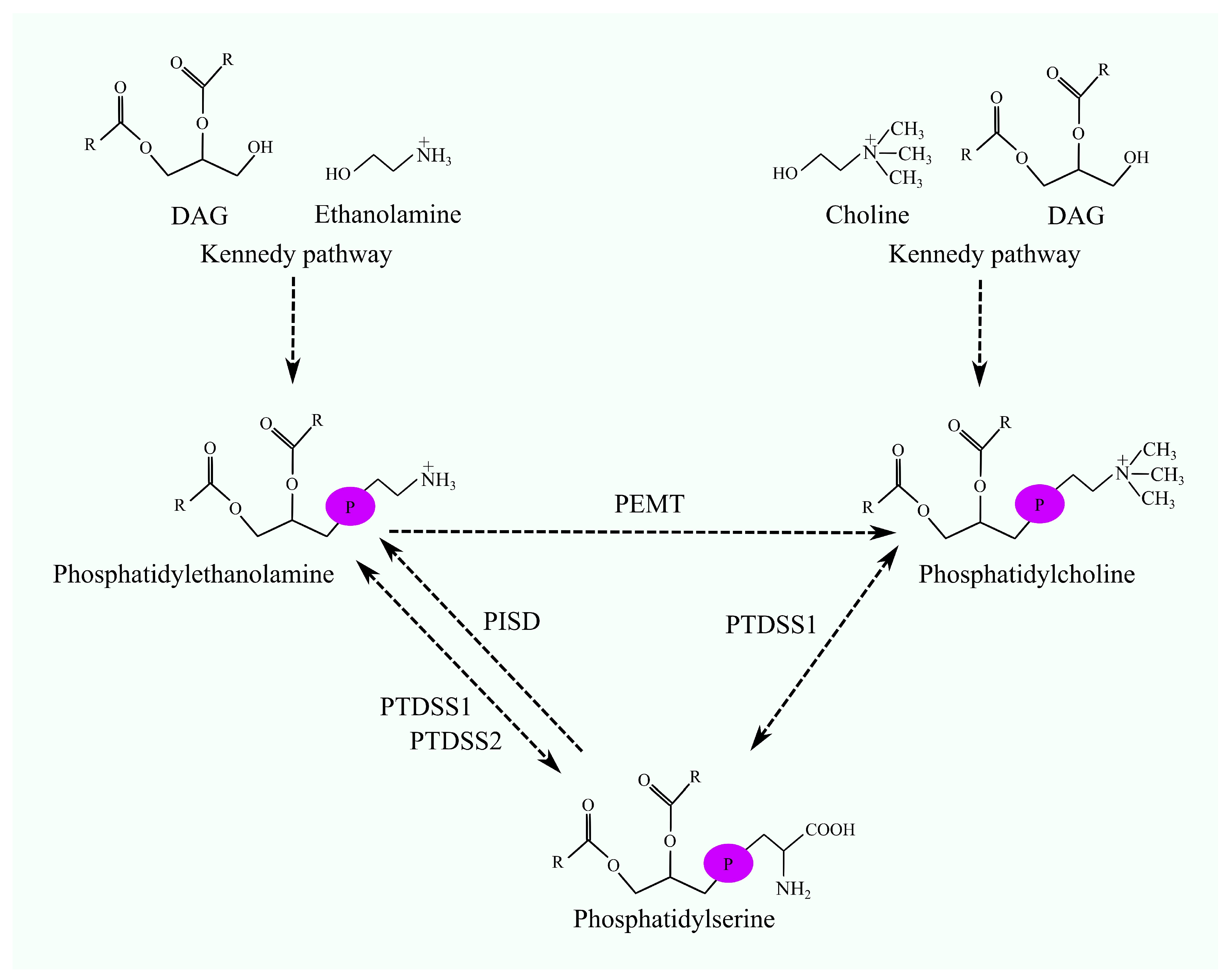
2. Phosphatidylethanolamine N-Methyltransferase
2.1. Biosynthesis of Phosphatidylcholine Involving Phosphatidylethanolamine N-Methyltransferase
2.2. Phosphatidylethanolamine N-Methyltransferase in Cancers
2.3. Phosphatidylethanolamine N-Methyltransferase in Metabolic and Hepatic Diseases
2.4. Phosphatidylethanolamine N-Methyltransferase in Type II Diabetes
2.5. Phosphatidylethanolamine N-Methyltransferase in Atherosclerosis
2.6. Phosphatidylethanolamine N-Methyltransferase in Neurological Diseases
2.7. Phosphatidylethanolamine N-Methyltransferase in Ontogeny
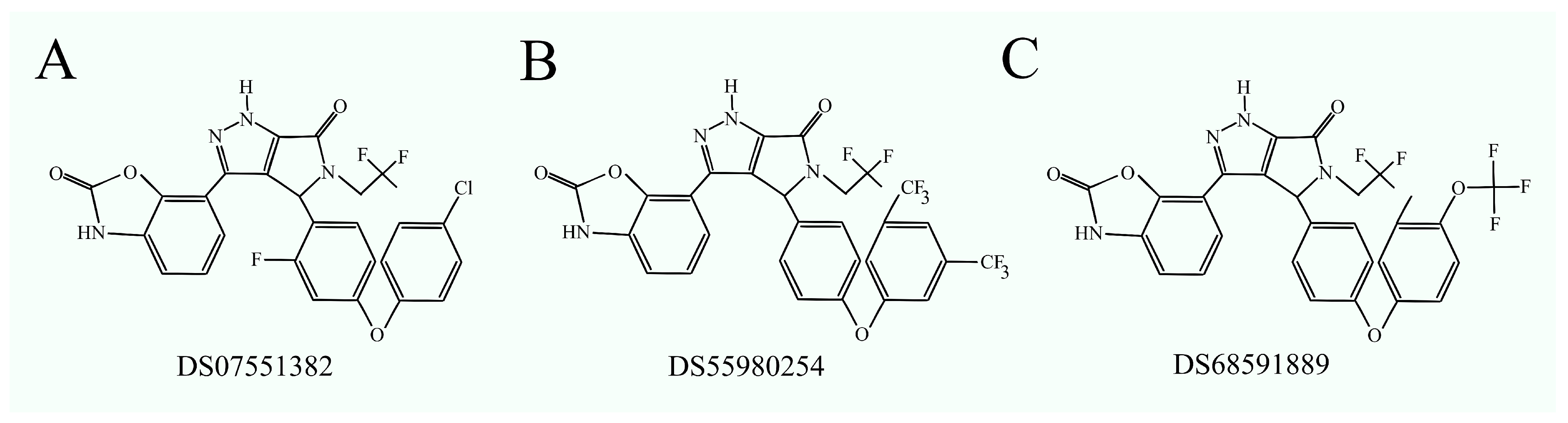
2.8. Inhibitors of Phosphatidylethanolamine N-Methyltransferase
3. Biosynthesis of Phosphatidylserine
3.1. Phosphatidylserine Synthase
3.2. Phosphatidylserine Synthases in Neurological Diseases
3.3. Phosphatidylserine Synthases in Metabolic Diseases
3.4. Phosphatidylserine Synthases in Myocardial Infarction
3.5. Phosphatidylserine Synthases in Cancer
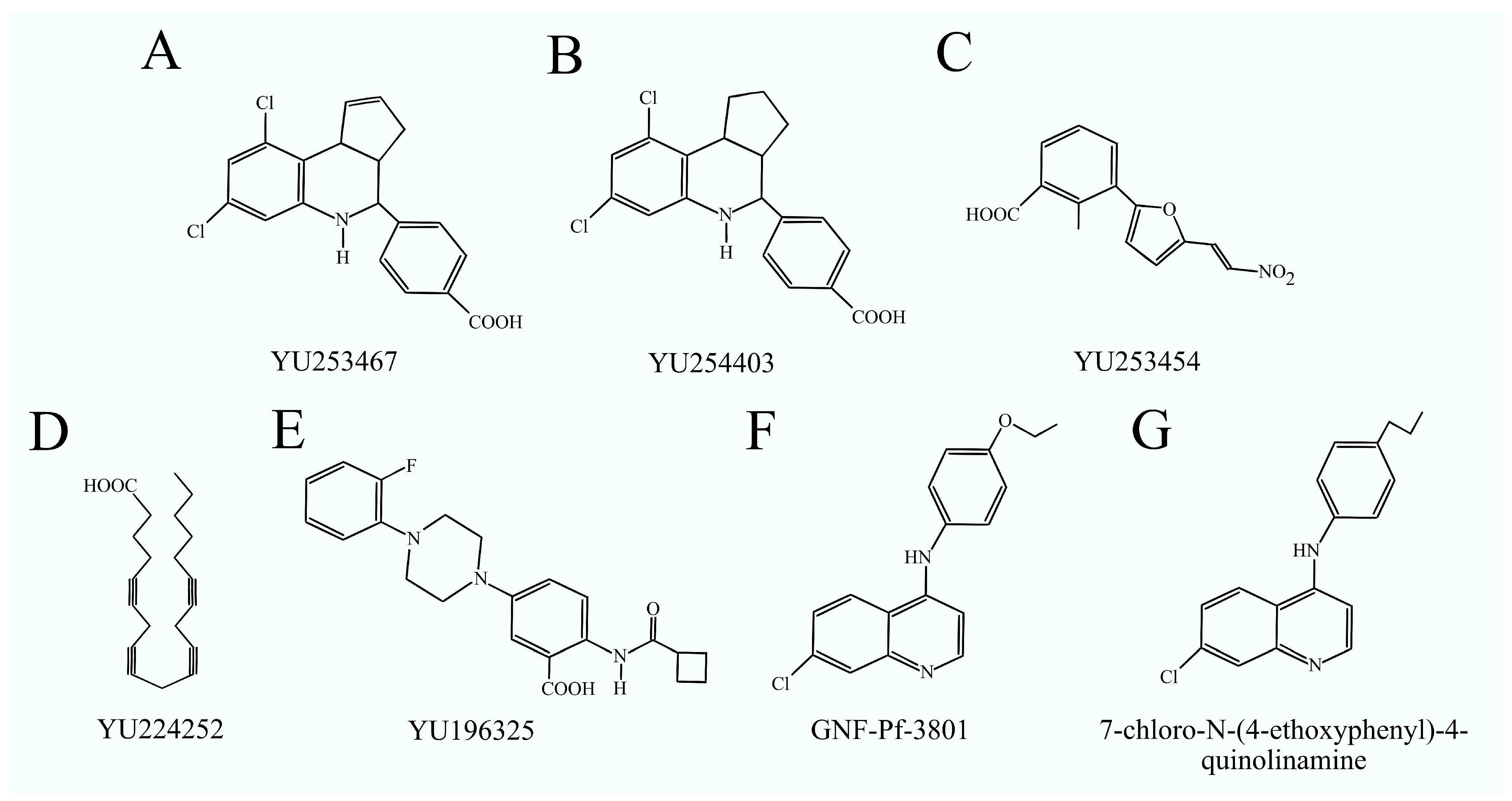
3.6. Phosphatidylserine Synthase Inhibitors as Anticancer Drugs
4. Biosynthesis of Phosphatidylethanolamine Involving Phosphatidylserine Decarboxylase
4.1. Phosphatidylserine Decarboxylase
4.2. Phosphatidylserine Decarboxylase in Cancer
4.3. Phosphatidylserine Decarboxylase Inhibitors
5. Bioinformatics Analysis of the Role of Enzymes Interconverting PC, PE, and PS in Cancer Processes
6. Conclusions
- -
- PEMT demonstrates both pro- and anticancer properties. It is also involved in conditions such as obesity, insulin resistance in obesity, HCV infection, type II diabetes, and atherosclerosis, while offering protection against NAFLD. Specific PEMT inhibitors have not yet been developed, but if they are in the future, they hold significant therapeutic potential, particularly for treating obesity. However, these inhibitors could have side effects, such as inducing NAFLD and impairing nervous system function. This latter issue could potentially be mitigated by designing PEMT inhibitors that do not cross the blood–brain barrier.
- -
- Phosphatidylserine synthases PTDSS1 and PTDSS2 play key roles in cancer progression, making them promising therapeutic targets. The inhibitors of PTDSS1 have shown anticancer potential in in vivo studies, suggesting they could be developed into effective cancer treatments. However, no clinical trials have been conducted with PTDSS1 inhibitors, meaning these compounds have not yet advanced to clinical use.
- -
- The role of PISD in disease is not well understood. The reduced activity of this enzyme in the brain may be associated with aging, highlighting the need for further research into its function in the brain and the consequences of altered activity in older individuals. Such studies could enhance our understanding of the aging process and potentially lead to strategies for slowing or mitigating its effects.
Author Contributions
Funding
Conflicts of Interest
References
- Liepkalns, V.A.; Myher, J.J.; Kuksis, A.; Leli, U.; Freysz, N.; Hauser, G. Molecular Species of Glycerophospholipids and Diacylglycerols of Cultured SH-SY5Y Human Neuroblastoma Cells. Biochem. Cell Biol. 1993, 71, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Author Correction: Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Y.; Fuentes, N.R.; Hou, T.Y.; Barhoumi, R.; Li, X.C.; Deutz, N.E.P.; Engelen, M.P.K.J.; McMurray, D.N.; Chapkin, R.S. Remodelling of Primary Human CD4+ T Cell Plasma Membrane Order by N-3 PUFA. Br. J. Nutr. 2018, 119, 163–175. [Google Scholar] [CrossRef]
- Hellwing, C.; Tigistu-Sahle, F.; Fuhrmann, H.; Käkelä, R.; Schumann, J. Lipid Composition of Membrane Microdomains Isolated Detergent-Free from PUFA Supplemented RAW264.7 Macrophages. J. Cell. Physiol. 2018, 233, 2602–2612. [Google Scholar] [CrossRef]
- Sakuragi, T.; Nagata, S. Regulation of Phospholipid Distribution in the Lipid Bilayer by Flippases and Scramblases. Nat. Rev. Mol. Cell Biol. 2023, 24, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.P.; Weiss, S.B. The Function of Cytidine Coenzymes in the Biosynthesis of Phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [CrossRef]
- Tomohiro, S.; Kawaguti, A.; Kawabe, Y.; Kitada, S.; Kuge, O. Purification and Characterization of Human Phosphatidylserine Synthases 1 and 2. Biochem. J. 2009, 418, 421–429. [Google Scholar] [CrossRef]
- Kuge, O.; Saito, K.; Nishijima, M. Cloning of a Chinese Hamster Ovary (CHO) cDNA Encoding Phosphatidylserine Synthase (PSS) II, Overexpression of Which Suppresses the Phosphatidylserine Biosynthetic Defect of a PSS I-Lacking Mutant of CHO-K1 Cells. J. Biol. Chem. 1997, 272, 19133–19139. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, N.D.; Vance, D.E. Specificity of Rat Hepatic Phosphatidylethanolamine N-Methyltransferase for Molecular Species of Diacyl Phosphatidylethanolamine. J. Biol. Chem. 1988, 263, 16856–16863. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, N.D.; Vance, D.E. Kinetic Mechanism of Phosphatidylethanolamine N-Methyltransferase. J. Biol. Chem. 1988, 263, 16864–16871. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, N.D.; Vance, D.E. Purification of Phosphatidylethanolamine N-Methyltransferase from Rat Liver. J. Biol. Chem. 1987, 262, 17231–17239. [Google Scholar] [CrossRef] [PubMed]
- Kuge, O.; Nishijima, M.; Akamatsu, Y. A Cloned Gene Encoding Phosphatidylserine Decarboxylase Complements the Phosphatidylserine Biosynthetic Defect of a Chinese Hamster Ovary Cell Mutant. J. Biol. Chem. 1991, 266, 6370–6376. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E. Physiological Roles of Phosphatidylethanolamine N-Methyltransferase. Biochim. Biophys. Acta 2013, 1831, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.J.; Shields, D.J.; Vance, D.E. Identification of Three Novel cDNAs for Human Phosphatidylethanolamine N-Methyltransferase and Localization of the Human Gene on Chromosome 17p11.2. Biochim. Biophys. Acta 1999, 1436, 405–412. [Google Scholar] [CrossRef]
- Shields, D.J.; Agellon, L.B.; Vance, D.E. Structure, Expression Profile and Alternative Processing of the Human Phosphatidylethanolamine N-Methyltransferase (PEMT) Gene. Biochim. Biophys. Acta 2001, 1532, 105–114. [Google Scholar] [CrossRef]
- Morita, S.; Takeuchi, A.; Kitagawa, S. Functional Analysis of Two Isoforms of Phosphatidylethanolamine N-Methyltransferase. Biochem. J. 2010, 432, 387–398. [Google Scholar] [CrossRef]
- DeLong, C.J.; Shen, Y.J.; Thomas, M.J.; Cui, Z. Molecular Distinction of Phosphatidylcholine Synthesis between the CDP-Choline Pathway and Phosphatidylethanolamine Methylation Pathway. J. Biol. Chem. 1999, 274, 29683–29688. [Google Scholar] [CrossRef]
- Shields, D.J.; Lehner, R.; Agellon, L.B.; Vance, D.E. Membrane Topography of Human Phosphatidylethanolamine N-Methyltransferase. J. Biol. Chem. 2003, 278, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Matsuyama, M.; Yamaguchi, S.; Katayama, A.; Eguchi, J.; Murakami, K.; Teshigawara, S.; Ogawa, D.; Wada, N.; Yasunaka, T.; et al. Insufficiency of Phosphatidylethanolamine N-Methyltransferase Is Risk for Lean Non-Alcoholic Steatohepatitis. Sci. Rep. 2016, 6, 21721. [Google Scholar] [CrossRef] [PubMed]
- Hörl, G.; Wagner, A.; Cole, L.K.; Malli, R.; Reicher, H.; Kotzbeck, P.; Köfeler, H.; Höfler, G.; Frank, S.; Bogner-Strauss, J.G.; et al. Sequential Synthesis and Methylation of Phosphatidylethanolamine Promote Lipid Droplet Biosynthesis and Stability in Tissue Culture and in Vivo. J. Biol. Chem. 2011, 286, 17338–17350. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakatsuka, A.; Murakami, K.; Inoue, K.; Terami, T.; Higuchi, C.; Katayama, A.; Teshigawara, S.; Eguchi, J.; Ogawa, D.; et al. Pemt Deficiency Ameliorates Endoplasmic Reticulum Stress in Diabetic Nephropathy. PLoS ONE 2014, 9, e92647. [Google Scholar] [CrossRef]
- Zhu, X.; Song, J.; Mar, M.-H.; Edwards, L.J.; Zeisel, S.H. Phosphatidylethanolamine N-Methyltransferase (PEMT) Knockout Mice Have Hepatic Steatosis and Abnormal Hepatic Choline Metabolite Concentrations despite Ingesting a Recommended Dietary Intake of Choline. Biochem. J. 2003, 370, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.L.; Zhao, Y.; Koonen, D.P.Y.; Sletten, T.; Su, B.; Lingrell, S.; Cao, G.; Peake, D.A.; Kuo, M.-S.; Proctor, S.D.; et al. Impaired de Novo Choline Synthesis Explains Why Phosphatidylethanolamine N-Methyltransferase-Deficient Mice Are Protected from Diet-Induced Obesity. J. Biol. Chem. 2010, 285, 22403–22413. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; van der Veen, J.N.; Bakala N’Goma, J.-C.; Nelson, R.C.; Vance, D.E.; Jacobs, R.L. Hepatic PEMT Activity Mediates Liver Health, Weight Gain, and Insulin Resistance. FASEB J. 2019, 33, 10986–10995. [Google Scholar] [CrossRef]
- Watkins, S.M.; Zhu, X.; Zeisel, S.H. Phosphatidylethanolamine-N-Methyltransferase Activity and Dietary Choline Regulate Liver-Plasma Lipid Flux and Essential Fatty Acid Metabolism in Mice. J. Nutr. 2003, 133, 3386–3391. [Google Scholar] [CrossRef]
- Serafim, V.; Chirita-Emandi, A.; Andreescu, N.; Tiugan, D.-A.; Tutac, P.; Paul, C.; Velea, I.; Mihailescu, A.; Șerban, C.L.; Zimbru, C.G.; et al. Single Nucleotide Polymorphisms in PEMT and MTHFR Genes Are Associated with Omega 3 and 6 Fatty Acid Levels in the Red Blood Cells of Children with Obesity. Nutrients 2019, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, F.; Kerbl-Knapp, J.; Korbelius, M.; Kuentzel, K.B.; Vujić, N.; Akhmetshina, A.; Hörl, G.; Paar, M.; Steyrer, E.; et al. Phosphatidylethanolamine N-Methyltransferase Knockout Modulates Metabolic Changes in Aging Mice. Biomolecules 2022, 12, 1270. [Google Scholar] [CrossRef]
- Johnson, J.M.; Verkerke, A.R.P.; Maschek, J.A.; Ferrara, P.J.; Lin, C.-T.; Kew, K.A.; Neufer, P.D.; Lodhi, I.J.; Cox, J.E.; Funai, K. Alternative Splicing of UCP1 by Non-Cell-Autonomous Action of PEMT. Mol. Metab. 2020, 31, 55–66. [Google Scholar] [CrossRef]
- Cui, Z.; Houweling, M.; Vance, D.E. Suppression of Rat Hepatoma Cell Growth by Expression of Phosphatidylethanolamine N-Methyltransferase-2. J. Biol. Chem. 1994, 269, 24531–24533. [Google Scholar] [CrossRef]
- Tessitore, L.; Marengo, B.; Vance, D.E.; Papotti, M.; Mussa, A.; Daidone, M.G.; Costa, A. Expression of Phosphatidylethanolamine N-Methyltransferase in Human Hepatocellular Carcinomas. Oncology 2003, 65, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, D.; Wang, F.; Liu, Y.; Xie, J.; Xie, J.; Xie, Y. Construction of a Novel Choline Metabolism-Related Signature to Predict Prognosis, Immune Landscape, and Chemotherapy Response in Colon Adenocarcinoma. Front. Immunol. 2022, 13, 1038927. [Google Scholar] [CrossRef] [PubMed]
- Zinrajh, D.; Hörl, G.; Jürgens, G.; Marc, J.; Sok, M.; Cerne, D. Increased Phosphatidylethanolamine N-Methyltransferase Gene Expression in Non-Small-Cell Lung Cancer Tissue Predicts Shorter Patient Survival. Oncol. Lett. 2014, 7, 2175–2179. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Liu, T.; Song, Y.; Chen, P.; Liu, L.; Wang, B.; Qu, J. The Association of Serum Choline Concentrations with the Risk of Cancers: A Community-Based Nested Case-Control Study. Sci. Rep. 2023, 13, 22144. [Google Scholar] [CrossRef]
- Yao, N.; Li, W.; Xu, G.; Duan, N.; Yu, G.; Qu, J. Choline Metabolism and Its Implications in Cancer. Front. Oncol. 2023, 13, 1234887. [Google Scholar] [CrossRef]
- Tajuddin, S.M.; Amaral, A.F.S.; Fernández, A.F.; Chanock, S.; Silverman, D.T.; Tardón, A.; Carrato, A.; García-Closas, M.; Jackson, B.P.; Toraño, E.G.; et al. LINE-1 Methylation in Leukocyte DNA, Interaction with Phosphatidylethanolamine N-Methyltransferase Variants and Bladder Cancer Risk. Br. J. Cancer 2014, 110, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant P53 as a Target for Cancer Treatment. Eur. J. Cancer 2017, 83, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gammon, M.D.; Zeisel, S.H.; Lee, Y.L.; Wetmur, J.G.; Teitelbaum, S.L.; Bradshaw, P.T.; Neugut, A.I.; Santella, R.M.; Chen, J. Choline Metabolism and Risk of Breast Cancer in a Population-Based Study. FASEB J. 2008, 22, 2045–2052. [Google Scholar] [CrossRef]
- Li, D.; Bi, F.-F.; Chen, N.-N.; Cao, J.-M.; Sun, W.-P.; Zhou, Y.-M.; Cao, C.; Li, C.-Y.; Yang, Q. Epigenetic Repression of Phosphatidylethanolamine N-Methyltransferase (PEMT) in BRCA1-Mutated Breast Cancer. Oncotarget 2014, 5, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Langberg, K.A.; Mondal, A.K.; Das, S.K. Phospholipid Biosynthesis Genes and Susceptibility to Obesity: Analysis of Expression and Polymorphisms. PLoS ONE 2013, 8, e65303. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, T.-Y.; Chen, Y.-C.; Huang, R.-F.S. PEMT Rs7946 Polymorphism and Sex Modify the Effect of Adequate Dietary Choline Intake on the Risk of Hepatic Steatosis in Older Patients with Metabolic Disorders. Nutrients 2023, 15, 3211. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Holstein, D.J.F.; Garcia-Cubero, N.; Moulla, Y.; Stroh, C.; Dietrich, A.; Schön, M.R.; Gärtner, D.; Lohmann, T.; Dressler, M.; et al. The Role of Phosphatidylethanolamine N-Methyltransferase (PEMT) and Its Waist-Hip-Ratio-Associated Locus Rs4646404 in Obesity-Related Metabolic Traits and Liver Disease. Int. J. Mol. Sci. 2023, 24, 16850. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.N.; Lingrell, S.; da Silva, R.P.; Jacobs, R.L.; Vance, D.E. The Concentration of Phosphatidylethanolamine in Mitochondria Can Modulate ATP Production and Glucose Metabolism in Mice. Diabetes 2014, 63, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.N.; Lingrell, S.; McCloskey, N.; LeBlond, N.D.; Galleguillos, D.; Zhao, Y.Y.; Curtis, J.M.; Sipione, S.; Fullerton, M.D.; Vance, D.E.; et al. A Role for Phosphatidylcholine and Phosphatidylethanolamine in Hepatic Insulin Signaling. FASEB J. 2019, 33, 5045–5057. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-Fat Diet-Induced Upregulation of Exosomal Phosphatidylcholine Contributes to Insulin Resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; van der Veen, J.N.; Hermansson, M.; Ordoñez, M.; Gomez-Muñoz, A.; Vance, D.E.; Jacobs, R.L. Decreased Lipogenesis in White Adipose Tissue Contributes to the Resistance to High Fat Diet-Induced Obesity in Phosphatidylethanolamine N-Methyltransferase-Deficient Mice. Biochim. Biophys. Acta 2015, 1851, 152–162. [Google Scholar] [CrossRef]
- Tasseva, G.; van der Veen, J.N.; Lingrell, S.; Jacobs, R.L.; Vance, D.E.; Vance, J.E. Lack of Phosphatidylethanolamine N-Methyltransferase in Mice Does Not Promote Fatty Acid Oxidation in Skeletal Muscle. Biochim. Biophys. Acta 2016, 1861, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Verkerke, A.R.P.; Ferrara, P.J.; Lin, C.-T.; Johnson, J.M.; Ryan, T.E.; Maschek, J.A.; Eshima, H.; Paran, C.W.; Laing, B.T.; Siripoksup, P.; et al. Phospholipid Methylation Regulates Muscle Metabolic Rate through Ca2+ Transport Efficiency. Nat. Metab. 2019, 1, 876–885. [Google Scholar] [CrossRef]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice Fed a Lipogenic Methionine-Choline-Deficient Diet Develop Hypermetabolism Coincident with Hepatic Suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef]
- Presa, N.; Dominguez-Herrera, A.; van der Veen, J.N.; Vance, D.E.; Gómez-Muñoz, A. Implication of Phosphatidylethanolamine N-Methyltransferase in Adipocyte Differentiation. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165853. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; da Costa, K.A.; Fischer, L.M.; Kohlmeier, M.; Kwock, L.; Wang, S.; Zeisel, S.H. Polymorphism of the PEMT Gene and Susceptibility to Nonalcoholic Fatty Liver Disease (NAFLD). FASEB J 2005, 19, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, J.; Li, C.; Hirose, A.; Nozaki, Y.; Takahashi, M.; Ono, M.; Akisawa, N.; Iwasaki, S.; Saibara, T.; et al. The Phosphatidylethanolamine N-Methyltransferase Gene V175M Single Nucleotide Polymorphism Confers the Susceptibility to NASH in Japanese Population. J. Hepatol. 2007, 46, 915–920. [Google Scholar] [CrossRef]
- Tan, H.-L.; Mohamed, R.; Mohamed, Z.; Zain, S.M. Phosphatidylethanolamine N-Methyltransferase Gene Rs7946 Polymorphism Plays a Role in Risk of Nonalcoholic Fatty Liver Disease: Evidence from Meta-Analysis. Pharmacogenet. Genom. 2016, 26, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bale, G.; Vishnubhotla, R.V.; Mitnala, S.; Sharma, M.; Padaki, R.N.; Pawar, S.C.; Duvvur, R.N. Whole-Exome Sequencing Identifies a Variant in Phosphatidylethanolamine N-Methyltransferase Gene to Be Associated with Lean-Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Piras, I.S.; Raju, A.; Don, J.; Schork, N.J.; Gerhard, G.S.; DiStefano, J.K. Hepatic PEMT Expression Decreases with Increasing NAFLD Severity. Int. J. Mol. Sci. 2022, 23, 9296. [Google Scholar] [CrossRef]
- Abomughaid, M.; Tay, E.S.E.; Pickford, R.; Malladi, C.; Read, S.A.; Coorssen, J.R.; Gloss, B.S.; George, J.; Douglas, M.W. PEMT Mediates Hepatitis C Virus-Induced Steatosis, Explains Genotype-Specific Phenotypes and Supports Virus Replication. Int. J. Mol. Sci. 2023, 24, 8781. [Google Scholar] [CrossRef]
- Hartz, C.S.; Nieman, K.M.; Jacobs, R.L.; Vance, D.E.; Schalinske, K.L. Hepatic Phosphatidylethanolamine N-Methyltransferase Expression Is Increased in Diabetic Rats. J. Nutr. 2006, 136, 3005–3009. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Su, B.; Jacobs, R.L.; Kennedy, B.; Francis, G.A.; Waddington, E.; Brosnan, J.T.; Vance, J.E.; Vance, D.E. Lack of Phosphatidylethanolamine N-Methyltransferase Alters Plasma VLDL Phospholipids and Attenuates Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K.; Dolinsky, V.W.; Dyck, J.R.B.; Vance, D.E. Impaired Phosphatidylcholine Biosynthesis Reduces Atherosclerosis and Prevents Lipotoxic Cardiac Dysfunction in ApoE−/− Mice. Circ. Res. 2011, 108, 686–694. [Google Scholar] [CrossRef]
- Zia, Y.; Al Rajabi, A.; Mi, S.; Ju, T.; Leonard, K.-A.; Nelson, R.; Thiesen, A.; Willing, B.P.; Field, C.J.; Curtis, J.M.; et al. Hepatic Expression of PEMT, but Not Dietary Choline Supplementation, Reverses the Protection against Atherosclerosis in Pemt−/−/Ldlr−/− Mice. J. Nutr. 2018, 148, 1513–1520. [Google Scholar] [CrossRef]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine Biosynthesis and Lipoprotein Metabolism. Biochim. Biophys. Acta 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- Zhu, X.; Zeisel, S.H. Gene Expression Profiling in Phosphatidylethanolamine N-Methyltransferase Knockout Mice. Brain Res. Mol. Brain Res. 2005, 134, 239–255. [Google Scholar] [CrossRef]
- Zhu, X.; Mar, M.-H.; Song, J.; Zeisel, S.H. Deletion of the Pemt Gene Increases Progenitor Cell Mitosis, DNA and Protein Methylation and Decreases Calretinin Expression in Embryonic Day 17 Mouse Hippocampus. Brain Res. Dev. Brain Res. 2004, 149, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Ju, G.; Zhang, X.; Xu, Q.; Liu, S.; Yu, Y.; Shi, J.; Boyle, S.; Wang, Z.; et al. A Study of the PEMT Gene in Schizophrenia. Neurosci. Lett. 2007, 424, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Liu, L.; Ju, G.; Jin, S.; Ye, L.; Zhang, X.; Wei, J. No Association of the Rs4646396 SNP in the PEMT Locus with Schizophrenia in a Chinese Case-Control Sample. Psychiatry Res. 2009, 169, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.-H.; Zhao, H.-L.; Zhang, Z.-X.; Zhang, J.-W. PEMT G523A (V175M) Is Associated with Sporadic Alzheimer’s Disease in a Chinese Population. J. Mol. Neurosci. 2012, 46, 505–508. [Google Scholar] [CrossRef]
- Guan, Z.Z.; Wang, Y.N.; Xiao, K.Q.; Hu, P.S.; Liu, J.L. Activity of Phosphatidylethanolamine-N-Methyltransferase in Brain Affected by Alzheimer’s Disease. Neurochem. Int. 1999, 34, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. J. Alzheimers Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Otoki, Y.; Kato, S.; Nakagawa, K.; Harvey, D.J.; Jin, L.-W.; Dugger, B.N.; Taha, A.Y. Lipidomic Analysis of Postmortem Prefrontal Cortex Phospholipids Reveals Changes in Choline Plasmalogen Containing Docosahexaenoic Acid and Stearic Acid between Cases with and without Alzheimer’s Disease. Neuromol. Med. 2021, 23, 161–175. [Google Scholar] [CrossRef]
- Meletis, C.D. Alkyl-Acylglycerols and the Important Clinical Ramifications of Raising Plasmalogens in Dementia and Alzheimer’s Disease. Integr. Med. 2020, 19, 12–16. [Google Scholar]
- Suazo, J.; Salamanca, C.; González-Hormazábal, P.; Cáceres-Rojas, G.; Pantoja, R.; Leiva, N.; Pardo, R. PEMT Variants Are Associated with Nonsyndromic Cleft Lip with or without Cleft Palate in Chile. Epigenomics 2022, 14, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, Y.-H.; He, X.-L.; Kohlmeier, M.; Zhou, L.-L.; Shen, L.-W.; Yi, X.-X.; Tang, Q.-Y.; Cai, W.; Wang, B. Dietary Choline Intake during Pregnancy and PEMT Rs7946 Polymorphism on Risk of Preterm Birth: A Case-Control Study. Ann. Nutr. Metab. 2020, 76, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Seremak-Mrozikiewicz, A.; Barlik, M.; Różycka, A.; Kurzawińska, G.; Klejewski, A.; Wolski, H.; Drews, K. Importance of Polymorphic Variants of Phosphatidylethanolamine N-Methyltransferase (PEMT) Gene in the Etiology of Intrauterine Fetal Death in the Polish Population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 43–47. [Google Scholar] [CrossRef] [PubMed]
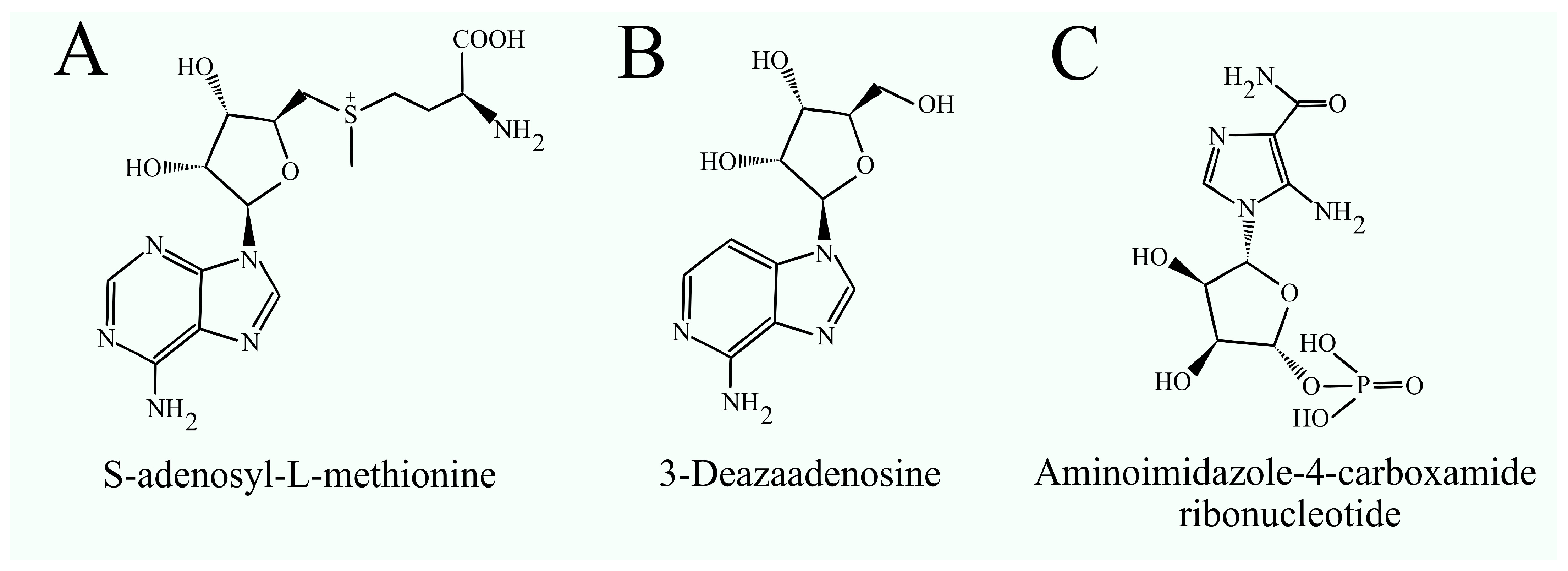
- Pritchard, P.H.; Chiang, P.K.; Cantoni, G.L.; Vance, D.E. Inhibition of Phosphatidylethanolamine N-Methylation by 3-Deazaadenosine Stimulates the Synthesis of Phosphatidylcholine via the CDP-Choline Pathway. J. Biol. Chem. 1982, 257, 6362–6367. [Google Scholar] [CrossRef]
- Samborski, R.W.; Ridgway, N.D.; Vance, D.E. Metabolism of Molecular Species of Phosphatidylethanolamine and Phosphatidylcholine in Rat Hepatocytes during Prolonged Inhibition of Phosphatidylethanolamine N-Methyltransferase. J. Lipid Res. 1993, 34, 125–137. [Google Scholar] [CrossRef]
- Jaroonwitchawan, T.; Visitchanakun, P.; Dang, P.C.; Ritprajak, P.; Palaga, T.; Leelahavanichkul, A. Dysregulation of Lipid Metabolism in Macrophages Is Responsible for Severe Endotoxin Tolerance in FcgRIIB-Deficient Lupus Mice. Front. Immunol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Backlund, P.S.; Carotti, D.; Cantoni, G.L. Effects of the S-Adenosylhomocysteine Hydrolase Inhibitors 3-Deazaadenosine and 3-Deazaaristeromycin on RNA Methylation and Synthesis. Eur. J. Biochem. 1986, 160, 245–251. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, H.S.; Rhim, H.; Kim, B.E.; Jeong, S.W.; Kim, I.K. Activation of Caspase-8 in 3-Deazaadenosine-Induced Apoptosis of U-937 Cells Occurs Downstream of Caspase-3 and Caspase-9 without Fas Receptor-Ligand Interaction. Exp. Mol. Med. 2001, 33, 284–292. [Google Scholar] [CrossRef]
- Stone, S.J.; Vance, J.E. Phosphatidylserine Synthase-1 and -2 Are Localized to Mitochondria-Associated Membranes. J Biol Chem 2000, 275, 34534–34540. [Google Scholar] [CrossRef]
- Saito, K.; Kuge, O.; Akamatsu, Y.; Nishijima, M. Immunochemical Identification of the pssA Gene Product as Phosphatidylserine Synthase I of Chinese Hamster Ovary Cells. FEBS Lett. 1996, 395, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N.; Kuge, O. Topology of Phosphatidylserine Synthase 1 in the Endoplasmic Reticulum Membrane. Protein Sci 2021, 30, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Bigelow, J.; Kevala, J.H. Substrate Preference in Phosphatidylserine Biosynthesis for Docosahexaenoic Acid Containing Species. Biochemistry 2004, 43, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.K.; Kim, H.-Y. Phosphatidylserine Synthase 2: High Efficiency for Synthesizing Phosphatidylserine Containing Docosahexaenoic Acid. J. Lipid Res. 2013, 54, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.B.; Jenkins, D.; Chanudet, E.; Tasseva, G.; Ishida, M.; Anderson, G.; Docker, J.; Ryten, M.; Sa, J.; Saraiva, J.M.; et al. Gain-of-Function Mutations in the Phosphatidylserine Synthase 1 (PTDSS1) Gene Cause Lenz-Majewski Syndrome. Nat. Genet. 2014, 46, 70–76. [Google Scholar] [CrossRef]
- Whyte, M.P.; Blythe, A.; McAlister, W.H.; Nenninger, A.R.; Bijanki, V.N.; Mumm, S. Lenz-Majewski Hyperostotic Dwarfism with Hyperphosphoserinuria from a Novel Mutation in PTDSS1 Encoding Phosphatidylserine Synthase 1. J. Bone Miner. Res. 2015, 30, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Gracie, S.; Sengupta, N.; Ferreira, C.; Pemberton, J.; Anderson, I.; Wang, X.; Rhodes, L.; Brown, K.; Balla, T.; Larson, A. De Novo Loss-of-Function Variant in PTDSS1 Is Associated with Developmental Delay. Am. J. Med. Genet. A 2022, 188, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yin, T.; Shinozaki, K.; Lampe, J.W.; Stevens, J.F.; Becker, L.B.; Kim, J. Comprehensive Analysis of Phospholipids in the Brain, Heart, Kidney, and Liver: Brain Phospholipids Are Least Enriched with Polyunsaturated Fatty Acids. Mol. Cell Biochem. 2018, 442, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Glade, M.J.; Smith, K. Phosphatidylserine and the Human Brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99–108. [Google Scholar] [CrossRef]
- Soueid, J.; Kourtian, S.; Makhoul, N.J.; Makoukji, J.; Haddad, S.; Ghanem, S.S.; Kobeissy, F.; Boustany, R.-M. RYR2, PTDSS1 and AREG Genes Are Implicated in a Lebanese Population-Based Study of Copy Number Variation in Autism. Sci. Rep. 2016, 6, 19088. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Huang, X. Identification of Major Depressive Disorder Disease-Related Genes and Functional Pathways Based on System Dynamic Changes of Network Connectivity. BMC Med. Genom. 2021, 14, 55. [Google Scholar] [CrossRef]
- Johnson, J.M.; Peterlin, A.D.; Balderas, E.; Sustarsic, E.G.; Maschek, J.A.; Lang, M.J.; Jara-Ramos, A.; Panic, V.; Morgan, J.T.; Villanueva, C.J.; et al. Mitochondrial Phosphatidylethanolamine Modulates UCP1 to Promote Brown Adipose Thermogenesis. Sci. Adv. 2023, 9, eade7864. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X.; Wang, T.; Cai, W.; Hua, T.; Duan, J.; Zhang, X.; Zhu, Y.; Yao, L. Metabolic Changes of Glycerophospholipids during the Reparative Phase after Myocardial Infarction Injury. Front. Cardiovasc. Med. 2023, 10, 1122571. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Curaj, A.; Staudt, M.; Cordes, F.; Dumitraşcu, A.R.; Rolles, B.; Beckers, C.; Soppert, J.; Rusu, M.; Simsekyilmaz, S.; et al. Phosphatidylserine Supplementation as a Novel Strategy for Reducing Myocardial Infarct Size and Preventing Adverse Left Ventricular Remodeling. Int. J. Mol. Sci. 2021, 22, 4401. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D.; Pound, J.D. Cell Death in the Neighbourhood: Direct Microenvironmental Effects of Apoptosis in Normal and Neoplastic Tissues. J. Pathol. 2011, 223, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-Mediated Cleavage of Phospholipid Flippase for Apoptotic Phosphatidylserine Exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef]
- Liang, X.; Luo, M.; Shao, B.; Yang, J.-Y.; Tong, A.; Wang, R.-B.; Liu, Y.-T.; Jun, R.; Liu, T.; Yi, T.; et al. Phosphatidylserine Released from Apoptotic Cells in Tumor Induces M2-like Macrophage Polarization through the PSR-STAT3-JMJD3 Axis. Cancer Commun. 2022, 42, 205–222. [Google Scholar] [CrossRef]
- Sekar, D.; Dillmann, C.; Sirait-Fischer, E.; Fink, A.F.; Zivkovic, A.; Baum, N.; Strack, E.; Klatt, S.; Zukunft, S.; Wallner, S.; et al. Phosphatidylserine Synthase PTDSS1 Shapes the Tumor Lipidome to Maintain Tumor-Promoting Inflammation. Cancer Res. 2022, 82, 1617–1632. [Google Scholar] [CrossRef]
- Xu, J.; Su, S.M.; Zhang, X.; Chan, U.I.; Adhav, R.; Shu, X.; Liu, J.; Li, J.; Mo, L.; Wang, Y.; et al. ATP11B Inhibits Breast Cancer Metastasis in a Mouse Model by Suppressing Externalization of Nonapoptotic Phosphatidylserine. J. Clin. Investig. 2022, 132, e149473. [Google Scholar] [CrossRef]
- Liu, G.; Li, H.; Zhang, W.; Yu, J.; Zhang, X.; Wu, R.; Niu, M.; Liu, X.; Yu, R. Csnk1a1 Inhibition Modulates the Inflammatory Secretome and Enhances Response to Radiotherapy in Glioma. J. Cell. Mol. Med. 2021, 25, 7395–7406. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Yoshihama, Y.; Namiki, H.; Kato, T.; Shimazaki, N.; Takaishi, S.; Kadoshima-Yamaoka, K.; Yukinaga, H.; Maeda, N.; Shibutani, T.; Fujimoto, K.; et al. Potent and Selective PTDSS1 Inhibitors Induce Collateral Lethality in Cancers with PTDSS2 Deletion. Cancer Res. 2022, 82, 4031–4043. [Google Scholar] [CrossRef]
- Omi, J.; Kato, T.; Yoshihama, Y.; Sawada, K.; Kono, N.; Aoki, J. Phosphatidylserine Synthesis Controls Oncogenic B Cell Receptor Signaling in B Cell Lymphoma. J. Cell Biol. 2024, 223, e202212074. [Google Scholar] [CrossRef]
- Hovius, R.; Faber, B.; Brigot, B.; Nicolay, K.; de Kruijff, B. On the Mechanism of the Mitochondrial Decarboxylation of Phosphatidylserine. J. Biol. Chem. 1992, 267, 16790–16795. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, R.; Nanowski, T.S.; Beigneux, A.; Kulinski, A.; Young, S.G.; Vance, J.E. Disruption of the Phosphatidylserine Decarboxylase Gene in Mice Causes Embryonic Lethality and Mitochondrial Defects. J. Biol. Chem. 2005, 280, 40032–40040. [Google Scholar] [CrossRef]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine Deficiency in Mammalian Mitochondria Impairs Oxidative Phosphorylation and Alters Mitochondrial Morphology. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef]
- Kumar, S.; Chitraju, C.; Farese, R.V.; Walther, T.C.; Burd, C.G. Conditional Targeting of Phosphatidylserine Decarboxylase to Lipid Droplets. Biol. Open 2021, 10, bio058516. [Google Scholar] [CrossRef]
- Bleijerveld, O.B.; Brouwers, J.F.H.M.; Vaandrager, A.B.; Helms, J.B.; Houweling, M. The CDP-Ethanolamine Pathway and Phosphatidylserine Decarboxylation Generate Different Phosphatidylethanolamine Molecular Species. J. Biol. Chem. 2007, 282, 28362–28372. [Google Scholar] [CrossRef] [PubMed]
- Kevala, J.H.; Kim, H.Y. Determination of Substrate Preference in Phosphatidylserine Decarboxylation by Liquid Chromatography-Electrospray Ionization Mass Spectrometry. Anal. Biochem. 2001, 292, 130–138. [Google Scholar] [CrossRef]
- Selathurai, A.; Kowalski, G.M.; Mason, S.A.; Callahan, D.L.; Foletta, V.C.; Della Gatta, P.A.; Lindsay, A.; Hamley, S.; Kaur, G.; Curtis, A.R.; et al. Phosphatidylserine Decarboxylase Is Critical for the Maintenance of Skeletal Muscle Mitochondrial Integrity and Muscle Mass. Mol. Metab. 2019, 27, 33–46. [Google Scholar] [CrossRef]
- Salvador, G.A.; López, F.M.; Giusto, N.M. Age-Related Changes in Central Nervous System Phosphatidylserine Decarboxylase Activity. J. Neurosci. Res. 2002, 70, 283–289. [Google Scholar] [CrossRef]
- Girisha, K.M.; von Elsner, L.; Neethukrishna, K.; Muranjan, M.; Shukla, A.; Bhavani, G.S.; Nishimura, G.; Kutsche, K.; Mortier, G. The Homozygous Variant c.797G>A/p.(Cys266Tyr) in PISD Is Associated with a Spondyloepimetaphyseal Dysplasia with Large Epiphyses and Disturbed Mitochondrial Function. Hum. Mutat. 2019, 40, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Humphries, B.; Brien, R.; Gibbons, A.E.; Chen, Y.-T.; Qyli, T.; Haley, H.R.; Pirone, M.E.; Chiang, B.; Xiao, A.; et al. Functional Isolation of Tumor-Initiating Cells Using Microfluidic-Based Migration Identifies Phosphatidylserine Decarboxylase as a Key Regulator. Sci. Rep. 2018, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Hendricson, A.; Umlauf, S.; Choi, J.-Y.; Thekkiniath, J.; Surovtseva, Y.V.; Fuller, K.K.; Reynolds, T.B.; Voelker, D.R.; Ben Mamoun, C. High-Throughput Screening for Phosphatidylserine Decarboxylase Inhibitors Using a Distyrylbenzene-Bis-Aldehyde (DSB-3)-Based Fluorescence Assay. J. Biol. Chem. 2019, 294, 12146–12156. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kumar, V.; Pachikara, N.; Garg, A.; Lawres, L.; Toh, J.Y.; Voelker, D.R.; Ben Mamoun, C. Characterization of Plasmodium Phosphatidylserine Decarboxylase Expressed in Yeast and Application for Inhibitor Screening. Mol. Microbiol. 2016, 99, 999–1014. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
| Name of the Cancer | PEMT | PTDSS1 | PTDSS2 | PISD |
|---|---|---|---|---|
| Adrenocortical carcinoma | - | ↓ | ↓ p = 0.10 | - |
| Bladder urothelial carcinoma | - | - | ↓ | ↑ |
| Breast invasive carcinoma | - | ↓ p = 0.088 | - | - |
| Cervical squamous cell carcinoma and endocervical adenocarcinoma | - | - | - | - |
| Cholangiocarcinoma | ↓ p = 0.085 | - | - | - |
| Colon adenocarcinoma | - | - | - | - |
| Lymphoid neoplasm diffuse large B-cell lymphoma | - | - | - | - |
| Esophageal carcinoma | - | - | - | - |
| Glioblastoma multiforme | - | - | ↓ | - |
| Head and neck squamous cell carcinoma | - | ↓ | - | - |
| Kidney chromophobe | - | ↓ | ↓ p = 0.088 | - |
| Kidney renal clear cell carcinoma | - | ↑ | - | ↓ p = 0.083 |
| Kidney renal papillary cell carcinoma | - | - | ↑ p = 0.094 | - |
| Acute myeloid leukemia | - | - | - | - |
| Brain lower grade glioma | - | ↓ | - | - |
| Liver hepatocellular carcinoma | - | ↓ | ↓ | ↓ |
| Lung adenocarcinoma | - | ↓ p = 0.072 | - | - |
| Lung squamous cell carcinoma | ↓ | - | - | - |
| Mesothelioma | ↓ p = 0.082 | ↓ | - | - |
| Ovarian serous cystadenocarcinoma | - | ↓ p = 0.089 | - | - |
| Pancreatic adenocarcinoma | ↑ | - | ↑ | ↑ |
| Pheochromocytoma and Paraganglioma | - | - | - | - |
| Prostate adenocarcinoma | - | ↓ | - | - |
| Rectum adenocarcinoma | - | - | - | ↑ |
| Sarcoma | - | ↓ | - | - |
| Skin cutaneous melanoma | ↓ | ↓ p = 0.057 | - | - |
| Stomach adenocarcinoma | - | - | - | - |
| Testicular germ cell tumors | - | - | - | - |
| Thyroid carcinoma | - | - | - | - |
| Thymoma | - | ↑ p = 0.093 | ↑ p = 0.061 | - |
| Uterine corpus endometrial carcinoma | - | - | - | - |
| Uterine carcinosarcoma | - | - | - | - |
| Uveal Melanoma | - | ↓ | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, J.; Bosiacki, M.; Kupnicka, P.; Barczak, K.; Ziętek, P.; Chlubek, D.; Baranowska-Bosiacka, I. Biochemistry and Diseases Related to the Interconversion of Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine. Int. J. Mol. Sci. 2024, 25, 10745. https://doi.org/10.3390/ijms251910745
Korbecki J, Bosiacki M, Kupnicka P, Barczak K, Ziętek P, Chlubek D, Baranowska-Bosiacka I. Biochemistry and Diseases Related to the Interconversion of Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine. International Journal of Molecular Sciences. 2024; 25(19):10745. https://doi.org/10.3390/ijms251910745
Chicago/Turabian StyleKorbecki, Jan, Mateusz Bosiacki, Patrycja Kupnicka, Katarzyna Barczak, Paweł Ziętek, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2024. "Biochemistry and Diseases Related to the Interconversion of Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine" International Journal of Molecular Sciences 25, no. 19: 10745. https://doi.org/10.3390/ijms251910745
APA StyleKorbecki, J., Bosiacki, M., Kupnicka, P., Barczak, K., Ziętek, P., Chlubek, D., & Baranowska-Bosiacka, I. (2024). Biochemistry and Diseases Related to the Interconversion of Phosphatidylcholine, Phosphatidylethanolamine, and Phosphatidylserine. International Journal of Molecular Sciences, 25(19), 10745. https://doi.org/10.3390/ijms251910745






